 Vol.2, No.3, 177-187 (2010) doi:10.4236/health.2010.23026 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Protection of groundwater from migration of infiltrates from a chromic waste storage site and methods of treating these infiltrates Zygmunt Kowalski, Adam Kozak, Marcin Banach, Agnieszka Makara Institute of Chemistry and Inorganic Technology, Cracow University of Technology, Cracow, Poland; zkow@chemia.pk.edu.pl Received 14 October 2009; revised 10 December 2009; accepted 14 December 2009. ABSTRACT This work presents the results of investigations to develop and implement methods to effectiv ely collect and purify infiltrates from hea ps, situated in the region of Alwernia near Cracow, where more than 3 million tonnes of waste material resulting from the production of chromium com- pounds have been stored. It describes a system for the protection of groundwater from these infiltrates which contain 50-400 g m-3 Cr6+, as well as the effectiveness of cheap and simple chemical methods to purify these chromic was- tewaters. The infiltrate collection system and the most effective method to decrease the concen- tration of Cr6+ to a level below 0.1 ppm, as re- quired by Polish and European Union regula- tions, were implemented in the Alwernia Che- mical Works S. A. in the years 1998-1999. Keywords: Was t e H eaps; Chromi c Infiltrates; Collect; Purify 1. INTRODUCTION The quantities of chromic waste accumulated in Poland are considerable. In Alwernia, in the course o f 50 years of production of chrome compounds, more than 3 million tonnes of waste have been stored. This waste contains more than 1% of carcinogenic Cr6+ compounds. The waste heap of a former pr oducer of chr omium compounds in Rudniki store a further 0.5 million t. Other 1.5 million t of waste from the production of ferro-chromium can be found in Siechnice. The amount of stored waste resulting from tanning and galvanic processes is estimated to be at least 50,000 tons per year [1-3]. Because of the position and the quantity of accumu- lated waste, the heap in Alwernia represents a serious hazard to the natural environment (Figure 1). The waste heap consists of two parts. The old one that was in use until 1967, and the n ew one, which has been in use since 1968. It is situated in the macroregion of the Cracow- Wi e l uń Upland (being a layer of u pper-Jurass i c l imestone) and the mesoregion of the Tenczyński Ridge [4,5]. The heap is located 2.5-3.0 km north of the Vistula River, in the valley of the meandering stream Regulanka. The dis- tance between this stream and the old heap is 75 to 150 metres and that between t he stream and the new heap is 75 to 200 m. The old heap at present forms a partly reculti- vated block, whi ch t owers 5 meters above the level of the Chemical Works plant. The new heap forms an irregular cone with a cut top. On its slopes special terraces have been prepared to improve the stability of the slope. In recent years ef forts aiming to limit the environmental impact of chromic waste accumulated in the heaps have mostly focused on the protection of groundwater Figure 1 . The “A lwernia” Chemical Works— a general view. An active heap of ch romic w astes ca n be seen in the f oreground. An old reclaimed he ap occupies the area b etween the factory and the heap.  Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 178 against infiltration from leachates containing Cr6+ and the development of an efficient and economical method to remove chromium from these wastewaters [6,7]. The present work describes the results of investigations which led to the implementation of a collection and treatment system for infiltrates from the chromic waste dumps, containing 50 to 400 g m-3 of Cr6+. The results of experiments to devise and implement a cheap and simple method of removing chromium from wastewaters, thus reducing its concentrations below 0.1 ppm, to order to comply with Polish and European Union regulations [8], also are presented. These methods were implemented in the Chemical Works “Alwernia” in 1998/1999. 2. RESEARCH ON THE SPREAD OF POLLUTION IN THE GROUNDWATER ENVIRONMENT IN THE REGION OF CHROMIC W ASTE MATE RIAL ST ORAGE The measurement of the electrical conductivity of the ground near the places of storage was conducted with the device 34-3XL produced by Geonics Ltd., Canada, based on the electromagnetic method (EM) utilising low fre- quencies [9]. Measurements were conducted every 20 m (old heap every 10 m ), with perpendicular arrangem ent of coils. The results of the specific conductivity of the ground t o a de pth of 15 m are given by Guli ński et al. [6]. The graphic illustration of the results indicates the p laces of potential groundwater pollution (Figure 2). Maps of groundwater pollution with ions of Cr6+ have been pre- pared on the basis of the analysis of samples with pie- zometers (archival and actual), and in some cases based on EM measurements [6,7], conducted simultaneously. On the basis of these investigations, both distribution and directi on of movem ent of poll ution have be en det erm ined. Based on the analysis of the data, a model of the dis- tribution of under ground pollution between the Chemical Works and the Regulanka River was identified. All pol- lutants affecting the soil in this region permeate to the “quaternary” water-bearing layer and are then carried further by t he un derground w aters. B etwee n the hea ps the water-bearing layers are