Pharmacology & Pharmacy
Vol.3 No.3(2012), Article ID:20649,6 pages DOI:10.4236/pp.2012.33048
Disruption of Drug Effects (Dopamine, Nicotine, Pilocarpine, (k-Opioid) in Planarians by UV Light
![]()
1Department of Pharmaceutical Sciences, School of Pharmacy, Temple University, Philadelphia, USA; 2Department of Pharmacology, Temple University School of Medicine and Center for Substance Abuse Research, Temple University, Philadelphia, USA.
Email: *robert.raffa@temple.edu
Received March 14th, 2012; revised April 25th, 2012; accepted May 11th, 2012
Keywords: UV Light; Drug-Receptor Interaction; Locomotor Activity; Seizure; Physical Dependence; Planarian
ABSTRACT
Based on previous work, it has been hypothesized that the energetics of ultraviolet (UV) light disrupts effects induced by receptor-binding ligands. If this hypothesis is true, then UV light should 1) disrupt a broad variety of endpoints and 2) disrupt effects produced by ligands that bind to diverse receptor types. This was tested directly in the present study by using ligands selective for four different receptors (one ionotropic, three metabotropic) and three different behavioral endpoints. The selective dopamine D2 receptor antagonist (–)sulpiride (0.1 mM) dose-relatedly decreased spontaneous locomotor velocity, the selective nicotinic acetylcholine receptor agonist nicotine (1, 3, 5 mM) and the selective muscarinic acetylcholine receptor agonist pilocarpine (20, 30, 50 mM) induced seizure-like activity, and the selective k-opioid receptor agonist U-50,488H (10 mM) produced physical dependence (manifested as abstinence-induced withdrawal) in planarian models. Each of these diverse ligand and receptor-mediated effects were attenuated by UV light (254 nm = 7.83 × 10–19 J = 4.89 eV). These findings provide further evidence that UV light disrupts ligand-receptor mediated interactions and that UV light might provide a useful tool for examining drug-receptor interactions.
1. Introduction
A photodynamic effect is produced whenever an isolated rabbit thoracic aorta, which has been previously contracted to a steady-state isometric tension by an aadrenoceptor agonist, is exposed to ultraviolet (UV) light [1,2]. The drug-induced isometric tension rapidly decreases, remains low during the duration of exposure to UV light, and spontaneously returns to baseline tension upon removal of the UV light. The phenomenon occurs independently of the agonist used, but in agonist-specific pharmacodynamic manner. We (RJT, RBR) have previously published evidence suggesting that the photorelaxation is due to the disruption of ligand-receptor bonds [3-6]. Some of the evidence includes (see the review [4]): comparison of agonist dissociation constants [7]; the use of selective receptor antagonists; potentiation of g aminobutyric acid (GABA)-induced current mediated by a1b2g2 GABA receptors [8]; and a decreased number of exposed antigenic determinants, which do not contain photoreactive amino acid residues, on bacteriophage MS2 [9].
We previously published on the utility of planarians for investigating drug action and physiological processes involved in drug abuse [10]. Planarians have a simplified nervous system and mammalian-like neurotransmitter systems, such as dopamine (e.g., [11,12]), acetylcholine [11,12], and opioid [13]. Planarians respond with doserelated behavioral changes to drug exposure or to withdrawal. As an example, dopamine D1- and D2-receptor agonists, antagonists, or inhibitors of neuronal dopamine reuptake alter planarian locomotor activity (“motility”) (e.g., [11,12,14-18]; anticonvulsants inhibit seizure-like activity (pSLA) [19]; and abstinenceand antagonistinduced withdrawal signs are elicited in planarian model of physical dependence [20-23]. Attenuation of these effects by receptor-selective antagonists supports recaptor-mediated mechanisms.
We have shown that a decrease in planarian spontaneous locomotor velocity (pLMV) produced by the selective dopamine D2 receptor antagonist sulpiride (5-(Aminosufonyl)-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxy benzamide) occurs in an enantiomeric-selective-((–)sulpiride >> (+)sulpiride) and in a dose-dependent manner [24], and that the effect is attenuated by UV light [25]. Amphetamine produces the opposite effect on pLMV (i.e., increases), and the increase is also attenuated by UV light [18]. That the UV light disrupts opposite drug-induced effects on pLMV is consistent with an action at receptors or transduction pathways. If true, then UV light should affect a variety of receptor-mediated effects. The purpose of the present study was to extend and challenge this hypothesis by determining if UV light would attenuate diverse endpoints––viz., pLMV [24], pSLA [19], and physical dependence (withdrawal signs) [20,21]––produced by multiple diverse drugs––viz., (–)sulpiride, nicotine, pilocarpine, and U-50,488H (trans-(±)-3,4-Dichlro-Nmethyl-N-(2-[1-pyrrolidinyl]cyclohexyl)-benzeneacetamide).
