Journal of Water Resource and Protection
Vol.5 No.12(2013), Article ID:41102,8 pages DOI:10.4236/jwarp.2013.512131
TiO2 PC500 Coated on Non Woven Paper with SiO2 as a Binder-Assisted Photocatalytic Degradation of Reactive Black 5 in Aqueous Solution
Laboratory of Water Chemistry, Faculty of Sciences, University of Lomé, Lomé, Togo
Email: tchakodotom@yahoo.fr
Copyright © 2013 Tomkouani Kodom et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 27, 2013; revised October 28, 2013; accepted November 25, 2013
Keywords: Photocatalytic Degradation; Non Woven Paper; TiO2 PC500; Reactive Black 5
ABSTRACT
Photocatalytic discoloration kinetics of Reactive Black 5 (RB5), a vinylsulfone dye, has been studied spectrophotometrically by following the decrease in dye concentration with time at ambient conditions using a flow loop reactor. UV lump, Black Light Blue (BLB) emitting at maximum wavelength of 365 nm and Ahlstrom Research Service paper consistent of TiO2 P500 coated on non woven paper was used respectively as source of UV light and photocatalyst. At natural pH, the result shows that photolysis of RB5 and its adsorption in the presence of photocatalyst was negligible while the photocatalytic oxidation (PCO) permits 30.8% of RB5 degradation. The degradation of dye was studied under a variety of conditions such as volumetric flow rate, initial pH, photocatalyst reuse, and in the presence of electron acceptor such as sodium persulphate ((Na)2S2O8). The degradation rates were found to be strongly influenced by all the above parameters. The circulation flow rate of 108 L/h was the best. The rate constant calculated when the initial pH was varied shows that pH 3 was more favorable for RB5 removal. Peroxydisulphate ions have the strong effect on RB5 discoloration even in dark without and with photocatalyst. When UV light was used in the presence of photocatalyst, 50 min was enough for quasi-total removal of RB5 with 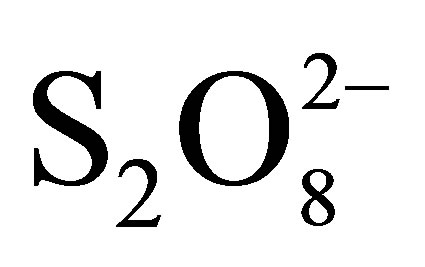 (0.2 M).
(0.2 M).
1. Introduction
Textile industries continue to be attracted nowadays due to the dress sense increasingly growing of human being over the world. In West Africa countries, tannery factories and loincloth craft industries are much developed to produce textile named “basin” very valued by many people. Organics dyes are one of raw materials used and they constitute one of the larger groups of pollutants in wastewater released from these processes. Except the carcinogenic effect of azo-dyes much used, textile wastewaters powdered into the ecosystem without any treatment involves environmental problems such as esthetic pollution and perturbation of aquatic life [1]. The more complex environmental problems associated with the textile industry are that organic dyes are resistant to microbial degradation and they can be converted to the toxic or carcinogenic compounds. Biological treatment becomes so inefficient for dye removal in water [2]. Therefore, advanced oxidation methods, such as photocatalysis, have became an attractive way for textile wastewaters treatment [3,4]. Titanium dioxide is the most popular photocatalyst with good activity under UV light or sunlight [5].
Despite the effectiveness of the heterogeneous photocatalysis process, the operating cost for the total mineralization of hazardous organic effluents remained high due to the obligation to separate the small TiO2 particles from the suspension after treatment [6], limitation of light penetration when the concentration of TiO2 increases (slurry system), reactor configuration which can maximize photocatalysis efficiency, weak activity under sunlight etc. To avoid the former situations, immobilization of catalyst has gained much attention using different methods and different inert supports. Support catalyst has advantage to be reused several times.
The aim of this study is to analyze the feasibility of discoloration of Reactive Black 5 (RB5) dye using a TiO2 PC500 supported non-woven fibers by Ahlstrom Firm (France). Some factors that influence the dye photocatalytic removal were investigated.
2. Material and Methods
2.1. Catalyst
The immobilized photocatalyst used in this study was a commercial titanium dioxide photocatalyst from Ahlstrom firm (France). It consists in PC500 TiO2 by Millennium inorganic chemicals (anatase: >99%, specific surface area 350 - 400 m2∙g−1, crystallites mean size = 5 - 10 nm). Titanium dioxide PC500 was coated on nonwoven fibers (254 µm of thickness) using an aqueous dispersion of colloidal SiO2 (EP1069950B1 European patent) as binder. A specific surface area extender (zeolite UOP, 2000 m2∙g−1) was used to increase adsorption properties of the photocatalyst. Table 1 shows some physical and chemical characteristic of the whole paper [6].
Before the first photocatalytic test, the paper (7.8 cm × 39.8 cm) is weighed, washed several times then dried in an oven to remove all adsorbed water. This operation is necessary to remove non bound particles which will create turbidity in solution. After drying, the lost in mass was ca. 0.136 g. Before each experiment, the paper is wash several times by distillated water. As resulting, there is a lengthening of the paper varying from 0.3 to 0.7 cm in length and in width.
The pH of zero charge of photocatalyst was monitored by introducing 0.2 g of photocatalyst in 40 mL of NaCl (0.1 M) solution at different pH0. Then the resulted pH was measured after one day, second day and finally third day. The pzc is identified as the pH at which ΔpH (pHf – pH0) is zero when plotting ΔpH versus the initial pH, pH0 [7].
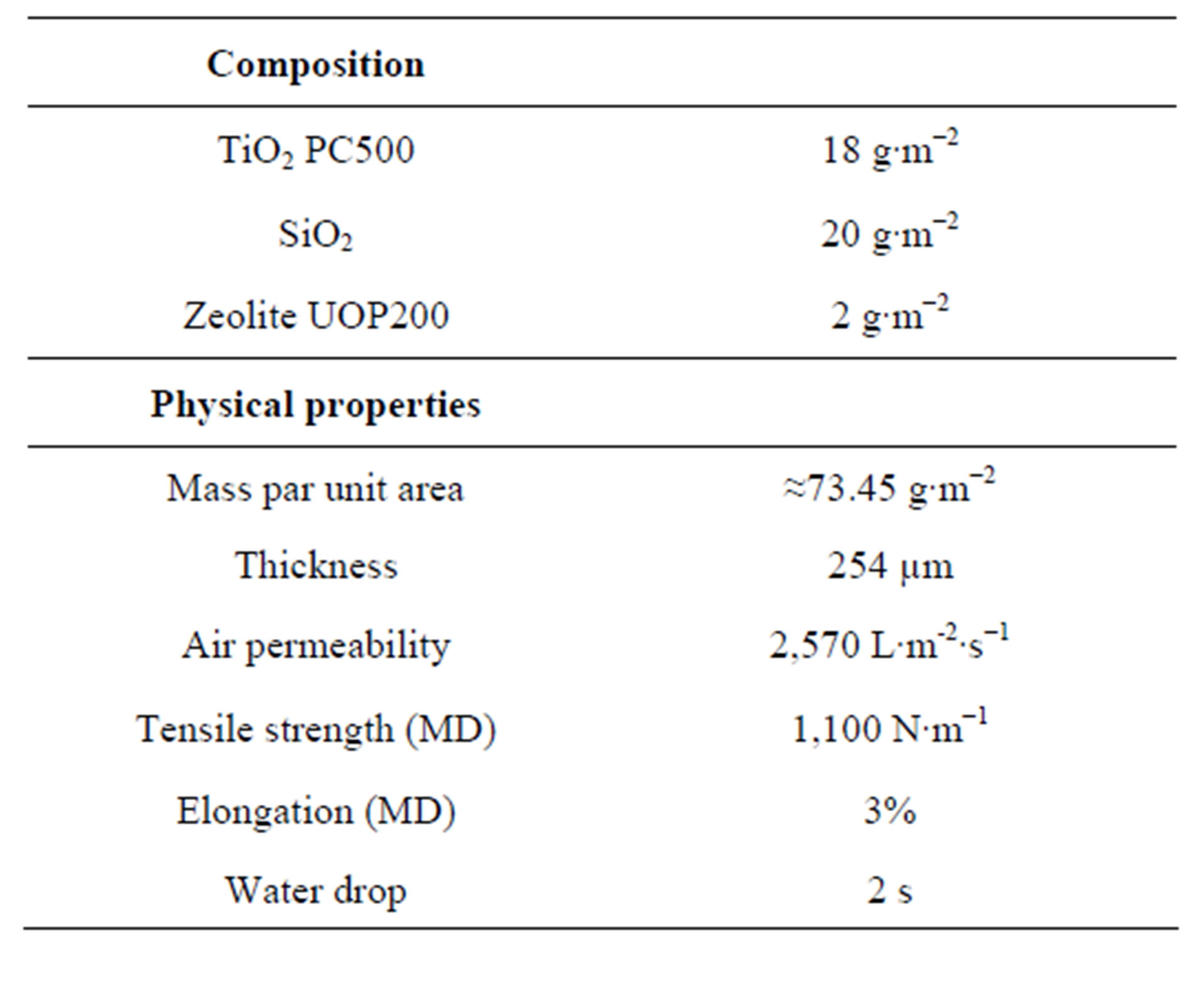
Table 1. Physical and chemical properties of Ahlstrom paper [6].
2.2. Dye
As a model dye compound Reactive Black 5 (RB5, purity 55%) purchased from Sigma Aldrich has been chosen. It is a water-soluble azo-dye with a molecular formula presented in Figure 1. The maximum absorption for RB5 is 599 nm, that wavelength was used for quantitative analysis. Solutions were prepared by dissolving requisite quantity of the dye in distilled water (3 - 4 µS∙cm−1). The pH was adjusted to a given value in the range 1 - 9 by addition of concentrated H2SO4 or NaOH (analytical grades) and was measured using a WTW pH 330i pH-meter.
The natural pH of 10 mg/L of the dye varies from 5.4 to 5.8 at our laboratory room temperature.
2.3. Photocatalytic Reactor
Photocatalysis experiments were performed in a flow loop reactor open to air, provided by a 310.44 cm2 surface of thin photocatalyst layer, volume of treatment 0.9 L solution.