connected by water-bearing gravel. Ho wever , the syst em of hydrois ohypses (lines on a map joining pl aces with the e qual groundwater elevation), together with the electrical conductivity distribution, indicates a disturbance in the flow of the waters from north to south. A small hill in the neighbourhood of the southern border of the Chemical Works which is charac- terized by minimal thickness of “quaternary” redundant contains impenetrable formations which causes the groundwater to flow in eastern direction. It is not ex- cluded that a part of the infiltrating wastewater flows in a south-easterly direction (Figure 2). It can be also stated Figure 2. Distribution of chromium pollution in the vicinity of the chromic heaps at the “Alwernia” Chemical Works (accord- ing to Guliński et al. [6]). that at the moment, in the region of Skowronek and Młyńczysko, the polluted waters from the Chemical Works are drained by the river Regulanka and carried away to the river Vistula. The hydrogeological conditions also indicate that, in the case of an attempt at regulating the riverbank and tightening its bed, the water may mi- grate to the Vistula through permeable sediments at the bottom of the Regulanka River valley. The results of the investigations are presented in Figure 2. The occurrence of three streams of polluted ground- water has been identified. These are the following: 1) From the grounds of the production facilities and the northern part of old hea p; 2) From the grounds of the production facilities and the southern pa rt of ol d heap; 3) From the grounds of the new heap. The main direction of the flow of pollution in the groundwater is from north to south, with some local de- viations. These flow directions a re indicated in Figure 2 [6]. Considering the fact that all the infiltrates might po- tentially enter the V istula, the possibility of pumping these polluted waters and cleaning them, or returning them to the technological processes has been considered to be an improvement to the natural environment. Decisions were taken to build the girding tren ches “A” and “C” and the two drainage wells “B” and S-25 in 1998 and at the beginni ng of 1999 (Figure 2). The original plan of changing the Regulanka riverbed over a distance of 1.5 km has been given up. Initiall y, the quantity o f infiltrates from these sources (intakes) was estimated to be between 600 to 2000 m3 per day.  Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 179 3. INVESTIGATIONS INTO THE REMOVAL OF Cr6+ FROM INFILTRATES ORIGINATING FROM CHROMIC WASTE HEAPS Taking into consideration the fact that it is possible to reuse o nly 20 0 m3 of chromic wastewater per 24 hours in the chromium compounds production, it was decided that the only feasible possibility to so lve the problem of infil- trates was to clean them from Cr6+ compounds. It was necessary, however, to develop a new method of cleaning chromic wastewaters. Such a method should be particu- larly adapted to low and variable chromium concentra- tions in wastewater and it should be cost effective to be used in practice. The laboratory investigations were carried out on model solutions, prepared on the basis of the composition of water t aken from the girding t rench “A” in the region o f the old chromic heap. The quantitative composition of the wastewater in the trench was taken from the analyses presented by Kania [7]. According to these analyses, the concentration of Cr6+ i n the wastew ater varie d between 1 1 to 112.5 g m-3 in th e first quarter of 1998 . The SO42- ani- ons in concentrations of 240 to 555 g m-3 can also be found in wastewater, and their presence has to be taken into consideration for the successful removal of chromate anions from the solution. In previous years the “Alwernia” Chemical Works in- vestigated ways of cleaning the wastewaters which con- tained more than 1 kg m-3 of Cr6+, using a classical chemical method, based on the reduction of Cr6+ to Cr3+ and the precipitation of Cr(OH)3. Sodium sulphite was used as a reducing agent at a pH between 2 and 4. Pre- cipitation of the reduced chromium as a chromium (III) hydroxide was the n accom plished with the us e of cal cium oxide [10]. The results of the laboratory investigations [11] on model solutions containing Cr6+ ions in concentrations of 50 to 200 g m-3 show that with the use of both, sodium sulphite and sodium pyrosulfite (Na2S2O5) as a reducing agent, lowe ring of the concen tration o f Cr6+ below 1 g m-3 was possible. In alm ost all the experim ents between 1 an d 12.5 g m-3 of Cr6+ were left in the solution after the re- duction, and in most of the analyses the concentration of Cr6+ ranged from 1 to 3 g m-3, with a tendency towards higher values. Many authors suggest the use of anion exchangers to remove Cr6+ [12-16]. The advantage of this method is that the Cr6+ compounds in the solution u sed to reg en erate the anion exchanger are more concentrated than in the ori- ginal wastewater. In the case of diluted wastewaters con- taining considerable quantities of suspended solids, this method is not practical because it requires the use of very large columns and the anion exchanger could be blocked by the suspended solid matter present in the infiltrates thus resulting in an increase in flow resistance. What is suggested most often is the purification of chromic wastewater by reducing hexavalent chromium to trivalent chromium in an acid environment and