2. Materials and Methods
2.1. Animals
All planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA) were and acclimated to laboratory conditions, including the ambient room temperature (21˚C). They were tested within two days of receipt. All chemicals were purchased from commercial sources.
2.2. pLMV
As previously described [24], each planarian was placed individually into a clear plastic petri dish (diameter = 60 mm and height = 15 mm) containing room-temperature water and placed over grid paper. pLMV was measured by counting the number of gridlines (0.5 cm apart) that each planarian crossed per minute over four four-minute observation periods (0 - 4 min, 4 - 8 min, 8 - 12 min, and 12 - 16 min). Four different conditions were tested: 1) water; 2) (–)sulpiride (0.1 mM) alone; 3) 254 nm UV light alone; and 4) (–)sulpiride (0.1 mM) plus UV.
2.3. pSLA
As was previously described [19], the planarians were placed individually into round (5.1 cm diameter) petri dishes containing water or test compound(s) and were observed over a 5-min period for the occurrence of pSLA events (for photographs of this behavior see [26]). The number of such events was counted. Twelve groups were tested (N = 8 planarians per group): 1) nicotine (1 mM); 2) nicotine (3 mM); 3) nicotine (5 mM); 4) nicotine (1 mM) plus 254 nm UV light; 5) nicotine (3 mM) plus 254 nm UV light; 6) nicotine (5 mM) plus 254 nm UV light; 7) pilocarpine (20 mM); 8) pilocarpine (30 m M); 9) pilocarpine (50 mM); 10) pilocarpine (20 mM) plus 254 nm UV light; 11) pilocarpine (30 mM) plus 254 nm UV light; and 12) pilocarpine (50 mM) plus UV.
2.4. Withdrawal (Physical Dependence)
Prior to testing, each of the planarians was individually pretreated by a 60-min exposure to either water or to U-50,488H (10 mM). The five test groups (N = 8 per group) were (pretreatment ® test): 1) water ® water; 2) water ® water plus 254 nm UV light; 3) water ® U-50,488H (10 mM); 4) water plus 254 UV light ® water; and 5) U-50,488H (10 mM) plus 254 nm UV light ® water. For each test group, the number of gridlines crossed during a 5-min observation period were counted.
2.5. UV Light Source
The UV lamp (UVP, model UVGL-58, Upland, CA) was positioned approximately 15 cm directly above the petri dish in which the planarians were tested.
2.6. Statistical Analysis
The group means were analyzed using one-way ANOVA followed, if P < 0.05, by Student-Newman-Keuls multiple comparisons post hoc test (InStat GraphPad Sofware, Inc). Criterion for significance was P < 0.05.
3. Results
3.1. Effect of UV Light on Drug-Altered pLMV
Consistent with prior findings from previous studies [10], planarians that were tested in water moved at an essentially constant locomotor velocity of about 15 - 20 grid crossings per minute. A slight progressive decline in pLMV occurred over the course of the four observation periods (0 - 4 min, 4 - 8 min, 8 - 12 min, and 12 - 16 min), but during each of the observation periods there was no significant difference (P > 0.05) between the pLMV of planarians not exposed to UV light and those exposed to 254 nm UV light, as shown in Figure 1.
Planarians that were tested in (–)sulpiride (0.1 mM) displayed significantly lower pLMV compared to planarians that were tested in water (69 ± 6 vs 54 ± 8 gridlines per 4 min, respectively). In (–)sulpiride (0.1 mM), pLMV continued to decline over each successive fourmin observation period to 34 ± 11 gridlines per 4 min in the 12 - 16 min observation period. In both periods in which a comparator group was exposed to UV light (4 - 8 min and 12 - 16 min), the pLMV of the UV-on group was significantly greater (P < 0.05) than of the UV-off group (Figure 1) and was comparable to the baseline water group (66 ± 8 vs 62 ± 4; 59 ± 11 vs 58 ± 4 gridlines per 4 min, respectively).