A UV-A Black light Blue Lamp, (15 W, and 365 nm) was used as light source under continuous flow conditions. The optical path through the solution was 1 cm, and the temperature was kept at ca. 29 - 31˚C. A Masterflex I/P Model 77601-00 pump was used for solution circulation during all the experiment. The scheme of the reactor is shown in Figure 2.
2.4. Photodegradation Procedure
The disappearance of the MO was measured using a Perkin Elmer UV/Vis spectrometer Lambda 2. All experiments were performed at room temperature and atmospheric pressure. The photocatalytic degradation experiments were carried out by loading 900 mL of dye solution in the reactor. In a typical experiment, the non woven paper coated (ca. 39.8 cm × 7.8 cm = 310.44 cm2) with TiO2 was fixed on the inner wall of the photoreactor. After adsorption equilibrium was carried out in the dark, the light was turned on to irradiate the solution and the first sample was taken (t = 0). Residual concentrations of the dye in solution at each time were determined from the calibration lines established.
3. Results and Discussion
3.1. Photocatalysis, Photolysis, Adsorption of Reactive Black 5 on Non Woven Paper Coated with TiO2 PC500
The decrease in concentration during a photocatalytic experiment would come from photolysis, adsorption and then photodegradation. It is important to evaluate the contribution of each phenomenon. Figure 3 presents the curves of photolysis of RB5 under UV light (365 nm),
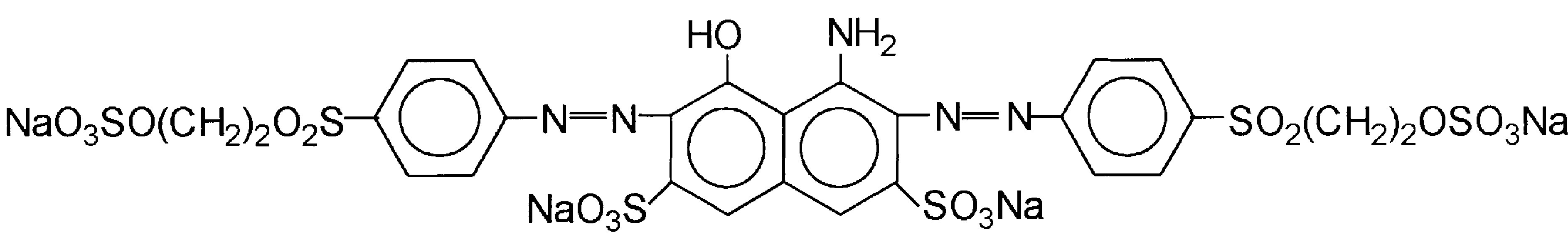
Figure 1. The molecular formula of Reactive Black 5 (λmax = 599 nm, molecular weight = 991.8 g∙mol−1).
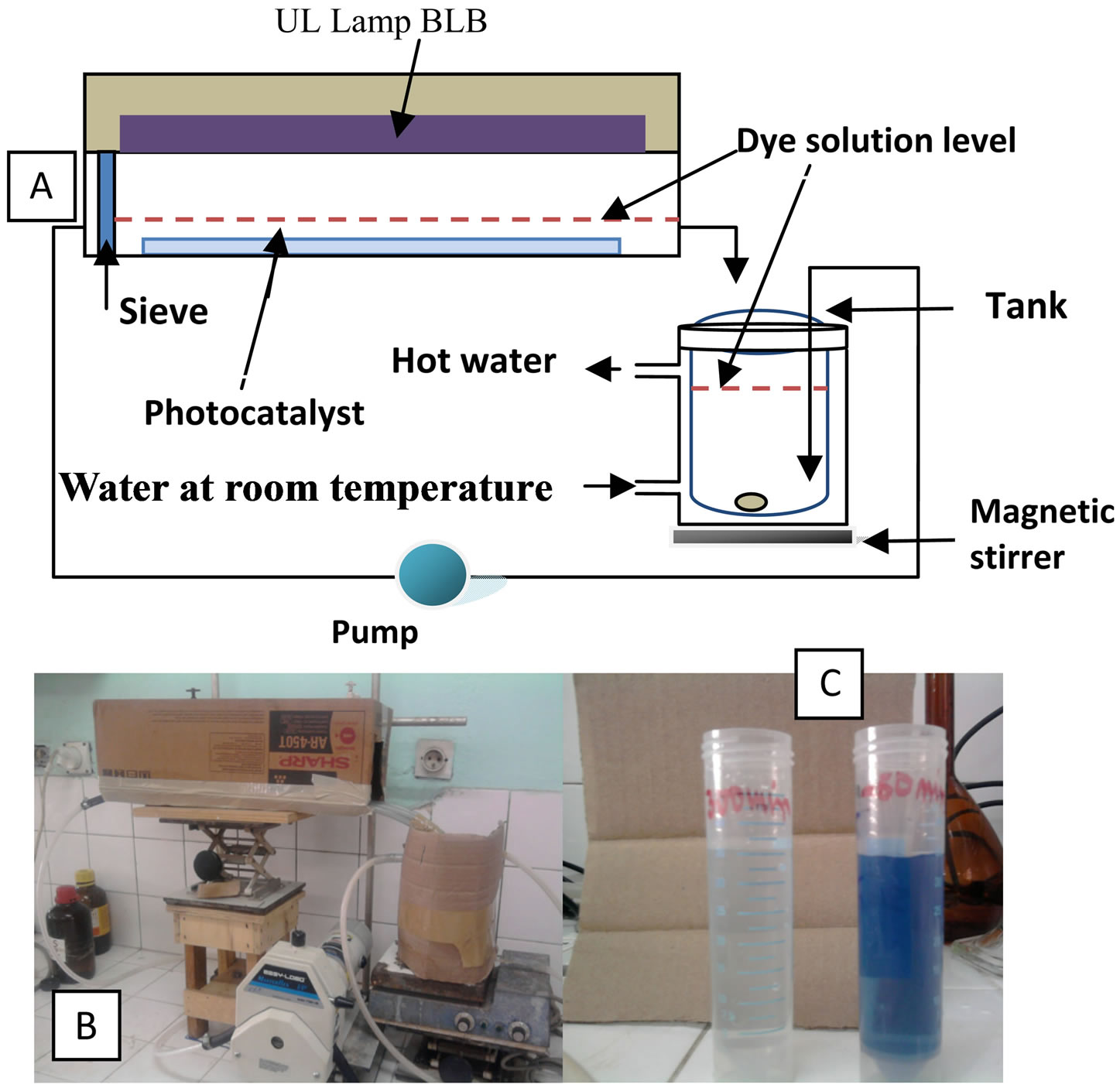
Figure 2. Schematic diagram of photocatalytic flow loop reactor (a), Photography of real reactor (b), Initial solution of RB5 (10 mg/L) and the resulting solution after photodegradation (t = 300 min, Experiment 8) using UV + Photocatalyst.
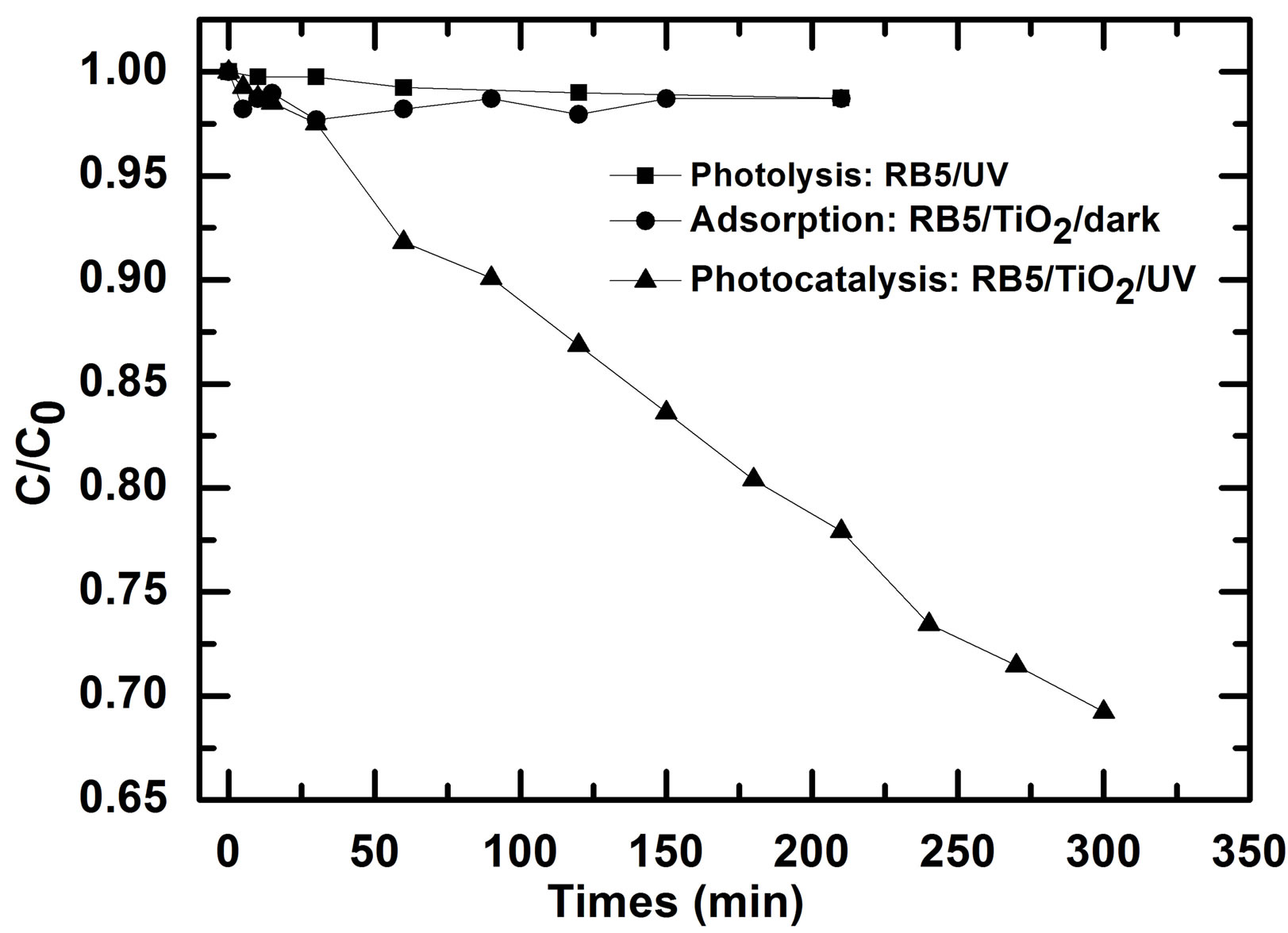
Figure 3. Photodegradation of RB5 (10 mg/L) versus photolysis and adsorption using TiO2 PC500 coated on non woven paper (Ahlstrom firm), V = 900 mL, pH0 ≈ 5.8, solution flow ≈ 108 L/h.
adsorption in darkness on non woven paper coated TiO2 PC500 and the photodegradation on the same paper.