then pre- cipitating the latter as chro mium (III) hydroxide. In addi- tion to the above-mentioned sodium sulphite and pyro- sulfite, the use of the reducing agents sulphur dioxide (SO2) and sodium acid sulphate (NaHSO3) at a lower pH range (2-2.5) is recommended [17-19]. Reduction of Cr6+ to Cr3+ may also be achieved in a basic environment using iron (II) sulphate as a reducing agent [14]. This reaction follows the equation: CrO42- + 3Fe2+ + 4OH- + 4H2O = Cr(OH)3 + 3Fe(OH)3 (1) Another interesting possibility of chromium removal is the precipitation of chromium from wastewater in the form of chromic ettryngite. This method can be used to purify chromic tanning wastewater containing Cr3+ [20-22]. The occurrence of ettr yngite was first observed in the research on cement mortar. Its chemical formula is 2CaOAl2O33CaSO432H2O [23,24]. Ettryngite forms during cement bonding and also after mixing of ashes from fluidised hearths with water [25]. It was stated by several authors that the aluminium in ettryngite can be substituted by Fe3+, Cr3+ and other metal ions [26-29]. Furthermore, the SO42- ion can be exchanged by other anions, i.e. chromate CrO42- [24-30]. In spite of some qualitative changes, such a compound retains the same structure. According to the above-mentioned authors the requirement for the formation of this compound, generally called ettryngite, is a high pH, and the maintenance of a CaO:Me2O3:anion(II) ratio of 6:2:3. A practical example of the use of the ettryngite precipitation to remove SO42- ions from wastewater has be en presente d by Woroszyńska et al. [31]. Among the chemical methods, the possibility of pre- cipitating chromate after first converting it into com- pounds with a very low solubilit y pro duct, i.e. BaCrO 4 or PbCrO4 [32], should also be mentioned. Four methods to purify wastewater containing Cr6+ compounds were tested under laboratory conditions in the “Alwernia” Chemical Works. Two of them were base d on the incorporation of the CrO42- ion into the ettryngite structure, in the presence of aluminium or iron (III) salts in a basic environment, and its subsequent precipitation. Another method was based on the precipitation of the ion of CrO42- by lead salts, utilising the low solubility of lead chromate. The last method was carried out in two stages: in the first stage the Cr6+ ion was reduced with iron (II) salts, after which the chromium ion was precipitated by adding CaO to the solution. The investigations were con- ducted at temperatures between 291 and 295 K. Model- ling solutions and wastewater from the band trench “A” in the “Alwernia” Chemical Works were used in our in- vestigations. 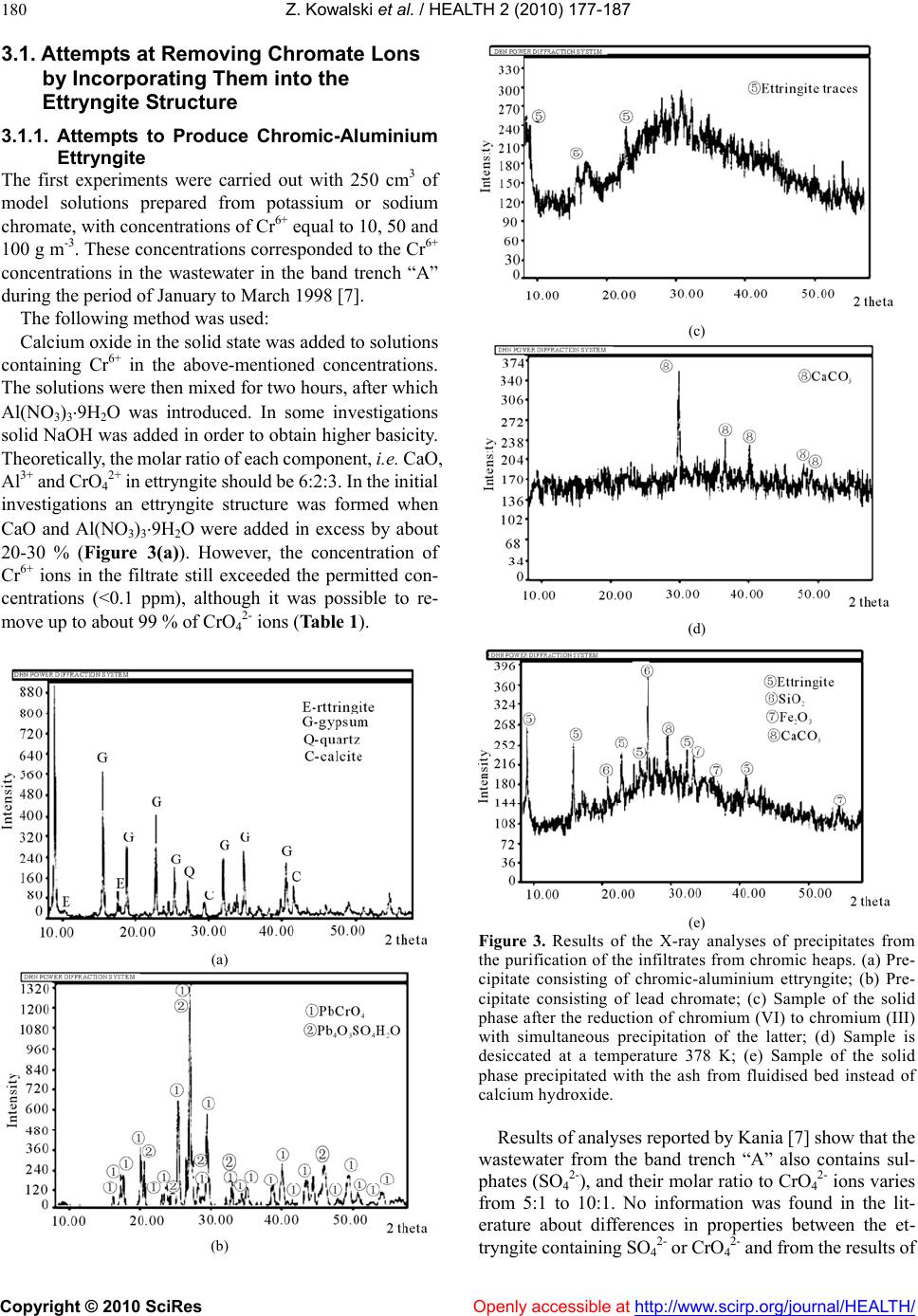 Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 180 3.1. Attempts at Removing Chromate Lons by Incorporating Them into the Ettryngite Structure 3.1.1. Attempts to Produce Chromic-Aluminium Ettryngite The first experiments were carried out with 250 cm3 of model solutions prepared from potassium or sodium chromate, with concentrations of Cr6+ equal to 10, 50 and 100 g m-3. These concentrations corresponded to the Cr6+ concentrations in the wastewater in the band trench “A” during the period of January to Mar ch 1998 [7]. The followin g method was used: Calcium oxide in the so lid state was add ed to solution s containing Cr6+ in the above-mentioned concentrations. The solutions were then mixed for two h ours, after which Al(NO3)39H2O was introduced. In some investigations solid NaOH was added in order to obtain higher basicity. Theoretically , the m olar ratio of each component, i.e. CaO, Al3+ and CrO42+ in ettryngite should be 6:2:3. In the initial investigations an ettryngite structure was formed when CaO and Al(NO3)39H2O were added in excess by about 20-30 % (Figure 3(a)). However, the concentration of Cr6+ ions in the filtrate still exceeded the permitted con- centrations (<0.1 ppm), although it was possible to re- move up to about 99 % of CrO42- ions (Table 1). (a) (b) (c) (d) (e) Figure 3. Results of the X-ray analyses of precipitates from the purification of the infiltrates from chromic heaps. (a) Pre- cipitate consisting of chromic-aluminium ettryngite; (b) Pre- cipitate consisting of lead chromate; (c) Sample of the solid phase a fter the reduction of chromium (VI) t o chromium (III ) with simultaneous precipitation of the latter; (d) Sample is desiccated at a temperature 378 K; (e) Sample of the solid phase precipitated with the ash from fluidised bed instead of calcium hydroxide. Results of analyses reported by Kania [7] show that the wastewater from the band trench “A” also contains sul- phates (SO42-), and their molar ratio to CrO42- ions varies from 5:1 to 10:1. No information was found in the lit- erature about differences in properties between the et- tryngite c ontai nin g SO42- or Cr O42- and from the results of  Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 181 our initial investigations it can be concluded that their properties are similar. Therefore, the quantities of added CaO and aluminium salts were referred to the sum of moles of CrO42- and SO42- in s ubseque nt experi ments. The results of investigations carried out on model solutions containing 104 mg of Cr6+ and 960 mg of SO42- in one litre of solution have been summarised in Table 2 and the results of investigations conducted on the wastewater from trench “A” are presented in Ta ble 3. In none of the above-mentioned investigations (with the exception of two-Table 1, No. 3 and 4), results below 1 g m-3 Cr 6+ were obtained. Using large excesses of added aluminium salts and calcium oxide only resulted in concentrations of Cr6+ in the range of 1 g m -3 (Table 3). When aluminium salts were added in excess of amounts theoretically needed for ettryngite formation, aluminium appeared in the filtrate. This was a result of the high pH of the solution, at which aluminium exists in the AlO2- form . 