3.2. Effect of UV Light on Drug-Altered pSLA
In order to assure that irradiation of water with 254 nm UV light did not alter the water in a manner that affected
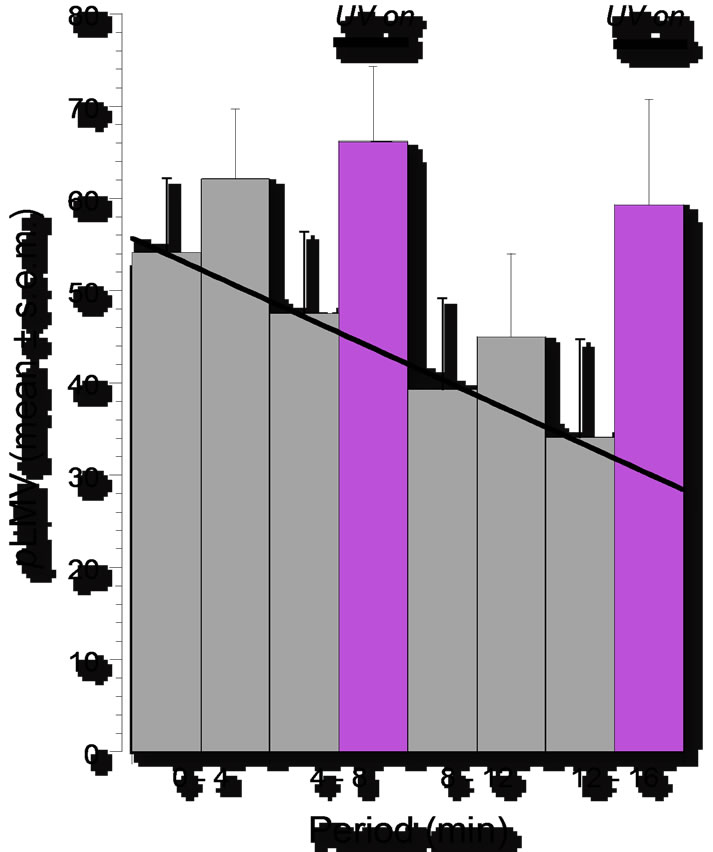
Figure 1. Locomotor velocity of planarians (pLMV) over 4-min intervals of 16-min observation period expressed as means ± S.E.M.: progressive decline in pLMV during exposure in (–)sulpiride (0.1 mM) and its reversal during periods of (–)sulpiride (0.1 mM) plus UV light (254 mm). N = 9 – 10 planarians per group.
the number of subsequent nicotine-induced seizures, two groups of planarians were placed into separate petri dishes, one of which was previously exposed to 254 nm UV light for 45 minutes and the other of which was previously unexposed for the same 45 minutes. The mean number of subsequent nicotine-induced seizures (± S.E.M.) (N = 8 planarians per group) in a five-minute observation period were 34 ± 1 and 36 ± 2 for the nonirradiated and irradiated groups, respectively (non-significant difference, P > 0.05). Thus 254 nm UV light had no discernable effect on water.
Nicotine (1, 3, and 5 mM) produced dose-related pSLA (N = 8 planarians per dose) as shown in Figure 2. The doses of 1, 3, and 5 mM were selected for subsequent study. At all three doses, the number of seizures produced by nicotine was less when the UV light was on than when the light was off. The differences were statistically significant (P < 0.05) at the doses of nicotine of 3 and 5 mM (Figure 2).
Pilocarpine (20, 30, and 50 mM) produced doserelated pSLA (N = 8 planarians per dose) and the number of seizures produced by pilocarpine was less when the UV light was on than when it was off. The differences were statistically significant (P < 0.05) at all three of the doses of pilocarpine (Figure 3).
3.3. Effect of UV Light on Physical Dependence (Drug Withdrawal)
The results are presented in Figure 4. Consistent with our prior results obtained in previous studies [10] drug-
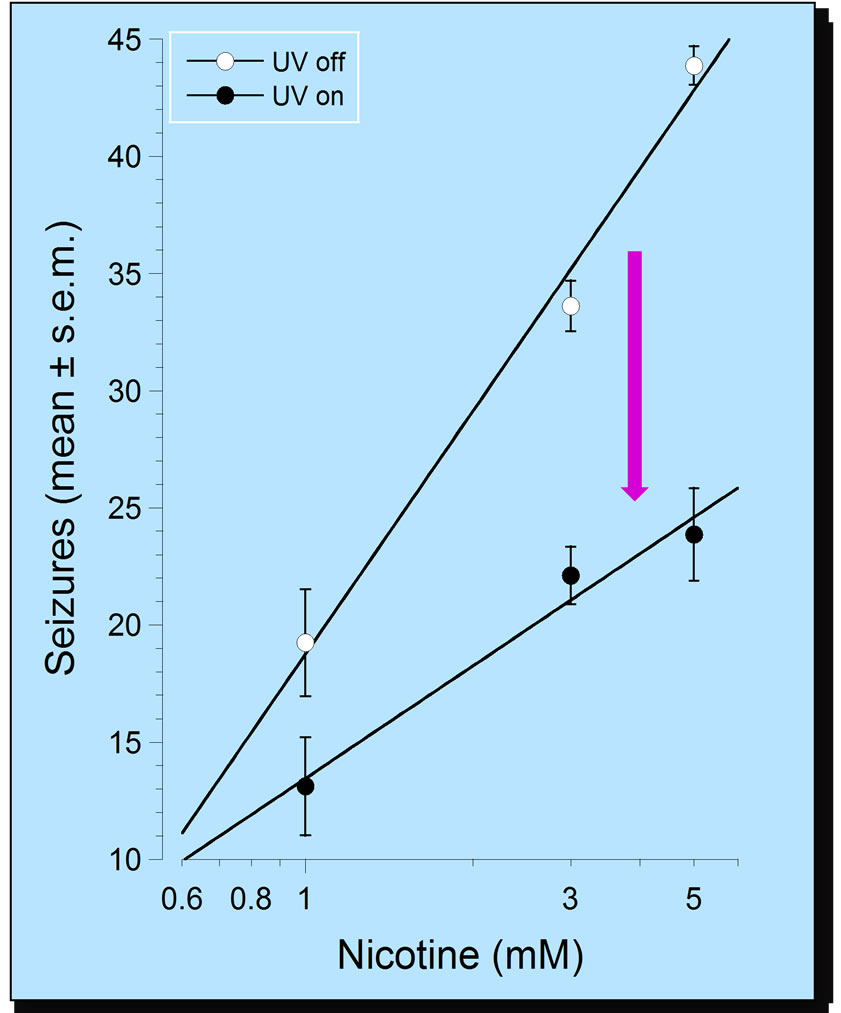
Figure 2. Dose-related (1, 3, 5 mM) nicotine-induced seizure-like activity in planarians (pSLA) over a 5-min observation period expressed as means ± S.E.M. of occurrences of pSLA in the absence and presence of UV light (254 mm). N = 8 planarians per group.