After 210 min, only 1.3% of RB5 undergoes decomposition (photolysis) when only UV light was used. This result is similar to results of Kodom et al. [4] and D. Chatterjee et al. [8] indicating clearly that RB5 cannot be remove efficiently by photolysis. However, removal percentage about 13.87% was reported by Zielinska et al. in the case when UV-Visible light was used probably due to dye sensitization in visible [9]. In the presence of photocatalyst none illuminated, the maximum amount of RB5 adsorption was about 2.5% at 30 min while at 210 min, the amount adsorbed was 1.3% probably because this big molecule can be desorbed as the solution continues flowing. Kinetics curves of the photocatalytic degradation of the dye shows clearly the ability of photocatalysis of RB5 removal in comparison to photolysis and adsorption. These curves followed an apparent first-order kinetics according to Langmuir-Hinshelwood model, confirmed by the linear transforms ln(C/C0) = f(t) not shown here. The rate constant of disappearance in min−1 corresponding to the slope of the later cure for RB5 photodegradation after 300 min was 12.4 × 10−4 min.
3.2. Effect of Solution Flow Speed on the Rate of RB5 Removal
One of the problems in the immobilized photocatalysis process is the mass transport limitation because the catalyst is immobilized on a solid carrier. This limitation was overcome by complete mixing by creating sufficient turbulence in batch system [10] or by solution circulation in flow loop reactor. In the last case, the flow rate can be a determinative factor for efficiently pollutant removal. In this study, the driving force of pump used permits different flow rate through modulation of a knob. It is also important to note that this pump works by pressure on the pipe creating a vibration of the entire reactor. The effect of flow rate on RB5 photodegradation is illustrated by Figure 4. This experiment was done with photocatalyst previously used three times.
The four speeds used correspond to 52.65 L/h, 108 L/h, 152.4 L/h and 186 L/h respectively. The apparent first orders kinetic, kapp, calculated for the four flow rates are 5.6 × 10−3 min−1, 12.7 × 10−3 min−1, 10.8 × 10−3 min−1 and 10.7 × 10−3 min−1 respectively. The flow rate of 108 L/h is more favorable for RB5 removal. It was found that the constant kapp calculated for the flow rate of 52.65 L/h was about twice lower than that for the other three flow rates, i.e. 108, 152.4 and 186 L/h.
During photocatalytic oxidation, organic substrates follow the steps of diffusion, adsorption, and reaction [11]. For the highest flow rates applied, it can be supposed that mass transport towards catalyst surface was beneficial to mass diffusion; therefore the rate constant k will be less affected by the flow rate. On the other hand, when the volumetric flow rate of 52.65 L/h was used, mass diffusion was a limiting factor of the examined process. However, we observed that RB5 degradation rate decrease for the last highest flow rates (152.4 L/h and 186 L/h) in comparison to the flow rate of 108 L/h. These flow rates corresponding to solution speed in the reactor on supported photocatalyst of 14.3 ms/s and 17.2 m/s respectively combined with the strong turbulence induced by the pump, are less favorable for pollutant diffusion-adsorption-reaction than 108 L/h. If we consider the removal efficiency at 186, 152.4, and 180 L/h, these
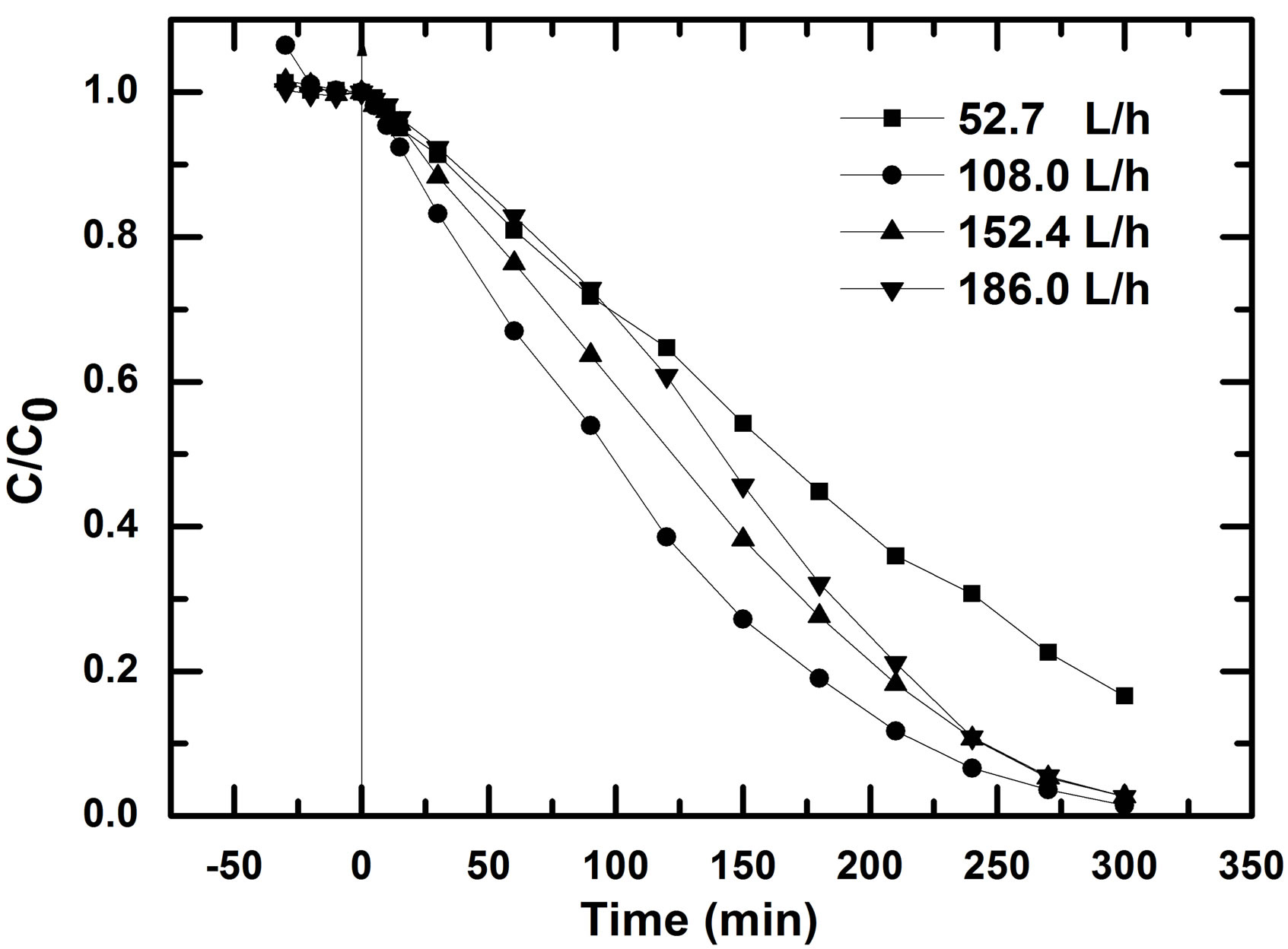
Figure 4. Changes of RB5 concentration in time for different solution flow rates; initial dye concentration: 10 mg/L, V = 900 mL, pH0 = natural pH.
results are in agreement with those of M. A. Behnajady and N. Modirshahla who reported that with decreasing volumetric flow rate from 120 to 60 ml∙mi−1, removal efficiency of Acid Orange 7 in a tubular continuous-flow photoreactor is increased [12]. However, their volumetric flow rates are less high than the values obtained in this study. Taking account the resident time of the reactant, one can clearly say that, the volumetric flow rate of 108 L/h (solution speed: 8.6 m/s over photocatalyst) was favorable for RB5 removal. So for the rest experiment, we have used as flow rate of solution, 108 L/h.
3.3. Determination of Point of Zero Charge (PZC or pHpZC)
The oxide surfaces of TiO2 and SiO2 in water are amphoteric and could be positively charged, negatively charged or neutral depending on the pH of the solution. For below the pH of zero charge (pHpzc), the oxide net surface charge is positive and above pHpzc the net surface charge is negative. Indeed, when a metal oxide is put in contact with an aqueous medium, it develops an electrical charge due to the amphoteric behavior of its surface sites, i.e., its hydroxyls groups. So it is important to know the isoelectrical point especially in heterogeneous catalysis allowing to control or explain some phenomenon bound to the variation of pH. The pH of zero charge was determined using the method described by M. Mullet et al. [7]. Figure 5 shows the variation in pH allowing the determination of pHpzc.