3.1.2. Attempts to Produce Iron-Chromic Ettryngite Several experiments were conducted to synthesise a compound composed of 3CaOFe2O32CaSO4nH2O, in which aluminium was substituted by the less amphoteric iron (III) (some authors are of the opinion that iron (III) is not even amphoteric [17]). The influence of iron (III)- added as ir on (III) chloride- was tested on a m odel solution containing 104 mg of Cr6+ and 960 mg of SO42- in one litre. The objective of the investigations was to determine the most economic ratio of calcium oxide and iron chloride added in relation to the sum of the ions of CrO42- and SO42- for the effective removal of Cr6+ ions. The experiments were also conducted on industrial wastewater containing 38.26 g m-3 of C r6+. All investigations were conducted in a manner sim ilar to the previous ones: a we ighed am ount of CaO was added to the Cr6+ solution and the solution was mixed. After 1 to 2 hours FeCl36H2O was ad ded and th e solution was mixed for a further 0.5 to 1.0 hours. The results of the investigations obtained with the model so- lutions are given in Table 4, and the results obtained with wastewater are presented in Table 5. Some improvement of filtrate quality can be observed. At a molar ratio of CaO and FeCl36H2O to the sum of chromates and sulphate equalling 20:1 and 3:1, respectively, the removal of the Cr6+ ions from the solution is about 94 %. 3.2. Precipitation of Chromate Lons in Form of Sparingly Soluble Compounds According to Ufimciewa and Smietanic [19], lead chro- mate is a sparingly soluble compound with a solubility product of 1.8 10-14. Thus, in the presence of lead the precipitation of C rO42- wi th subseque nt decre ase of Cr 6+ to values below 1 g m-3 Cr6+ is expected. The tests with model solutions rendered the expected results. The model solution contained 0.194 g of K2CrO4 and 1.62 g of Na2SO410H2O in 500 cm3 of water, which corresponded to concentrations of Cr6+ ions equal to 104 g m -3 and SO42- ions equal to 960 g m-3. Solid PbCl2 was added to the solution. The molar ratios of Pb2+:Cr6+ were 1.5:1, 2.0:1 and 2.5:1. The sol ution was then mixed for two ho urs, after which the coagulant was introduced. Separating the solid phase from the solution did not present any difficulties. Filtrate analysis showed Cr6+ concentrations of 0.99, 0.27 and 0.025g m-3 for the Pb2+: Cr6+ ratios 1.5:1, 2.0:1 and 2.5:1, respectively. Further experiments were conducted with wastewater from the trench “A”, which had a Cr6+ concentration of 17 .28 g m-3. The molar ratios of Pb2+: Cr6+ were 1.5:1, 2.0: 1 and 2.5:1 . T he best resul ts were obt ained when 2.5 moles of lead chloride were introduced per 1 mole of Cr6+ (about 97% of Cr6+ removal ). The quality of the filtrate is also influenced by its pH. The pH of the wastewater is weakly alkaline and under these conditions the formation and precipitation of lead hydroxide takes place (solubility product equal to 1.1 10-20) thus reducing the participation of lead (II) in the formation of lead chromate, which results in an incomplete removal of CrO42- from the solution. Under acidic conditions (pH < 4) Cr6+ ions are still present in the so lution. There- fore, the most profitable precipitating conditions are ob- tained at a pH between 4 and 6 (acidification of the wastewater with 2-3 dro ps of conce ntrated muriatic acid). However, the presence of SO42- in the wastewater causes the precipitation of Pb2+ ions as hardly soluble lead sul- phate (Ir =1.610-8), thus reducing the efficiency of this method. The results of the experiments are presented in Table 6. T he best treatment results (Cr6+ c ontent belo w0.1 ppm) are obtained when molar ratio Pb2+/Cr6+ was 2.5: 1 and pH was between 5.0 and 6.1. X-ray analysis shows (Figure 3(b)) that the precipitate consists of lead chro mate (PbCrO4) and of another, not precisely identified, solid phase, probably Pb4O3SO4H2O. 3.3. The Reduction of Cr6+ to Cr3+ in Alkaline Environment with Simultaneous Precipitation of Cr(OH)3 The reduction of Cr6+ to Cr3+ with simultaneous precipi- tation of the latter can be described by the following equation: Na2CrO4+2Ca(OH)2+3FeSO4+8H2O=Cr(OH)3+3Fe(OH)3 +2CaSO42H2O+Na2SO4 (2) To determine the optimum quantities of iron (II) sul- phates and calcium hydroxide, the experiments were initially conducted with model solutions. The solution simulating was t ewate r co ntai ned potassium chromate and sodium sulphate in concentrations corresponding to 104 mg Cr 6+ and 960 mg of SO42- in one litre of the solution. The added components are subsequently only referred to as Cr6+. In a first at t empt calcium hydr oxide was added to the solution in a molar ratio of Ca(OH)2 to Cr6+ of 25:1. 