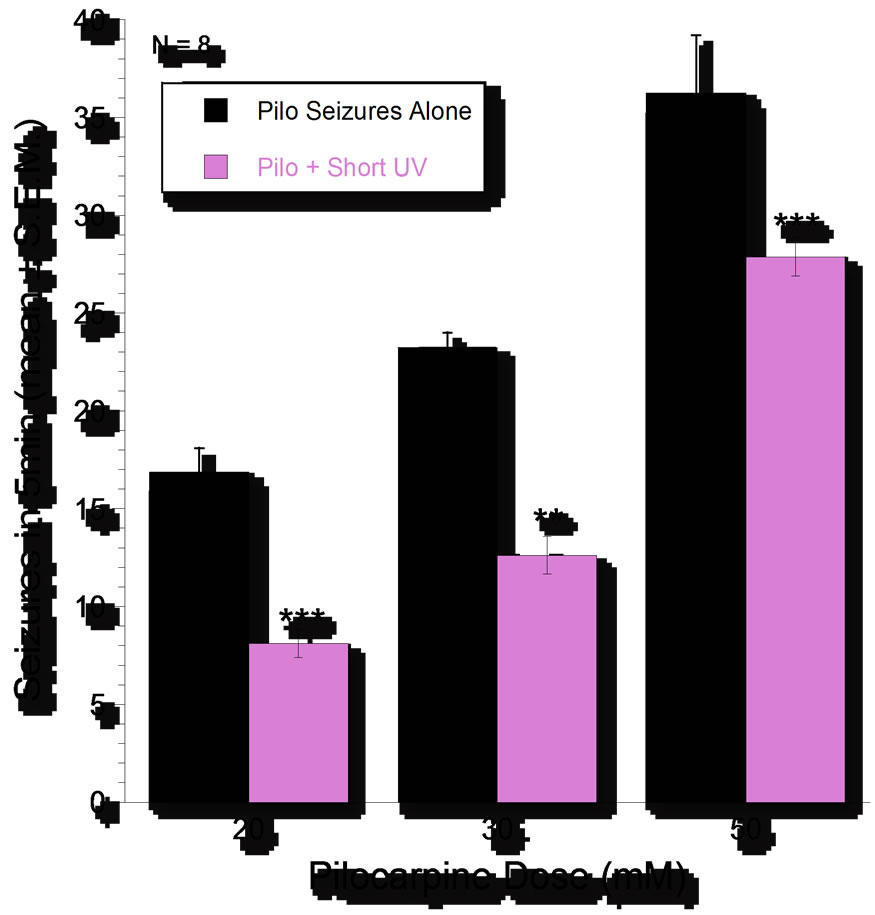
Figure 3. Dose-related (20, 30, 50 mM) pilocarpine-induced seizure-like activity in planarians (pSLA) over 5-minutes expressed as means ± S.E.M. of occurrences of pSLA in the absence or presence of UV light (254 mm). N = 8 per group. Asterisks indicate significant (P < 0.05) reversal by UV.