The variations in pH are explained by the proton equilibria that occur at the surface of photocatalyst. As expected, at pH0 values below the pzc of material, an increase in pH (ΔpH > 0) is observed. In these range of pH, the hydroxyls groups at the surface of the paper become protonated and so positively charged. On the contrary, at pH0 values above the pzc, a decrease in pH (ΔpH < 0) is observed. In this range of pH, the hydroxyls groups be-
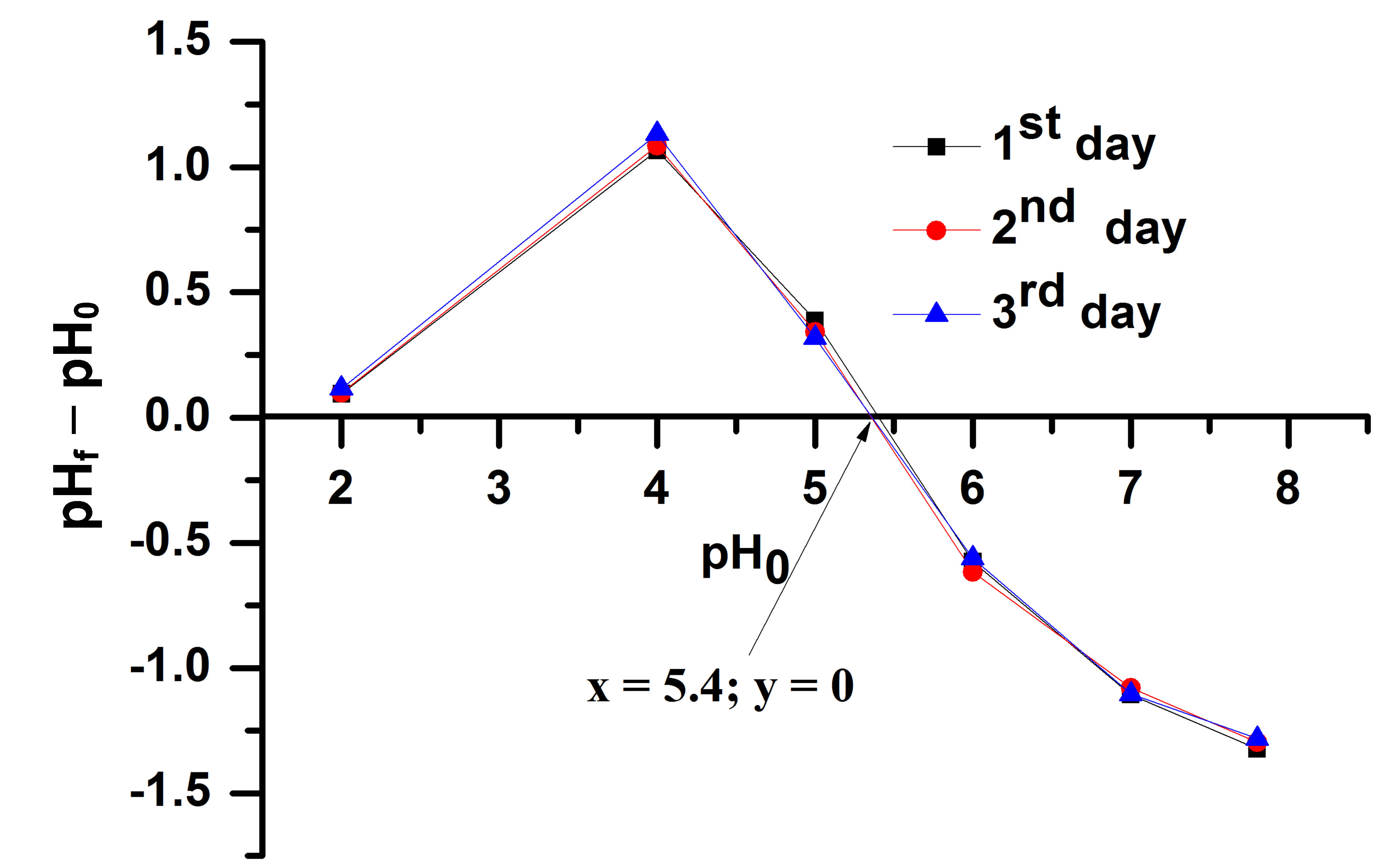
Figure 5. Plot of the variation in ΔpH = pHf – pH0 in 0.1 M NaCl solution, 2 g of photocatalyst.
come deprotonated and so negatively charged [7]. The pzc is identified as the pH at which ΔpH is zero. It occurs at a pH of about 5.4. This value results from four materials (non woven paper, TiO2 PC500, SiO2 and Zeolite). The small variation at pH 2 is due to the influence of SiO2 which pzc is 2.5 while for TiO2 the pzc is reported to be 6.2 [1].
3.4. Effect of Initial pH of the Solution on the Rate of RB5 Photooxidation
According to the previous result on point of zero charge, the behavior of the material will change when pH will vary. It is well known that pH is a determinative factor that needs to be controlled in order to enhance the rate of degradation [2]. Six different pH included natural or free pH of RB5 were chosen to determined the effect of initial pH of solution on the rate of RB photooxidation. The kinetics of RB5 removal obtained at these pHs are presented in Figure 6. Natural pH of RB5 measured at 10 mg/L varies from 5.4 to 5.8 probably due to the influence of laboratory atmosphere. Figure 6 shows clearly the pH dependence of RB5 photodegradation in the range of 1 to 9. The photodegradation rate was better in acid medium. However, decolorization of RB5 was the fastest at pH 3 than pH 2 and pH 1. Nevertheless, the adsorption rate was higher in the latter pHs showing that silica (pHpzc = 2.5 [1]) as a binder perturbs activity in acidic medium.
pH may affect photocatalytic oxidation (PCO) in a number of ways [13].
First, the charge of the dye molecules with ionizable functional groups and the surface of the TiO2 catalyst are both pH dependent. pH changes can thus influence the adsorption of dye molecules onto the catalyst surfaces, an important step for PCO to take place. The phenomenon explains well the increase in rate of RB5 removal in acid medium since RB5 is an acid (anionic) dye which adsorption is strong. Indeed, in acid media, TiO2 surface is positively charged and thus electrostatic attraction be-
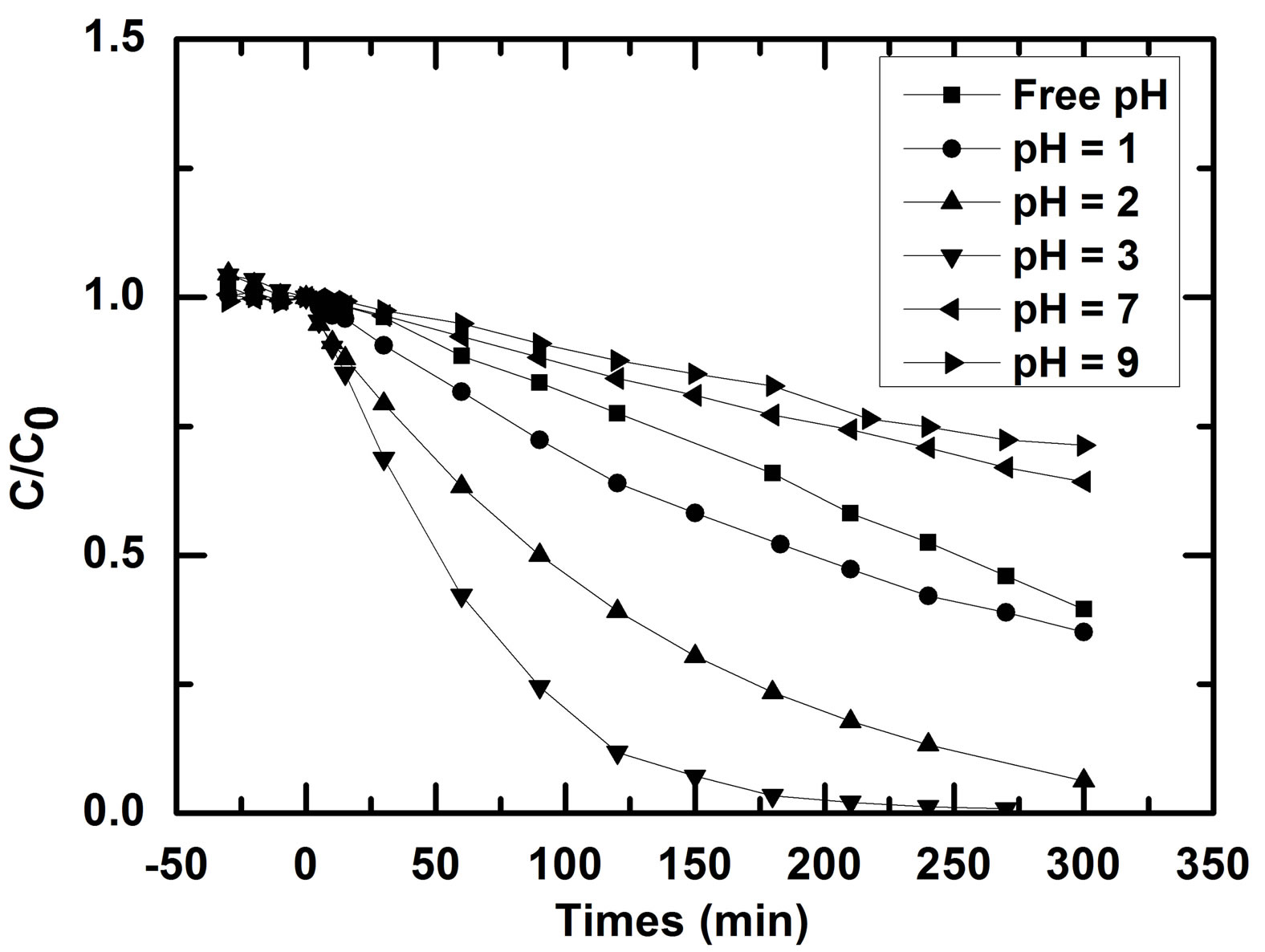
Figure 6. Effect of initial pH on the rate of RB5 degradation with non woven paper coated with TiO2 PC500, RB5 (10 mg/L, Vsolution = 900 mL).
tween sulfonic moiety of the azo dye and positive charges of the oxide is increased.
For the second view,  can be formed by the reaction between hydroxide ion and positive hole in valence band (h+VB) according to Equation 3.
can be formed by the reaction between hydroxide ion and positive hole in valence band (h+VB) according to Equation 3.
An alkaline condition would thus favor  formation and enhance degradation. However, we observe that the rate of RB5 degradation was not positively affected in alkaline media and the peuso-first order constant decreases when pH increases (Figure 7). In Alkaline condition, there is repulsion between RB5 and TiO2 avoiding adsorption step.
formation and enhance degradation. However, we observe that the rate of RB5 degradation was not positively affected in alkaline media and the peuso-first order constant decreases when pH increases (Figure 7). In Alkaline condition, there is repulsion between RB5 and TiO2 avoiding adsorption step.
Third, the TiO2 particles tend to agglomerate under acidic condition and the surface area available for dye adsorption and photon absorption would be reduced [14]. This behavior would explain the decrease in rate when pH varies from 2 to 1. Moreover, the adsorption at these pHs was important probably because of the presence of SiO2, as binder with a point of zero charge around 2.5. This study shows that the photocatalytic process occurs mainly on the photocatalyst surface (direct photocatalysis), and not in the bulk solution (indirect photocatalysis), thus adsorption of substrates is essential for their photocatalytic degradation [15].