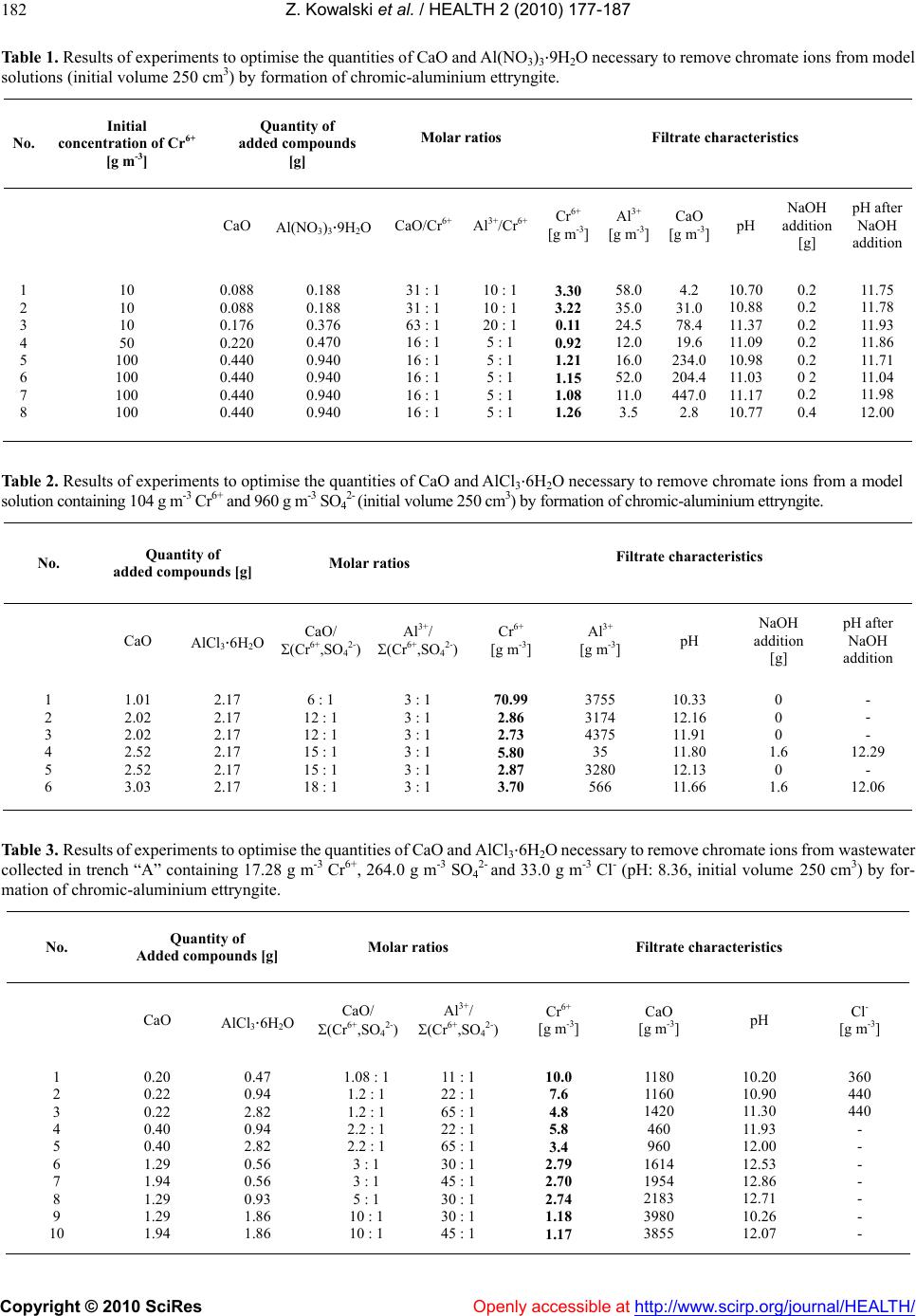 Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes http://www.scirp.org/journal/HEALTH/Openly accessible at 182 Table 1. Results of exper im ents to optim ise th e q ua ntit ies of C aO and Al(NO3)39H2O necessary to remove chromate ions from model solutions (initial volume 250 cm3) by formation of chromic-aluminium ettryngite. No. Initial concentration of Cr6+ [g m-3] Quantity of added compounds [g] Molar ratios Filtrate characteristics CaO Al(NO3)39H2OCaO/Cr6+ Al3+/Cr6+ Cr6+ [g m-3]Al3+ [g m-3]CaO [g m-3] pH NaOH addition [g] pH after NaOH addition 1 2 3 4 5 6 7 8 10 10 10 50 100 100 100 100 0.088 0.088 0.176 0.220 0.440 0.440 0.440 0.440 0.188 0.188 0.376 0.470 0.940 0.940 0.940 0.940 31 : 1 31 : 1 63 : 1 16 : 1 16 : 1 16 : 1 16 : 1 16 : 1 10 : 1 10 : 1 20 : 1 5 : 1 5 : 1 5 : 1 5 : 1 5 : 1 3.30 3.22 0.11 0.92 1.21 1.15 1.08 1.26 58.0 35.0 24.5 12.0 16.0 52.0 11.0 3.5 4.2 31.0 78.4 19.6 234.0 204.4 447.0 2.8 10.70 10.88 11.37 11.09 10.98 11.03 11.17 10.77 0.2 0.2 0.2 0.2 0.2 0 2 0.2 0.4 11.75 11.78 11.93 11.86 11.71 11.04 11.98 12.00 Table 2. Results of experiments to optimise the quantities of CaO and AlCl36H2O necessary to remove chroma te ions from a model solution contain ing 104 g m-3 Cr6+ and 960 g m-3 SO42- (initial volum e 250 cm 3) by formation of chromic-aluminium ettryngite. No. Quantity of added compounds [g] Molar ratios Filtrate characteristics CaO AlCl36H2O CaO/ (Cr6+,SO42-)Al3+/ (Cr6+,SO42-)Cr6+ [g m-3] Al3+ [g m-3] pH NaOH addition [g] pH after NaOH addition 1 2 3 4 5 6 1.01 2.02 2.02 2.52 2.52 3.03 2.17 2.17 2.17 2.17 2.17 2.17 6 : 1 12 : 1 12 : 1 15 : 1 15 : 1 18 : 1 3 : 1 3 : 1 3 : 1 3 : 1 3 : 1 3 : 1 70.99 2.86 2.73 5.80 2.87 3.70 3755 3174 4375 35 3280 566 10.33 12.16 11.91 11.80 12.13 11.66 0 0 0 1.6 0 1.6 - - - 12.29 - 12.06 Table 3. Results of exp eriments to optimise the quantities of CaO and AlCl 36H2O necessary to rem ove chromate ions from wastewater collected in trench “A” containing 17.28 g m-3 Cr6+, 264.0 g m-3 SO42- and 33.0 g m-3 Cl- (pH: 8.36, initial volume 250 cm3) by for- mation of chromic-aluminium ettryngite. No. Quantity of Added compounds [g] Molar ratios Filtrate characteristics CaO AlCl36H2O CaO/ (Cr6+,SO42-)Al3+/ (Cr6+,SO42-) Cr6+ [g m-3] CaO [g m-3] pH Cl- [g m-3] 1 2 3 4 5 6 7 8 9 10 0.20 0.22 0.22 0.40 0.40 1.29 1.94 1.29 1.29 1.94 0.47 0.94 2.82 0.94 2.82 0.56 0.56 0.93 1.86 1.86 1.08 : 1 1.2 : 1 1.2 : 1 2.2 : 1 2.2 : 1 3 : 1 3 : 1 5 : 1 10 : 1 10 : 1 11 : 1 22 : 1 65 : 1 22 : 1 65 : 1 30 : 1 45 : 1 30 : 1 30 : 1 45 : 1 10.0 7.6 4.8 5.8 3.4 2.79 2.70 2.74 1.18 1.17 1180 1160 1420 460 960 1614 1954 2183 3980 3855 10.20 10.90 11.30 11.93 12.00 12.53 12.86 12.71 10.26 12.07 360 440 440 - - - - - - - 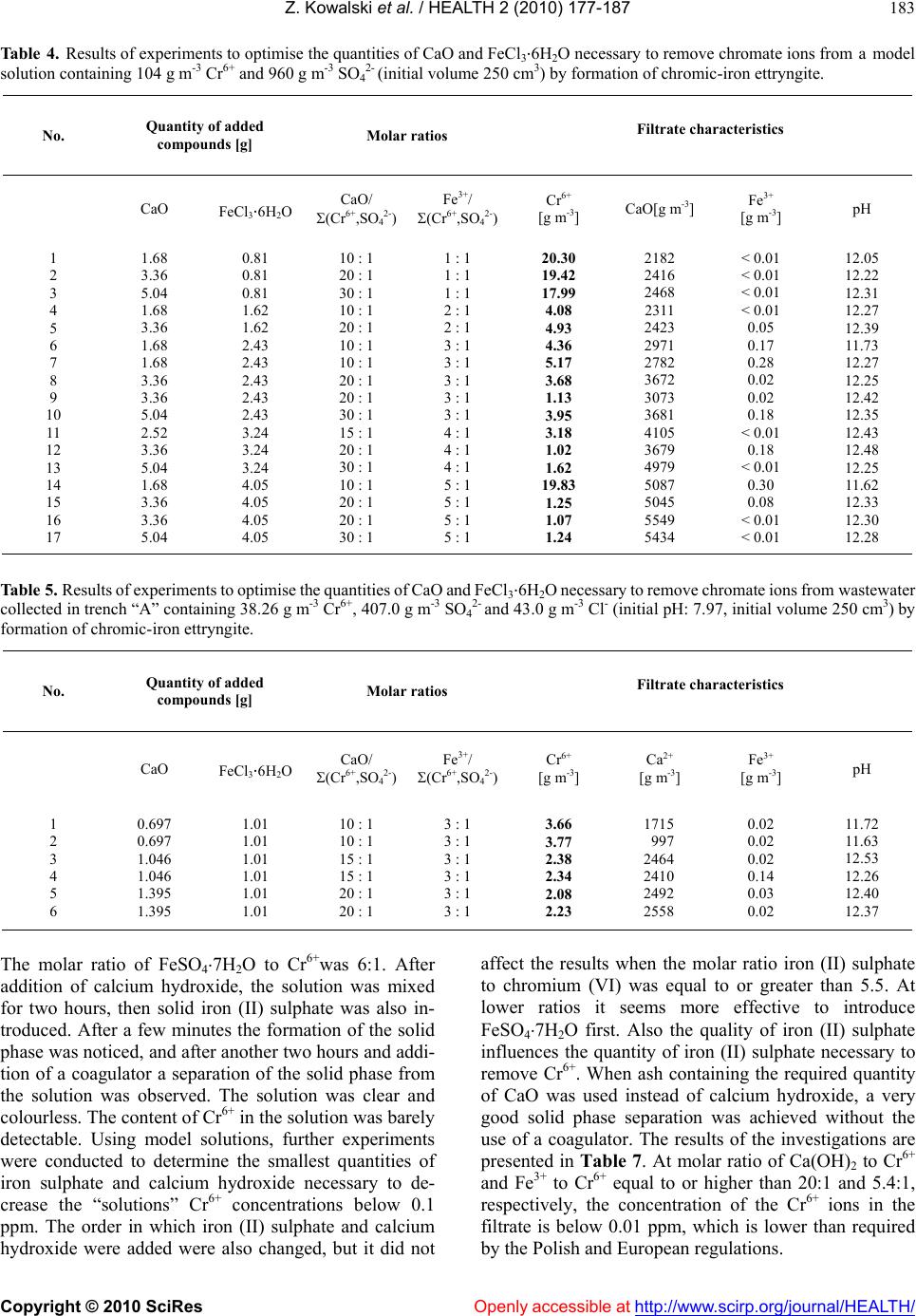 Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 183 Table 4 . Results of experiments to optimise the quantities of CaO and FeCl36H2O necessary to remove chromate ions from a model solution containing 104 g m-3 Cr6+ and 960 g m-3 SO42- (initial volume 250 cm3) by formation of chromic-iron ettryngite. No. Quantity of added compounds [g] Molar ratios Filtrate characteristics CaO FeCl36H2O CaO/ (Cr6+,SO42-)Fe3+/ (Cr6+,SO42-) Cr6+ [g m-3] CaO[g m-3] Fe3+ [g m-3] pH 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 1.68 3.36 5.04 1.68 3.36 1.68 1.68 3.36 3.36 5.04 2.52 3.36 5.04 1.68 3.36 3.36 5.04 0.81 0.81 0.81 1.62 1.62 2.43 2.43 2.43 2.43 2.43 3.24 3.24 3.24 4.05 4.05 4.05 4.05 10 : 1 20 : 1 30 : 1 10 : 1 20 : 1 10 : 1 10 : 1 20 : 1 20 : 1 30 : 1 15 : 1 20 : 1 30 : 1 10 : 1 20 : 1 20 : 1 30 : 1 1 : 1 1 : 1 1 : 1 2 : 1 2 : 1 3 : 1 3 : 1 3 : 1 3 : 1 3 : 1 4 : 1 4 : 1 4 : 1 5 : 1 5 : 1 5 : 1 5 : 1 20.30 19.42 17.99 4.08 4.93 4.36 5.17 3.68 1.13 3.95 3.18 1.02 1.62 19.83 1.25 1.07 1.24 2182 2416 2468 2311 2423 2971 2782 3672 3073 3681 4105 3679 4979 5087 5045 5549 5434 < 0.01 < 0.01 < 0.01 < 0.01 0.05 0.17 0.28 0.02 0.02 0.18 < 0.01 0.18 < 0.01 0.30 0.08 < 0.01 < 0.01 12.05 12.22 12.31 12.27 12.39 11.73 12.27 12.25 12.42 12.35 12.43 12.48 12.25 11.62 12.33 12.30 12.28 Table 5. Results of expe riments to optimis e the quantities of CaO and FeCl36H2O necessary to r emove chromate ions from wastewater collected in trench “A” containing 38.26 g m-3 Cr6+, 407.0 g m-3 SO42- and 43.0 g m-3 Cl- (initial pH: 7.97, initial volume 250 cm3) by formation of chromic-iron ettryngite. No. Quantity of added compounds [g] Molar ratios Filtrate characteristics CaO FeCl36H2O CaO/ (Cr6+,SO42-)Fe3+/ (Cr6+,SO42-) Cr6+ [g m-3] Ca2+ [g m-3] Fe3+ [g m-3] pH 1 2 3 4 5 6 0.697 0.697 1.046 1.046 1.395 1.395 1.01 1.01 1.01 1.01 1.01 1.01 10 : 1 10 : 1 15 : 1 15 : 1 20 : 1 20 : 1 3 : 1 3 : 1 3 : 1 3 : 1 3 : 1 3 : 1 3.66 3.77 2.38 2.34 2.08 2.23 1715 997 2464 2410 2492 2558 0.02 0.02 0.02 0.14 0.03 0.02 11.72 11.63 12.53 12.26 12.40 12.37 The molar ratio of FeSO47H2O to Cr6+was 6:1. After addition of calcium hydroxide, the solution was mixed for two hours, then solid iron (II) sulphate was also in- troduced. After a few minutes the formation of the solid phase was noticed, and after another two hours and addi- tion of a coagulator a separation of the solid phase from the solution was observed. The solution was clear and colourless. The content of Cr6+ in th e solution was barely detectable. Using model solutions, further experiments were conducted to determine the smallest quantities of iron sulphate and calcium hydroxide necessary to de- crease the “solutions” Cr6+ concentrations below 0.1 ppm. The order in which iron (II) sulphate and calcium hydroxide were added were also changed, but it did not affect the results when the molar ratio iron (II) sulphate to chromium (VI) was equal to or greater than 5.5. At lower ratios it seems more effective to introduce FeSO47H2O first. Also the quality of iron (II) sulphate influences the quantity of iron (II) sulphate necessary to remove Cr6+. When ash containing the required quantity of CaO was used instead of calcium hydroxide, a very good solid phase separation was achieved without the use of a coagulator. The results of the investigations are presented in Table 7. At molar ratio of Ca(OH)2 to Cr6+ and Fe3+ to Cr6+ equal to or higher than 20:1 and 5.4:1, respectively, the concentration of the Cr6+ ions in the filtrate is below 0.01 ppm, which is lower than required by the Polish and European regulations.  Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 184 Table 6. Results of experiments to optimise pH and Pb2+/Cr6+ ratio in order to remove chromate ions from wastewater col- lected in trench “A” containing 264.0 g m-3 Cr6+ and 33.0 g m-3 SO42- (initial pH: 7.28, initial volume 250 cm3) by precipitation as lead chromate. No. Molar ratio Pb2+/Cr6+ pH after correction Filtrate pH Filtrate content [g m-3] Cr6+ Pb2+ 1 2 3 4 5 6 7 8 9 10 11 12 1.5 : 1 1.5 : 1 1.5 : 1 1.5 : 1 2.0 : 1 2.0 : 1 2.0 : 1 2.0 : 1 2.5 : 1 2.5 : 1 2.5 : 1 2.5 : 1 4.0 5.4 6.0 - 4.0 5.0 6 0 - 4.3 5.0 6.1 - - - - 8.72 - - - 8.68 - - - 8.34 4.800 0.470 1.440 2.110 1.220 1.010 0.066 2.710 0.910 0.092 0.074 2.700 0.258 < 0.2 - < 0.2 - - - < 0.2 < 0.2 < 0.2 < 0.2 4.57 Further investigations were conducted with wastewa- ter from trench “A”. Calcium hydroxide was added to the solution in molar ratios of Ca(OH)2 to Cr6+ of 15:1, 20:1,27:1 and 68:1. The molar ratios of Fe3+ to Cr6+ were 3.5:1, 4:1, 5.5:1, 6:1, 7.5:1, 13.3:1 and 15:1. After the addition of calcium hydroxide, the solution was mixed for two hours, then solid iron (II) sulphate was intro- duced. After a few minutes, without addition of a co- agulator, the formation of the solid phase was noticed and after another two hours a separation of the solid phase from the solution was observed. The solution was clear and colourless. The results of these investigations are presented in Table 8. The experiment conducted with the natural wastewater proved that by adding cal- cium hydroxide and iron (II) sulphate in molar ratios of Ca(OH)2 to Cr6+ and Fe3+ to Cr6+ equal to or higher than 20:1 and 4:1, respectively, the Cr6+ concentration of the wastewater can be decreased to bel o w 0. 1 pp m. The results of the X-ray analysis of the precipitates showed that the solid phase, which had formed had an amorphous character with some ettryngite traces (Figure 3(c)). The same sample, having been desiccated at a temperature of 378 K still retained its amorphous char- acter (Figure 3(d)). According to the chemical reaction presented above on e can expect the presence of iron (III) and chromium (III) hydroxides and gypsum in the solid phase. Calcium hydroxide is also expected to be present due to the high quantities of Ca(OH)2 which were added to the solution. The fact that the X-ray analysis could not confirm the presence of calcium compounds, especially of gypsum, which shows peaks even in small concentra- tions, indicates either their absence (which is very unlikely), or the creation of compounds of the spine type, which can be amorphous. This problem will be the object of further investigations. Introduction of the ash from fluidised hearths instead of calcium hydroxide, led to formation of the components of ash: SiO2 and Fe2O3, in addition to the ettryngite and the amorphous phases (Figur e 3(e)). Gypsum was not detected either, in spite of the fact that the anhydrite is detectable in ash by X-ray radiography. In this case CaO and SO42- had been incor- porated in the e ttryngite structure. The calci um carbonate, which appeared on the X-ray graphs, was a secondary product, caused by carbonisation. 4. RECAPITULATION AND CONCLUSIONS The geophysical investigations show that the pollutants from chromic heaps are drained by the Regulanka River and carried to the Vistula River. The hydrogeological conditions also ind icate, that in the case of an attempt to regulate the river Regulanka and tighten its river-bed, the polluted water is going to migrate to the Vistula through the permeable sediments on the bo ttom of the Regu lanka River valley. Assuming that all the infiltrates could potentially mi- grate to the Vistula River, the possibility of draining the polluted wate rs and cleanin g them has b een recognized a s an option to improve the environment. Decisions were taken to build the two trenches “A” and “C” and two draining well s “B” and S- 25 in 1 998 an d at t he be gi nni ng of 1999. At the same time the initial plan to regulate the Regulanka riverbed along the distance of 1.5 km, had been given up. The quantity of drained and purified infiltrates, coming from these trenches and draining wells in 1999, was av- erage 900 m3 per day. Results of investigations into the purification of infil- trates containing Cr6+ demonstrated t hat such wastewaters could be efficiently purified to chromium concentrations below 0.1 ppm using the following methods: 1) Precipitation of CrO42- as lead chromate (PbCrO4) using lead (II) salts. The removal of CrO42- by precipita- tion with lead salts should take place at a pH of 4 to 5. At a lower pH, Pb2+ ions can be observed in solution which at a higher pH, on the other hand, an increased consumption of lead salts is observed, since lead pre- cipitates as hydroxide (Pb(OH)2) as a result of hydroly sis. The best results were obtained when the molar ratio of Pb2+ to Cr6+ was to 2.5:1. 