naïve planarians displayed relatively constant pLMV of about 15 gridlines per minute when tested in water (group: water ® water). UV light (254 nm) had no effect on the pLMV of U-50,488H-naïve planarians (group:
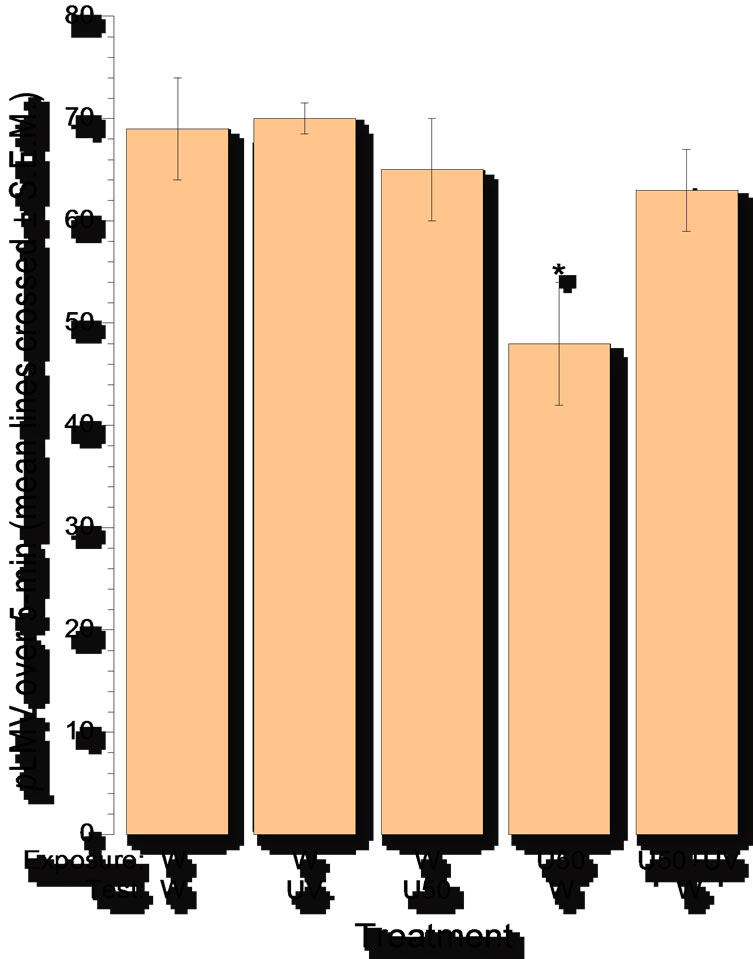
Figure 4. Abstinence-induced withdrawal after 60-min exposure to the k-opioid receptor agonist U-50,488H (10 mM). Withdrawal was assessed by decrease in spontaneous locomotor velocity (pLMV), measured as the mean (± S.E.M.) number of gridlines crossed over a 5-min period. N = 8 planarians per group. Asterisk indicates significant (P < 0.05) difference from baseline (U-50,488H naïve). U50 = U-50,488H; W = water.
water ® water plus UV light). Likewise, the acute exposure to U-50,488H (10 mM) had no effect on the pLMV of U-50,488H-naïve planarians (group: water ® U-50, 488H). In contrast, U-50,488H pretreated planarians that were tested in water (group: U-50,488H ® water) displayed a significant (p < 0.05) reduction in pLMV. Critically, planarians that were exposed to UV light while being pretreated with U-50,488H and then tested in water (group: U-50,488H (10 mM) plus 254 nm UV light ® water) displayed pLMV not different from U-50, 488H-naïve planarians (P > 0.05).
4. Discussion
The phenomenon of UV light-induced photorelaxation in mammalian isolated tissue preparations has been reviewed previously [4]. Briefly, a strip or ring of rabbit thoracic aorta in state of active contraction induced by norepinephrine or other a-adrenoceptor agonist will exhibit a rapid decrease in the agonist-induced tension when exposed to UV light of sufficient intensity [1,2,5]. The photorelaxation occurs within 1 sec and is reversible, that is, tension is restored to pre-radiation levels upon cessation of the UV light exposure. A series of studies using this preparation has led to the proposal that the energetics of UV light disrupts drug-receptor binding equilibrium [27]. Some of the evidence in support of this includes: values of agonist-receptor dissociation constants determined by photorelaxation agree with corresponding values determined by other method [5]; photorelaxation kinetics of agonists in the presence of rcversiblc and irreversible antagonists are in agreement with theoretical predictions [3,28]; a-adrenoceptor antagonists do not alter the time constant of agonists at other (e.g., angiotensin) receptors [3]. To test the possibility that UV light induces the synthesis of an agonistselective ‘relaxing’ substance, two muscle chambers, each containing a norepinephrine-contracted aorta strip, were aligned vertically, such that the bathing solution of the lower chamber could be quickly replaced by that of the upper chamber. The strip in the upper chamber was exposed to UV radiation, producing photorelaxation––no effect was produced on the strip in the lower chamber. We also tested if UV light degrades ligand by exposing norepinephrine to UV light for 10 min, then testing its ability to contract aorta strips. There was no difference in the magnitude of response (tension development) [4].