3.5. Reuse of the Photocatalyst during RB5 Photodegradation
The possibility of reusing the photocatalyst was examined to determine the cost effectiveness of the method. Successive tests of the photocatalytic degradation of Reactive Black 5 using the same photocatalyst were performed. The regeneration of the catalyst can be done in a very simple way. After the degradation, the photocatalyst was then thoroughly washed with distilled water and reused for degradation with a fresh lot of dye solution. The experimental results of eleven successive experiences interrupted by photocatalyst washing are shown in Figure 8.
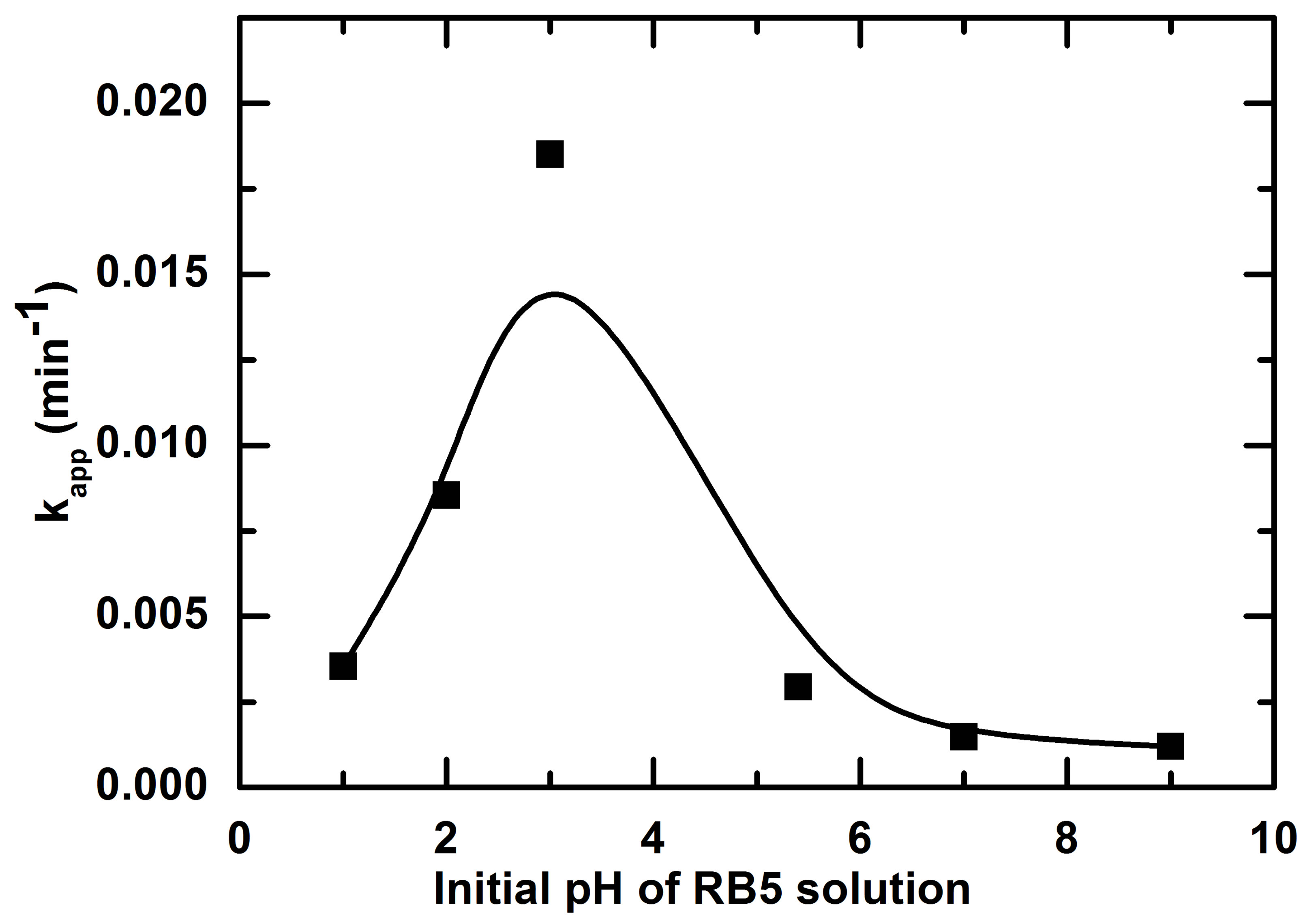
Figure 7. Effect of initial pH on the pseudo-first-order rate constant for the photocatalytic oxidation (PCO) of RB5 (normalized curve).
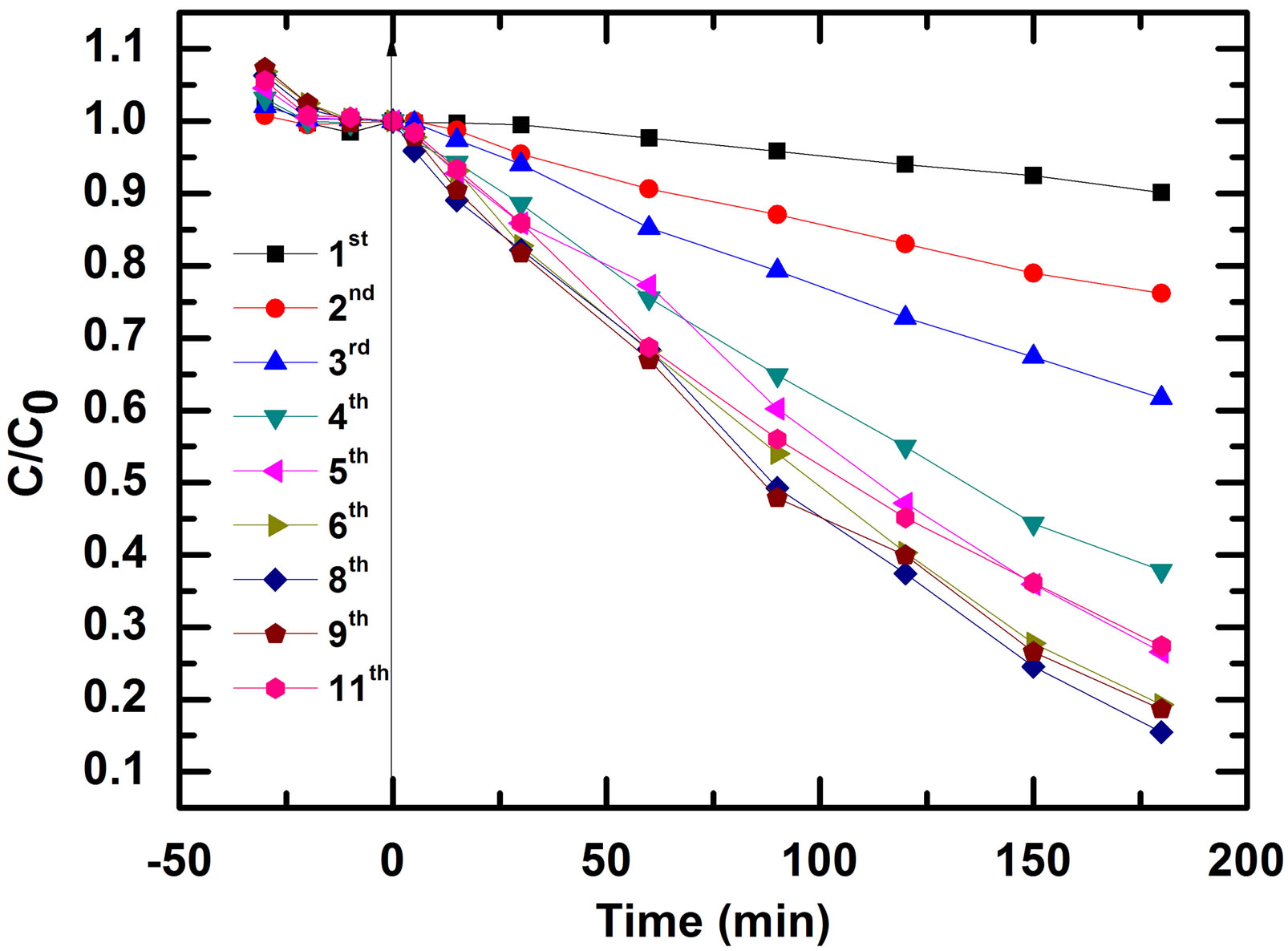
Figure 8. Successive tests of the photocatalytic degradation of Reactive Black 5 using the same photocatalyst.
An increase of photocatalytic efficiency was observed from the first experiment until eighth (8th) experiment and then a slight loss of efficient was observed. The evolution of the apparent first order constant is represented in Figure 9.
One can clearly see that a strong and gradual increase of efficiency occurs form beginning to eighth test. The apparent first order constant was increased 6.6 times at 8th test in comparison to the first test. The monitoring of adsorption during 30 min in darkness indicated that the amount adsorbed at successive experiment increases in the same direction as photocatalytic oxidation rate (Table 2).
Indeed, when the photocatalytic was wetted with water, there was an elongation of the paper about 3.6% in length and 2.5% in width explaining the increase of adsorption and then photoactivity.
On the other hand, the amorphous silica used as binder would have an important influence on the photoactivity of TiO2 P500. In fact, F. Thevenet et al. [16] noticed, in their study done in gas phase, that the inhibitor effect of SiO2 is not to be neglected taking care about efficiency. In liquid phase as in present work, repetitive use coupled with intermediate washing could cause silica detachment.