2) Reduction of Cr6+ with iron (II) salts and precipita- tion as chromium hydroxide. The most economic com- bination of reagents assuring the complete precipitation of Cr6+ in this reaction is obtained when the molar ratio of Fe2+ to Cr6+ is 4.5:1 and that of CaO to Cr6+ is 20:1. Such ratios guarantee a complete removal of all Cr6+ ions from the solution, even when part of the iron (II) salt is oxidised. With the method based on the incorporation of CrO42- into an ettryngite compound, it was possible to remove 94 % of Cr6+, although not to concentrations below 0.1 ppm, when iron (III) salt and calcium oxide had been added in large quantities. 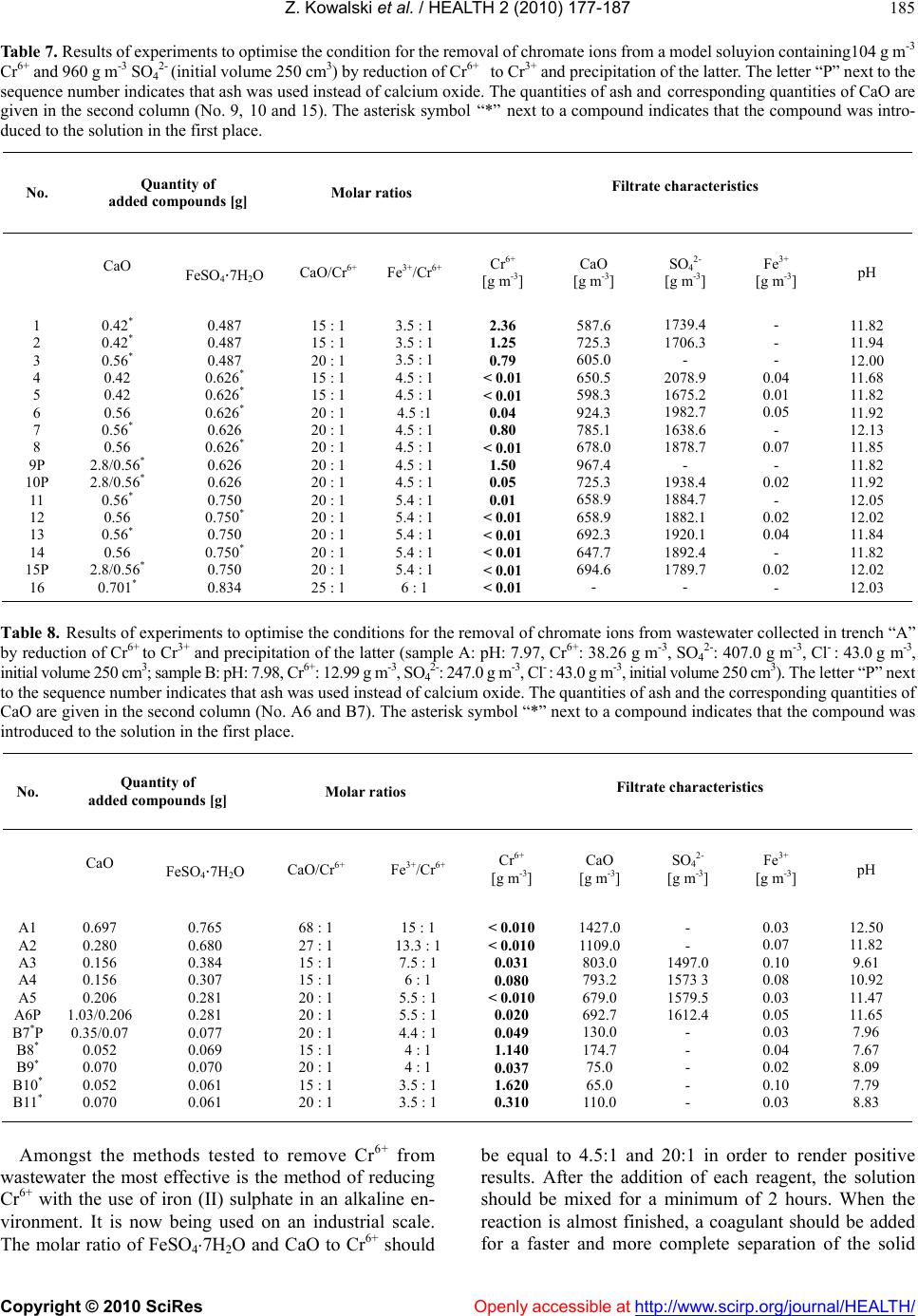 Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes http://www.scirp.org/journal/HEALTH/Openly accessible at 185 Table 7. Results of experimen ts to opti mise the co ndition fo r the r emova l of chromate ions from a model solu yion co ntaining104 g m-3 Cr6+ and 960 g m -3 SO42- (initial vo lume 250 cm 3) b y redu c tion of Cr6+ to Cr3+ and precipitation o f th e la tter. The lett er “P ” next to the sequence number indicates that ash was used instead of calcium oxide. The quantities of ash and corresponding quantities of CaO are given in the second column (No. 9, 10 and 15). The asterisk symbol “*” next to a compound indicates that the compound was intro- duced to the solution in the first place. No. Quantity of added compounds [g] Molar ratios Filtrate characteristics CaO FeSO47H2O CaO/Cr6+ Fe3+/Cr6+ Cr6+ [g m-3] CaO [g m-3] SO42- [g m-3] Fe3+ [g m-3] pH 1 2 3 4 5 6 7 8 9P 10P 11 12 13 14 15P 16 0.42* 0.42* 0.56* 0.42 0.42 0.56 0.56* 0.56 2.8/0.56* 2.8/0.56* 0.56* 0.56 0.56* 0.56 2.8/0.56* 0.701* 0.487 0.487 0.487 0.626* 0.626* 0.626* 0.626 0.626* 0.626 0.626 0.750 0.750* 0.750 0.750* 0.750 0.834 15 : 1 15 : 1 20 : 1 15 : 1 15 : 1 20 : 1 20 : 1 20 : 1 20 : 1 20 : 1 20 : 1 20 : 1 20 : 1 20 : 1 20 : 1 25 : 1 3.5 : 1 3.5 : 1 3.5 : 1 4.5 : 1 4.5 : 1 4.5 :1 4.5 : 1 4.5 : 1 4.5 : 1 4.5 : 1 5.4 : 1 5.4 : 1 5.4 : 1 5.4 : 1 5.4 : 1 6 : 1 2.36 1.25 0.79 < 0.01 < 0.01 0.04 0.80 < 0.01 1.50 0.05 0.01 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 587.6 725.3 605.0 650.5 598.3 924.3 785.1 678.0 967.4 725.3 658.9 658.9 692.3 647.7 694.6 - 1739.4 1706.3 - 2078.9 1675.2 1982.7 1638.6 1878.7 - 1938.4 1884.7 1882.1 1920.1 1892.4 1789.7 - - - - 0.04 0.01 0.05 - 0.07 - 0.02 - 0.02 0.04 - 0.02 - 11.82 11.94 12.00 11.68 11.82 11.92 12.13 11.85 11.82 11.92 12.05 12.02 11.84 11.82 12.02 12.03 Table 8. Results of experiments to optimise the conditions for the removal of chromate ions from wastewater collected in trench “A” by reduction of Cr6+ to Cr3+ and precipitation of the latter (sample A: pH: 7.97, Cr6+: 38.26 g m-3, SO42-: 407.0 g m-3, Cl- : 43.0 g m-3, initial volume 250 cm3; sample B: pH: 7.98, Cr6+: 12.99 g m-3, SO42-: 247.0 g m-3, Cl- : 43.0 g m-3, initial volume 250 cm3) . The letter “P” next to the sequence n umber indicat es that as h was used inst ead of ca lciu m oxi de. T he qu antities of as h and t he corre spo ndin g quan ti ties of CaO are given in the second column (No. A6 and B7). The asterisk symbol “*” next to a compound indicates that the compound was introduced to the solution in the first place. No. Quantity of added compounds [g] Molar ratios Filtrate characteristics CaO FeSO47H2O CaO/Cr6+ Fe3+/Cr6+ Cr6+ [g m-3] CaO [g m-3] SO42- [g m-3] Fe3+ [g m-3] pH A1 A2 A3 A4 A5 A6P B7*P B8* B9* B10* B11* 0.697 0.280 0.156 0.156 0.206 1.03/0.206 0.35/0.07 0.052 0.070 0.052 0.070 0.765 0.680 0.384 0.307 0.281 0.281 0.077 0.069 0.070 0.061 0.061 68 : 1 27 : 1 15 : 1 15 : 1 20 : 1 20 : 1 20 : 1 15 : 1 20 : 1 15 : 1 20 : 1 15 : 1 13.3 : 1 7.5 : 1 6 : 1 5.5 : 1 5.5 : 1 4.4 : 1 4 : 1 4 : 1 3.5 : 1 3.5 : 1 < 0.010 < 0.010 0.031 0.080 < 0.010 0.020 0.049 1.140 0.037 1.620 0.310 1427.0 1109.0 803.0 793.2 679.0 692.7 130.0 174.7 75.0 65.0 110.0 - - 1497.0 1573 3 1579.5 1612.4 - - - - - 0.03 0.07 0.10 0.08 0.03 0.05 0.03 0.04 0.02 0.10 0.03 12.50 11.82 9.61 10.92 11.47 11.65 7.96 7.67 8.09 7.79 8.83 Amongst the methods tested to remove Cr6+ from wastewater the most effective is the method of reducing Cr6+ with the use of iron (II) sulphate in an alkaline en- vironment. It is now being used on an industrial scale. The molar ratio of FeSO47H2O and CaO to Cr6+ should be equal to 4.5:1 and 20:1 in order to render positive results. After the addition of each reagent, the solution should be mixed for a minimum of 2 hours. When the reaction is almost finished, a coagulant should be added for a faster and more complete separation of the solid  Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 186 Figure 4. Block dia gram sh owing the removal p rocedure of chro - mium (VI) from wastewater in the “Alwernia” Chemical Works. phases from the solution. When ash is used, no coagulant is necessary. The decisive argument for selecting this method was the fact that it is possible to use precipitates from the infiltrates as raw material for the production of sodium chromate [33]. The following quantities of chemicals are necessary for the wastewater purification: For each kmol Cr6+ it is necessary to add 4.5 kmols FeSO47H2O and 20.0 kmols CaO. For each kg (tonne) Cr6+ it is necessary t o ad d 24.06 kg (ton nes) FeSO47H2O and 21.54 k g (tonnes) CaO. 10 00 m3 of waste f rom trench “A” with