To further test the concept that the UV light photorelaxation phenomenon is intimate to the ligandreceptor interaction or transduction, we speculated that it should be manifested by a variety of diverse drugs acting at different receptors and producing different effects. This is difficult to achieve using mammalian models, so we chose to use an invertebrate model with which we have extensive experience [10]. We selected four drugs: three of them agonists (nicotine, U-50,488H, and pilocarpine) and––in order to examine the influence of the absence of signal transduction processes––an antagonist ((–)sulpiride). The four drugs act at different receptors: dopamine D2 (DAR2), nicotinic cholinergic (nAChR), muscarinic cholinergic (mAChR), and k-opioid (KOR). Three of the receptors are metabotropic, namely, G protein-coupled (GPCRs), and the other one is ionotropic (i.e., nAChR). Three diverse drug-induced quantifiable endpoints were selected for study: spontaneous locomotor activity, seizures, abstinence-induced withdrawal (physical dependence).
Dopamine has been identified in planarians [12,14]. It is present in relatively high concentrations [29], and it is localized to dense-core vesicles in synapses [30]. Dopamine receptor agonists increase, dopamine receptor antagonis decrease cAMP levels in planarians [11,15]. Agonist-induced behavioral effects are mediated through dopamine D1- and D2-like receptors and are attenuated by selective receptor antagonists [15]. Regarding the endpoint used in this study planarian locomotor activity, an association between changes in and dopamine receptors has been reported by several groups (e.g., [11,12, 14-17]), and dopamine agonist-induced changes on locomotor activity are antagonized by dopamine receptor antagonist haloperidol [14,17]. Acetylcholine has been identified in planarians [12,14] and behavioral effects are elicited by cholinergic agonists and antagonists and acetylcholinesterase inhibitors [29,31]. The presence of endogenous opioids in planarians has been demonstrated [13], opioid ligands elicit behavioral effects [16] antagonized by the opioid receptor antagonist naloxone and the selective k-opioid receptor antagonist Nor-BNI (Norbinaltorphimine) [14]. Physical dependence development and abstinence-induced withdrwal from U-50,488H has been described previously [21]. Seizure induction in planarians has also been described previously [19].
We demonstrated previously [25] that (–)sulpiride decreases pLMV in an enantiomer-specific manner and the effect is attenuated by UV light. This was duplicated in the present study, which demonstrated that the effect of UV light is reversible. Since amphetamine-induced increase in pLMV is also attenuated by UV light [18], the effect of UV light on this endpoint does not appear to be at the level of 2nd-messenger transduction pathways, but rather at the level of the receptor. We demonstrated that UV light attenuation of the amphetamine-induced effect is not due to degradation of amphetamine by running a spectrophotometric scan (model DR/4000U, HACH Co., Loveland, CO) over 10-min exposure to UV light. There was no decrement in peak height or area and no new peak appeared (that would have been indicative of a breakdown product) [18]. Saline was used to provide a control for osmolarity. Neither saline (0.1 mM) during treatment nor saline (0.1 mM) during testing had effect on pLMV [21]. Involvement of opioid receptors in U-50, 488H-induced effect in planarians was demonstrated in prior work by a dose-related naloxone-sensitivity and the specific involvement of k-opioid receptors in physical dependence development was demonstrated in planarians that were co-incubated with nor-BNI together with U-50,488H and then tested in water displayed pLMV that was not different from naïve planarians [21]. The pH of solutions containing same concentrations of U-50,488H or naloxone were not different from the pH of the test water [21].
5. Conclusion
In summary, the spontaneous locomotor activity of planarians was decreased by (–)-sulpiride and was reverted back toward the control levels when the planarians were exposed to UV light; seizures were induced in planarians by nicotine and pilocarpine and were attenuated when the planarians were exposed to UV light; and development of physical dependence to U-50,488H (manifested by abstinence-induced withdrawal) was attenuated by UV light. The observed effects of UV light cannot be attributed to toxic effects, since the UV light had no effect of its own on the behaviors. The observed effects of UV light have also been shown to attenuate antagonist—as well as agonist-induced effects—which argues against an effect of UV light only on ligands having intrinsic activity (efficacy). The results also argue against UV light releasing some endogenous substance, since UV light counteracts opposite effects. Based on the demonstration in the present study of attenuation by UV light of four different ligands selective for four different receptors and at three different endpoints, the results are compatible with the view that UV light disrupts ligand-receptor binding.
6. Acknowledgements
The authors thank Timothy Shickley, Ph.D., for suggesting Planaria as a model. This work was supported by NIDA grants DA15378 (RBR) and DA022694 (SMR).
REFERENCES
- R. F. Furchgott, W. Sleator, M. W. McCaman and J. Elchlepp, “Relaxation of Arterial Strips by Light, and the Influence of Drugs on This Photodynamic Effect,” Journal of Pharmacology and Experimental Therapeutics, Vol. 113, 1955, pp. 22-23.