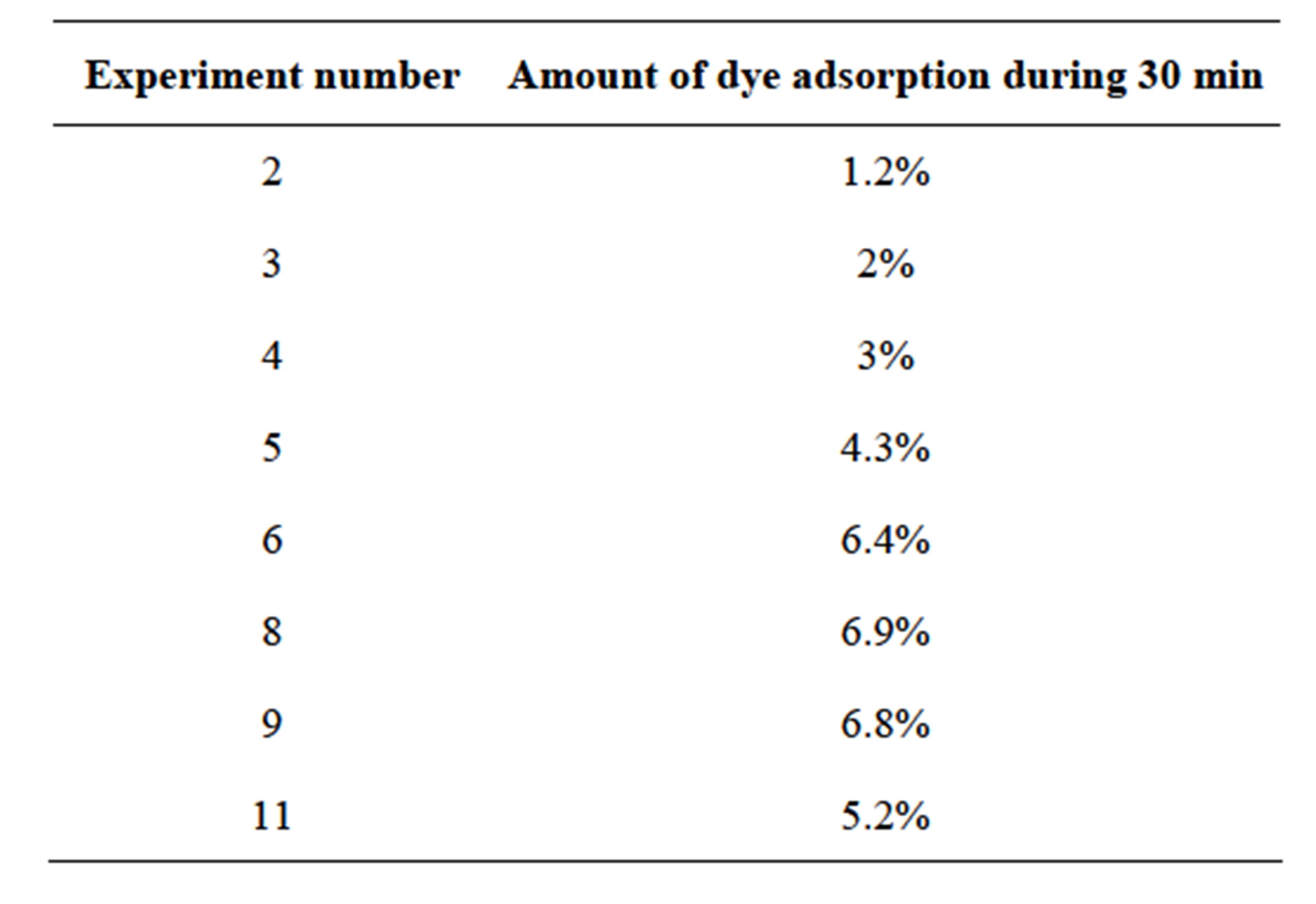
Table 2. Adsorption amount at successive experiments of RB5 oxidation with the same paper.
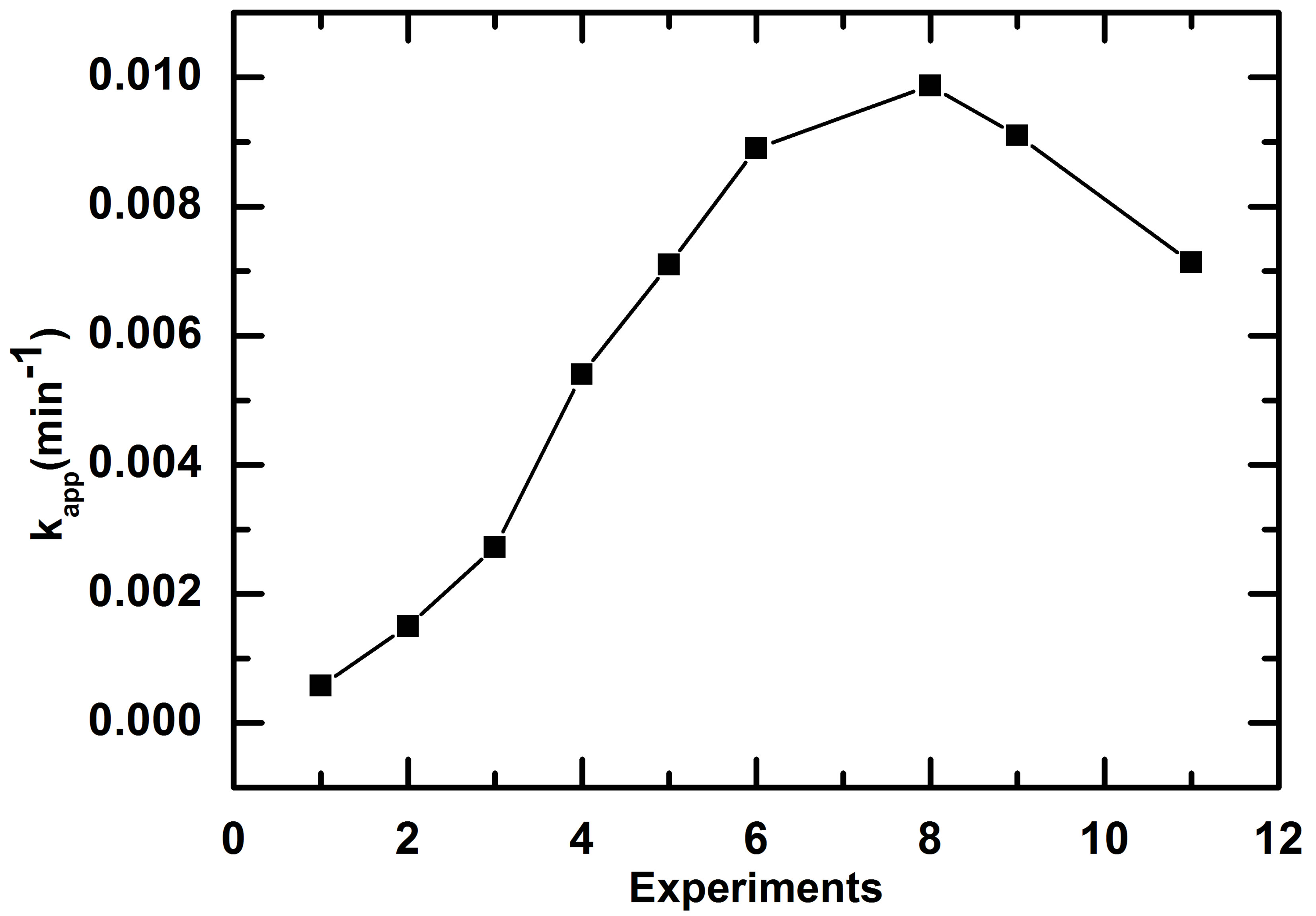
Figure 9. Evolution of apparent first order constant, kapp, according to successive tests of RB5 removal with the same paper coated with TiO2 PC500.
TiO2 particles are then denuded leading to an increase of photoactivity. However, there is a limitation consistent of layer saturation with byproducts which could be removed by washing the paper in acid solution. This result shows that the photocatalyst can be used several times. However, it is necessary to take care of the manipulation of the paper to avoid all liberation of the powder.