concentration 12.99 g m-3 Cr6+ (see Table 8) contained for example 0.25 k mol, i.e. 12.99 kg Cr6+. The quantities of the main produc ts received from the precipitat ion of 1 kmol Cr6+ are 1.0 kmol Cr(OH)3 and 4.5 kmols Fe(OH )3. The precipitation of 1 kg (tonnes) of Cr6+ results in the formation of 1.98 kg (tonnes) of Cr(OH)3 and 3.08 kg (tonnes) of Fe(OH)3. These quantities were produced for example from 4000 m3 above-mentioned waste from trench “A”. A schematic diagram of this process is presented in Figure 4. The reduction and precipitation of Cr6+ from waste- water can be conducted at ambient temperature even in winter since the introduction of CaO causes a rise in temperature. The addition of the chemicals should take place under intensive mixing with the stirrers being in- stalled in such a way that the reagents can be evenly distributed throughout the container. REFERENCES [1] Kowalski, Z. and Mazanek, C. (1998) Sodium chromate- material flow analysis and technology assessment. Jour- nal of Cleaner Production, 6, 135-142. [2] Kowalski, Z. and Mazanek, Cz. (1998) Conception of the national model for reusing of chromium wastes. Pro- ceedings of International Conference Environmental Pro- tection in Non-Ferrous Metals and Coal Industry, Szk- larska Poręba, Poland. [3] Kowalski, Z. and Wantuch, W. (1999) Process of solid chrome waste utilization: A Polish experience. Chemical Business, 1, 34-36. [4] Hydrogeological map of Poland, sheet Kraków (1986). Wydawnictwo Geologiczne, Warszawa. [5] Kondracki, J. (1978) Geografia fizyczna Polski. PWN, Warszawa. [6] Guliński, M., Kobiela, K. and Kasicki, T. (1998) Spra- wozdanie z badań rozprzestrzeniania się zanieczyszczeń w środowisku gruntowo-wodnym w rejonie składowisk odpadów Z.Ch. Alwernia, Instytut Gospodarki Odpadami w Katowicach (not published). [7] Kania, S. (1998) Gospodarka wodno ściekowa w Z.Ch. Alwernia, Alwernia (not published). [8] Offici al Journ al of the Euro p ean Com m uniti es (1 994) No. L356/15. Hazardous Wastes According to Article 1(4) of Directive 91689/EEC. [9] McNeill, J.D. (1980) Electrical conductivity of soil and rocks. Geonics LTD. [10] Awierbuch, T. and Pawłow, P. (1969) Tiechnołogia soje- dinienij chroma. Chimija, Lieningrad. [11] Szafraniec, J., Szczerburska, T. and Nagel, P. (1986) Badania nad odchromianiem wodnych roztworów na drodze redukcji (VI) siarczynem względnie pirosiaczy- nem sodowym z wydzieleniem chromu (III) w postaci wodorotlenku chromowego. Instytut Chemii Nieorganic- znej, Gliwice, Poland, (not published). [12] Humphreys, F.E. and Bailey, A.D. (1969) Tannery efflu- ents: What are the problem s? Water Pollution Control, 68, 93-98. [13] Hunter, R.E. and Spraul, O.J. (1969) Water Pollution Control, 41, 1716. [14] Koziorowski, A. (1975) Oczyszczanie ścieków przemy- słowych. WNT, Warszawa. [15] Mulokozi, A.M. (1972) The quantitative separation of chromium (VI) from other elements with a strongly basic anion-exchange res in. Analyst, 97, 820-822. [16] Nriagu, J. and Nieboer, E. (1995) Chromium in natural and human environment. John Wiley et Sons, New York. [17] Dittrich, V. (1971) Wasser, Luft und Betrieb, 15, 15. [18] Eru, K. (1964) Wasser, Luft und Betrieb, 8, 603. [19] Ufimciewa, W.P. and Smietanic, A.D. (1969) Oczistka proizwodstwiennych stocznych wod. Sbornik Nr 4, Stroi- jizdat, Moskwa. [20] Bulewicz, E.M., Kozak, A. and Kowalski, Z. (1997) Treatment of chromic tannery wastes using coal ashes from fluidized bed combustion of coal. Industrial and  Z. Kowalski et al. / HEALTH 2 (2010) 177-187 Copyright © 2010 SciRes http://www.scirp.org/journal/HEALTH/Openly accessible at 187 Engineering Chemistry Research, 36, 4381-4384. [21] Kowalski, Z., Kozak, A. and Bulewicz, E.M. (1997) Me- thod of treating liquid wastes containing chromium compounds. Polish Patent Application, 319201. [22] Kowalski, Z. and Kozak, A. (1998) A new method for treatment of chromium containing wastes, in Pawlowski et al. (eds.), Chemistry for the Protection of the Environ- ment 3, Plenum Press, New York and London. [23] Kurowski, W. (1978) Chemia cementu. PWN, Warszawa . [24] Taylor, H.F.W. (1964) The chemistry of cements. Aca- demic Press, London and New York. [25] Anthony, E.J., Bulewicz, E.M., Dudek, K. and Kozak, A. (1997) Proceedings of the 14th International Conference Fluidized Bed Combustion, Vancouver , Canada. [26] Bensted, A. and Prakosh Varma, S. (1971) Studies of ettringite and its derivatives. Cement Technology, 2, 73-76. [27] Bensted, A. and Prakosh Varma, S. (1973) The lowsul- phate form of calcium sulphoaluminate (monosulphate). Cement Te chnol ogy, 4, 112-115. [28] Fela, K., Mazurek, A. and Kozak, A. (1997) Praca Insty- tutu Chemii i Technologii Chemicznej Politechniki Kra- kowskiej, Cracow (not published). [29] Zdral, R. and Kozak, A. (1996) Praca Instytutu Chemii i Technologii Chemicznej Politechniki Krakowskiej, Cra- cow, Poland (not published). [30] Hassett, D.J., Pflughoe ft-Hasse tt , D.F. and McGarthy, G.J. (1991) Papers of the 9th International Ash Use Sympo- sium. Vancouver 2. [31] Woroszyńska, A., Van Leuven, K. and Wilms, D. (1995) Proceedings of the 10th International Conference Chem- istry for Protection of the Environment. Lublin, Poland. [32] Aleksiejew, W. (1966) Analiza jakościowa. PWN, War- szawa. [33] Kowalski, Z. and Walawska, B. (1997) Utilization of chrome tannery wastes into sodium chromate production process. Polish Journal of Applied Chemistry, 41, 385-418.
|