- R. F. Furchgott, S. J. Ehrreich and E. Greenblatt, “The Photoactivated Relaxation of Smooth Muscle of Rabbit Aorta,” Journal of General Physiology, Vol. 44, 1961, pp. 499-519. doi:10.1085/jgp.44.3.499
- L. S. Jacob and R. J. Tallarida, “Further Studies on the Action of Ultraviolet Light on Vascular Smooth Muscle: Effect of Partial Irreversible Receptor Blockade,” Archives Internationales Pharmacodynamie et de Thérapie, Vol. 225, 1977, pp. 166-176.
- R. B. Raffa, M. J. Robinson and R. J. Tallarida, “Ultraviolet Light-Induced Photorelaxation of Agonist-Contracted Rabbit Aorta: Further Characterization and the Estimation of Drug-Receptor Rate Constants,” Drug Development Research, Vol. 5, No. 4, 1985, pp. 359-369. doi:10.1002/ddr.430050409
- R. J. Tallarida, R. W. Sevy, C. Harakal and M. H. Loughnane, “Characteristics of Photorelaxation in Vascular Smooth Muscle: Evidence Supporting the Hypothesis of Drug-Receptor Equilibrium Disturbance,” IEEE Transactions on Biomedical Engineering, Vol. 22, No. 6, 1975, pp. 493-501. doi:10.1109/TBME.1975.324471
- R. J. Tallarida, O. L. Laskin and L. S. Jacob, “Perturbation of Drug Receptor Equilibrium in the Presence of Competitive Blocking Agents,” Journal of Theoretical Biology, Vol. 61, No. 1, 1979, pp. 211-219. doi:10.1016/0022-5193(76)90115-6
- R. F. Furchgott and P. Bursztyn, “Comparison of Dissociation Constants and Relative Efficacies of Selected Agonists Acting on Parasympathetic Receptors,” Annals of the New York Academy of Sciences, Vol. 144, 1967, pp. 882-899. doi:10.1111/j.1749-6632.1967.tb53817.x
- Y. Chang, Y. Xie and D. S. Weiss, “Positive Allosteric Modulation by Ultraviolet Irradiation on GABAA, But Not GABAC, Receptors Expressed in Xenopus Oocytes,” Journal of Physiology, Vol. 536, No. 2, 2001, pp. 471- 478. doi:10.1111/j.1469-7793.2001.0471c.xd
- E. I. Budovskii and G. V. Kostiuk, “Principles of Selective Inactivation of the Virus Genome. IV. The Effect of UV-Irradiation of Phage MS2 on Its Binding with AntiMS2-Immunoglobulins,” Molekulyarnaya Biologiya, Vol. 19, 1985, pp. 1216-1222.
- R. B. Raffa and S. M. Rawls, “Planaria: A Model for Drug Action and Abuse,” Landes Bioscience, Austin, 2008.
- S. Algeri, A. Carolei, P. Ferretti, C. Gallone, G. Palladini and G. Venturini, “Effects of Dopaminergic Agents on Monoamine Levels and Motor Behaviour in Planaria,” Comparative Biochemistry and Physiology C, Vol. 74, No. 1, 1983, pp. 27-29. doi:10.1016/0742-8413(83)90142-1
- J. H. Welsh and L. D. Williams, “Monoamine-Containing Neurons in Planaria,” Journal of Comparative Neurology, Vol. 138, No. 1, 1970, pp. 103-115. doi:10.1002/cne.901380108
- G. Venturini, A. Carolei, G. Palladini, V. Margotta and M. G. Lauro, “Radioimmunological and Immunocytochemical Demonstration of Met-Enkephalin in Planaria,” Comparative Biochemistry and Physiology C, Vol. 74, No. 1, 1983, pp. 23-25. doi:10.1016/0742-8413(83)90141-X
- A. Carolei, V. Margotta and G. Palladini, “Proposal of a New Model with Dopaminergic-Cholinergic Interactions for Neuropharmacological Investigations,” Neuropsychobiology, Vol. 1, No. 6, 1975, pp. 355-364. doi:10.1159/000117512
- G. Palladini, S. Ruggeri, F. Stocchi, M. F. De Pandis, G. Venturini and V. Margotta, “A Pharmacological Study of Cocaine Activity in Planaria,” Comparative Biochemistry and Physiology C, Vol. 115, No. 1, 1996, pp. 41-45. doi:10.1016/S0742-8413(96)00053-9
- F. Passarelli, A. Merante, F. E. Pontieri, V. Margotta, G. Venturini and G. Palladini, “Opioid-Dopamine Interaction in Planaria: A Behavioral Study,” Comparative Biochemistry and Physiology C, Vol. 124, No. 1, 1999, pp. 51-55.doi:10.1016/S0742-8413(99)00048-1