3.6. Effect of Addition Peroxydisulphate Ions
Upon irradiation of TiO2, electrons on the surface of the semiconductor are produced at the conduction band, and positive holes are formed in the valance band. The electrons and holes can either recombine and produce thermal energy, or interact with other molecules. The holes may react either with electron donors in the electrolyte or with hydroxide ion to produce powerful oxidizing species such as hydroxyl radicals, which oxidize organic compounds on the surface [17]. According to this, the relevant reactions at the semiconductor surface causing the degradation of dyes can be expressed as follows:
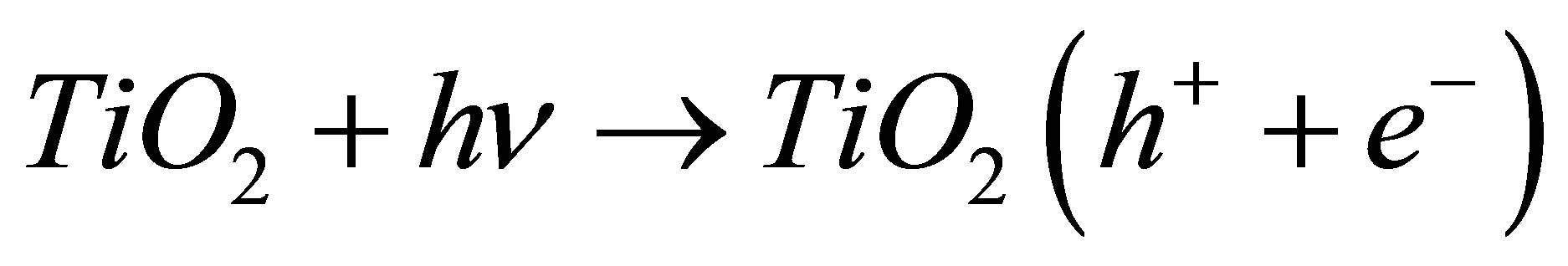 (1)
(1)
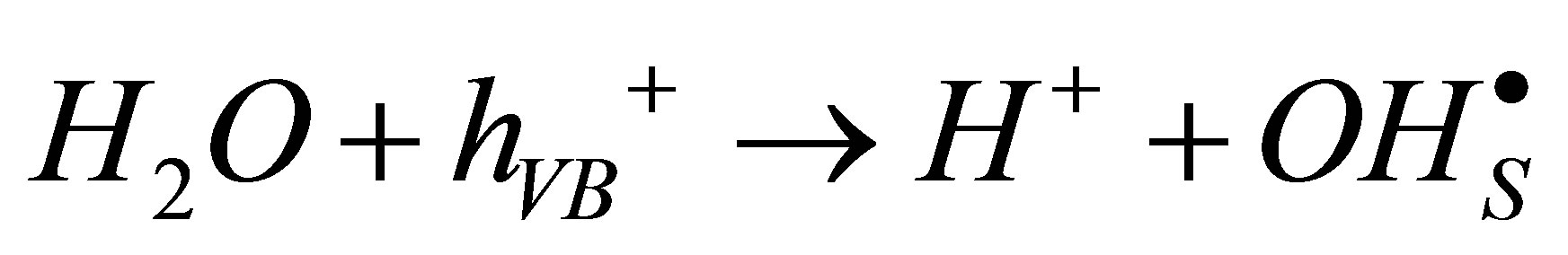 (2)
(2)
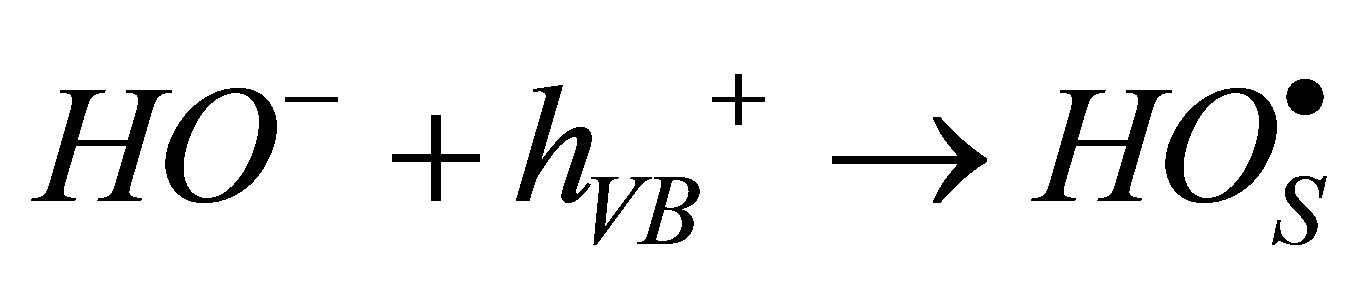 (3)
(3)
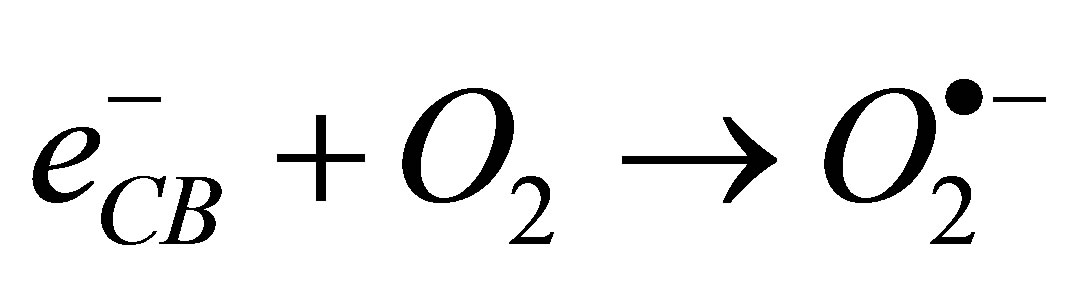 (4)
(4)
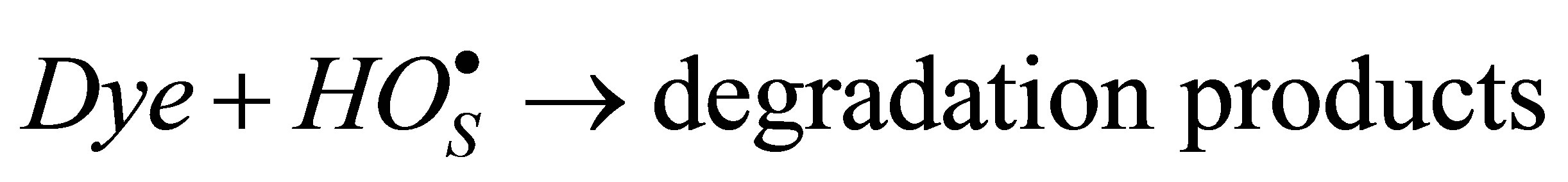 (5)
(5)
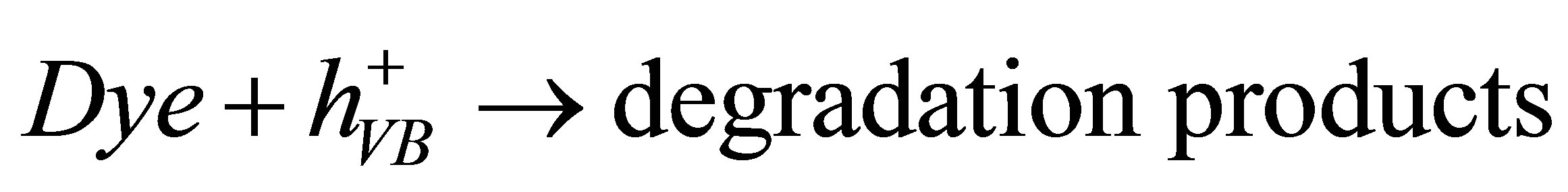 (6)
(6)
To avoid recombination, so increase photocatalytic efficiency, additional of a good electron scavenger is the suitable way. Hydrogen peroxide (H2O2) and peroxydisulphate ions (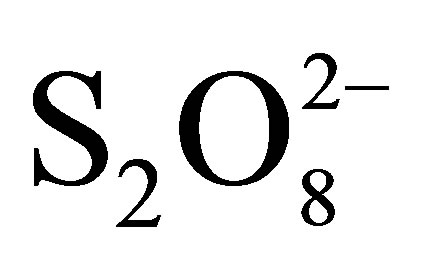 ), strong oxidants, haven been proposed to play this role. We have focused this study on effect of peroxydisulphate on the rate of RB5 photodegradation. Kinetics of RB5 disappearance in the presence of
), strong oxidants, haven been proposed to play this role. We have focused this study on effect of peroxydisulphate on the rate of RB5 photodegradation. Kinetics of RB5 disappearance in the presence of 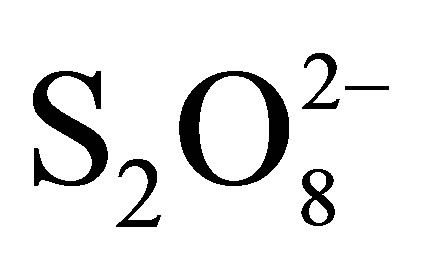 are presented in Figure 10.
are presented in Figure 10.
Figure 10 shows that peroxydisulphate has a strong effect on the rate of RB5 oxidation. We remark that the solution faded even in dark and without TiO2 showing that these ions are able to oxidize RB5 themselves. V. Augugliaro et al. have reported this behavior on Methyl Orange photodegradation using TiO2 P25 in slurry form. However, it was found that there was no mineralization in these conditions when total organic carbon was monitored. Due to this behavior, experiments were conducted in the presence of 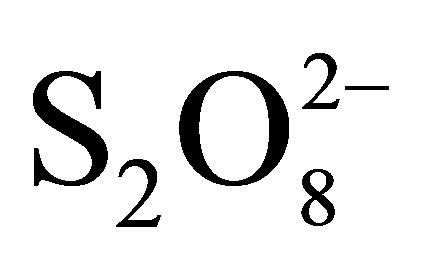 but without TiO2 and without irradiation, in the presence of
but without TiO2 and without irradiation, in the presence of 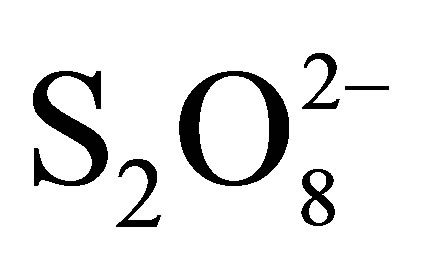 with TiO2 and without irradiation and finally in the presence of
with TiO2 and without irradiation and finally in the presence of 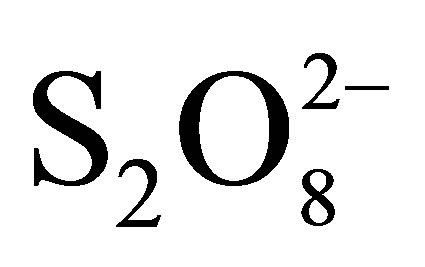 with TiO2 under irradiation. The percentages of color abatement at 20 min are reported in Table 3.
with TiO2 under irradiation. The percentages of color abatement at 20 min are reported in Table 3.
As the initial concentrations of 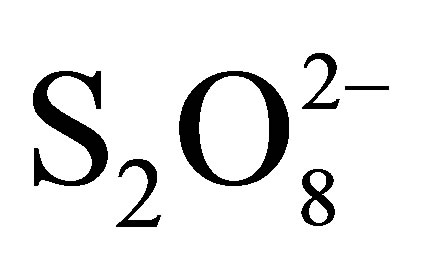 increases, the effect on the rate of RB5 abatement increases in every condition. These result compared to the case of absence of
increases, the effect on the rate of RB5 abatement increases in every condition. These result compared to the case of absence of  show that RB5 disappearance was very fast, although is showed to be more significant in respect to that of corresponding heterogeneous systems:
show that RB5 disappearance was very fast, although is showed to be more significant in respect to that of corresponding heterogeneous systems: 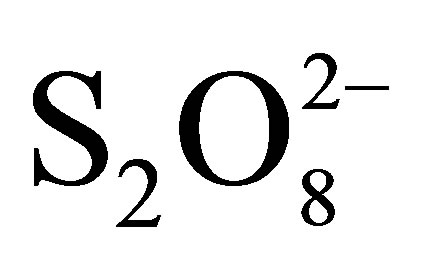 + TiO2 and
+ TiO2 and 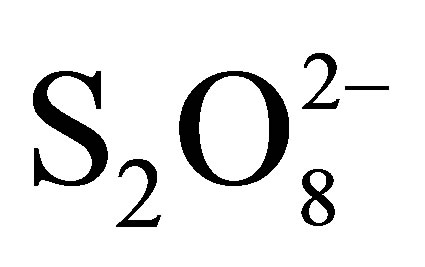 + TiO2 + hν. In presence of
+ TiO2 + hν. In presence of 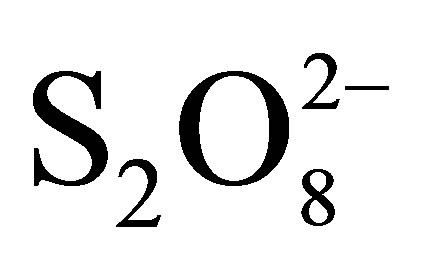 the solution of RB5 faded quickly and turns to rose before total discoloration.
the solution of RB5 faded quickly and turns to rose before total discoloration.