- G. Venturini, F. Stocchi, V. Margotta, S. Ruggieri, D. Bravi, P. Bellantuono and G. Palladini, “A Pharmacological Study of Dopaminergic Receptors in Planaria,” Neuropharmacology, Vol. 28, No. 12, 1989, pp. 1377- 1382. doi:10.1016/0028-3908(89)90013-0
- R. B. Raffa and A. F. Martley, “Amphetamine-Induced Increase in Planarian Locomotor Activity and Block by UV Light,” Brain Research, Vol. 1031, No. 1, 2005, pp. 138-140. doi:10.1016/j.brainres.2004.10.051
- S. M. Rawls, T. Thomas, M. Adeola, T. Patil, N. Raymondi, A. Poles, M. Loo and R. B. Raffa, “Topiramate Antagonizes NMDAand AMPA-Induced Seizure-Like Activity in Planarians,” Pharmacology Biochemistry and Behavior, Vol. 93, 2009, pp. 363-367. doi:10.1016/j.pbb.2009.05.005
- R. B. Raffa and J. M. Valdez, “Cocaine Withdrawal in Planaria,” European Journal of Pharmacology, Vol. 430, No. 1, 2001, pp. 143-145. doi:10.1016/S0014-2999(01)01358-9
- R. B. Raffa, G. W. Stagliano and S. Umeda, “kappaOpioid Withdrawal in Planaria,” Neuroscience Letters, Vol. 349, No. 3, 2003, pp. 139-142. doi:10.1016/S0304-3940(03)00814-0
- S. Umeda, G. W. Stagliano and R. B. Raffa, “Cocaine and Kappa-Opioid Withdrawal in Planaria Blocked by D-, But Not L-, Glucose,” Brain Research, Vol. 1018, No. 2, 2004, pp. 181-185. doi:10.1016/j.brainres.2004.05.057
- R. B. Raffa and P. Desai, “Description and Quantification of Cocaine Withdrawal Signs in Planaria,” Brain Research, Vol. 1032, No. 1-2, 2005, pp. 200-202. doi:10.1016/j.brainres.2004.10.052
- R. B. Raffa, L. J. Holland and R. J. Schulingkamp, “Quantitative Assessment of Dopamine D2 Antagonist Activity Using Invertebrate (Planaria) Locomotion as a Functional Endpoint,” Journal of Pharmacological and Toxicological Methods, Vol. 45, No. 3, 2001, pp. 223-226. doi:10.1016/S1056-8719(01)00152-6
- R. B. Raffa, J. M. Valdez, L. J. Holland and R. J. Schulingkamp, “Energy-Dependent UV Light-Induced Disruption of (–)Sulpiride Antagonism of Dopamine,” European Journal of Pharmacology, Vol. 406, No. 3, 2000, pp. R11-12. doi:10.1016/S0014-2999(00)00730-5
- R. B. Raffa, K. E. Finno, C. S. Tallarida and S. M. Rawls, “Topiramate-Antagonism of L-Glutamate-Induced Paroxysms in Planarians,” European Journal of Pharmacology, Vol. 649, No. 1-3, 2010, pp. 150-153. doi:10.1016/j.ejphar.2010.09.021
- R. J. Tallarida, R. W. Sevy and C. Harakal, “Relaxation Methods for the Determination of Drug Receptor Affinities,” Bulletin of Mathematics and Biophysics, Vol. 32, No. 1, 1970, pp. 65-69. doi:10.1007/BF02476793
- R. J. Tallarida, O. L. Laskin and L. S. Jacob, “Perturbation of Drug Receptor Equilibrium in the Presence of Competitive Blocking Agents,” Journal of Theoretical Biology, Vol. 61, No. 1, 1976, pp. 211-219. doi:10.1016/0022-5193(76)90115-6
- P. Ribeiro, F. El-Shehabi and N. Patocka, “Classical Transmitters and Their Receptors in Flatworms,” Parasitology, Vol. 131, Suppl. 1, 2005, pp. S19-S40. doi:10.1017/S0031182005008565
- G. Palladini, V. Margotta, A. Carolei, F. Chiarini, M. Del Piano, G. M. Lauro, L. Medolago-Albani and G. Venturini, “The Cerebrum of Dugesia Gonocephala s.1. Platyhelminthes, Turbellaria, Tricladida. Morphological and Functional Observations,” Journal für Hirnforschung, Vol. 24, 1983, pp. 165-172.
- F. R. Buttarelli, F. E. Pontieri, V. Margotta and G. Palladini, “Acetylcholine/Dopamine Interaction in Planaria,” Comparative Biochemistry and Physiology C, Vol. 125, 2000, pp. 225-231. doi:10.1016/S0742-8413(99)00111-5
NOTES
*Corresponding author.