Without possibility to monitor TOC measurement due to the lake of TOC analyzer, we have measured quickly the absorbance at different wavelength when the solution was rose at 20 min in two cases (Figure 11).
These absorbencies lower than the absorbance of RB5 at initial concentration of 10 mg/L (0.395 - 0.4 a.u), show clearly the disappearance of Reactive Black 5. The by products absorb very weakly in the range of wave lengths studied (Visible). This shows that these byproducts, certainly still organic in nature, are derived from the cleavage of azo bonds, because the cleavage of -N=N-bonds leads to the decolorization of dyes [18]. In darkness 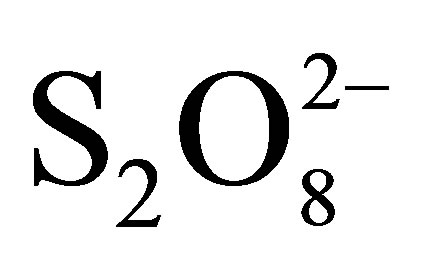 ions are able to oxidize directly RB5 molecules. In the presence of photocatalyst, the increase of RB5
ions are able to oxidize directly RB5 molecules. In the presence of photocatalyst, the increase of RB5
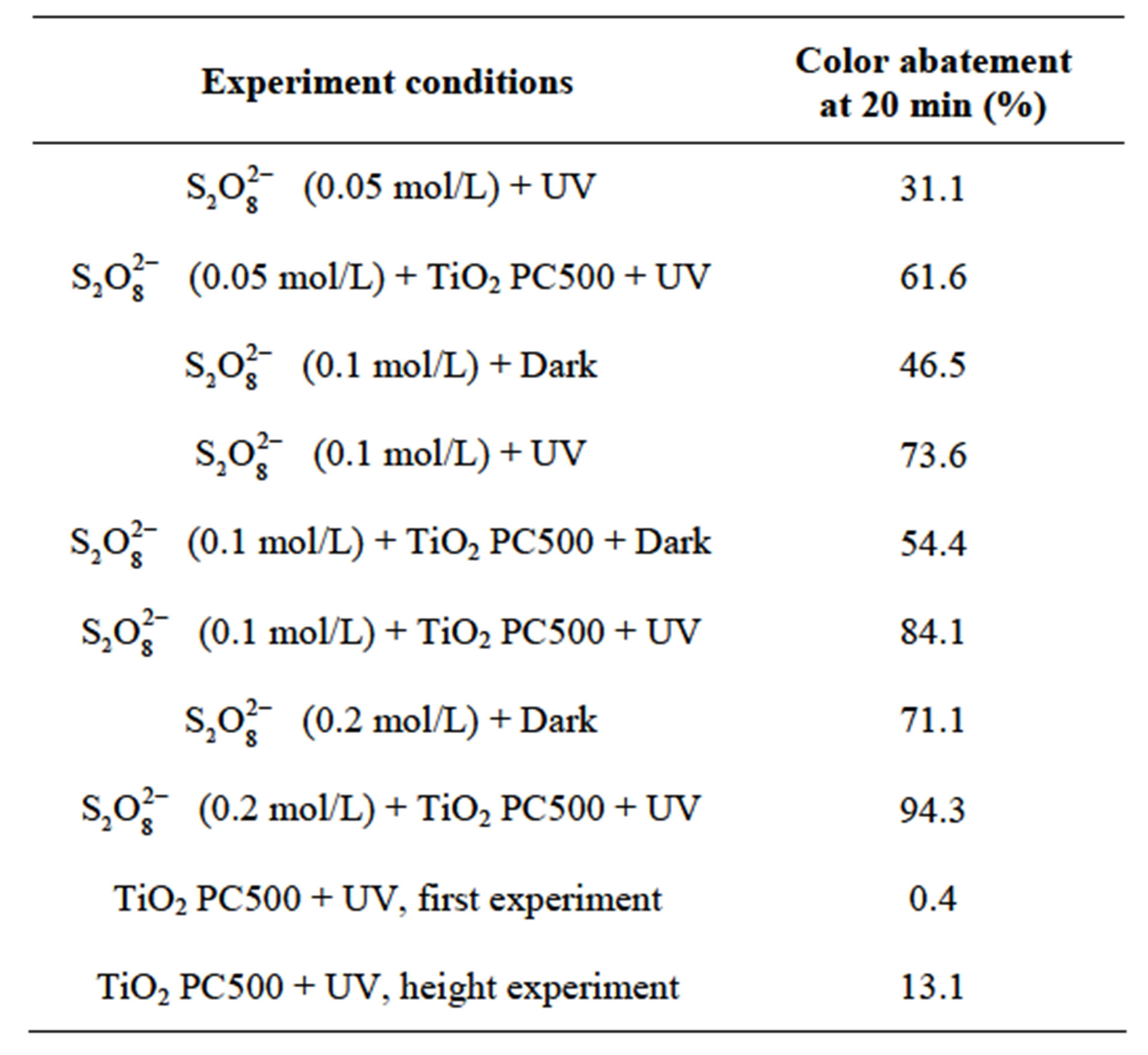
Table 3. RB5 (10 mg/L) abatement at 20 min in presence of peroxydisuphate ions.
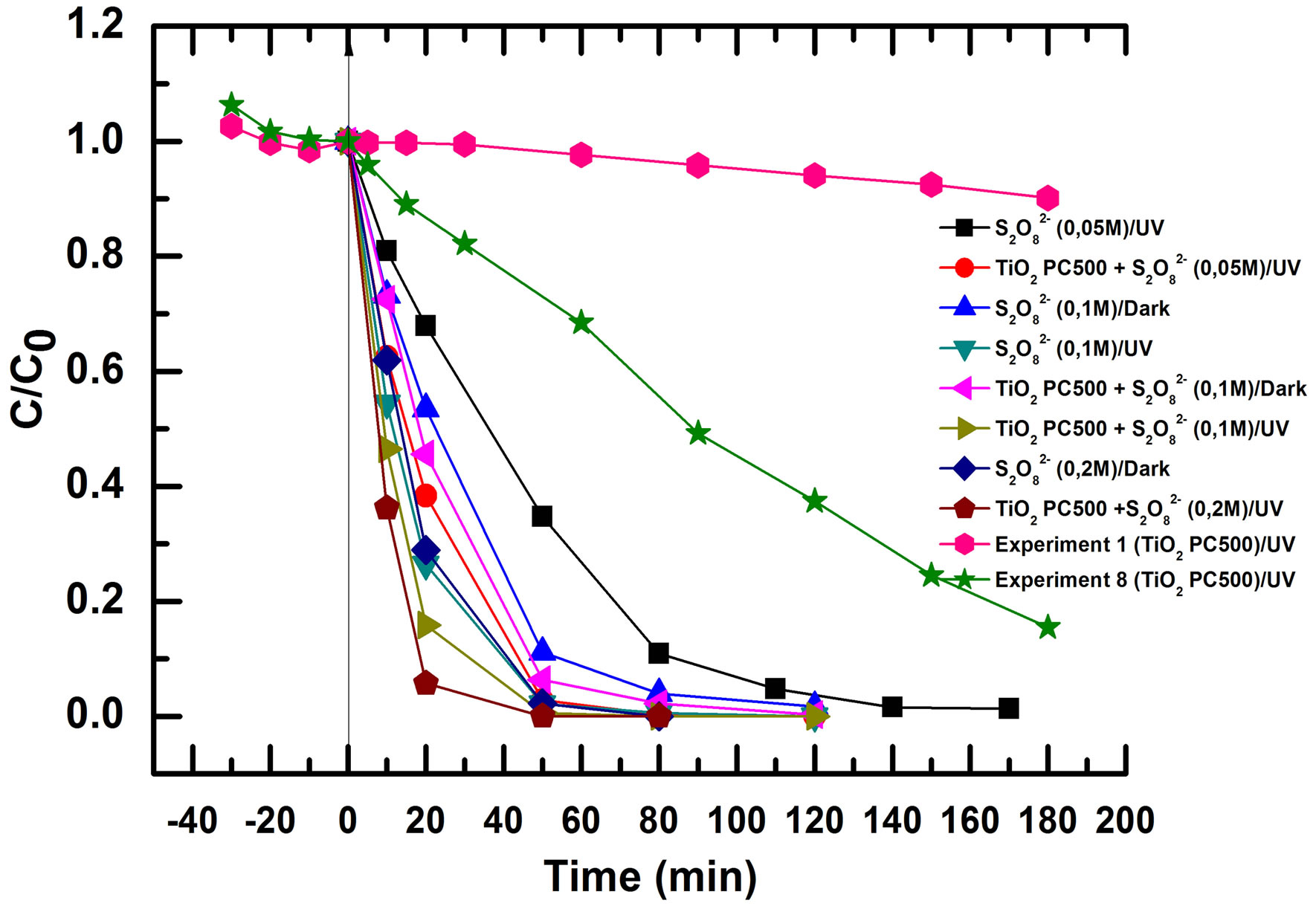
Figure 10. 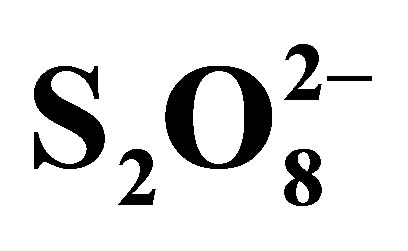 effect on PCO of RB5, 10 mg/L, V = 900 mL,
effect on PCO of RB5, 10 mg/L, V = 900 mL, 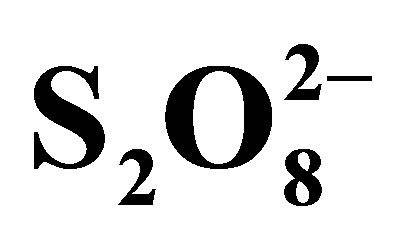 concentrations (0, 0.1, and 0.2 mol/L).
concentrations (0, 0.1, and 0.2 mol/L).
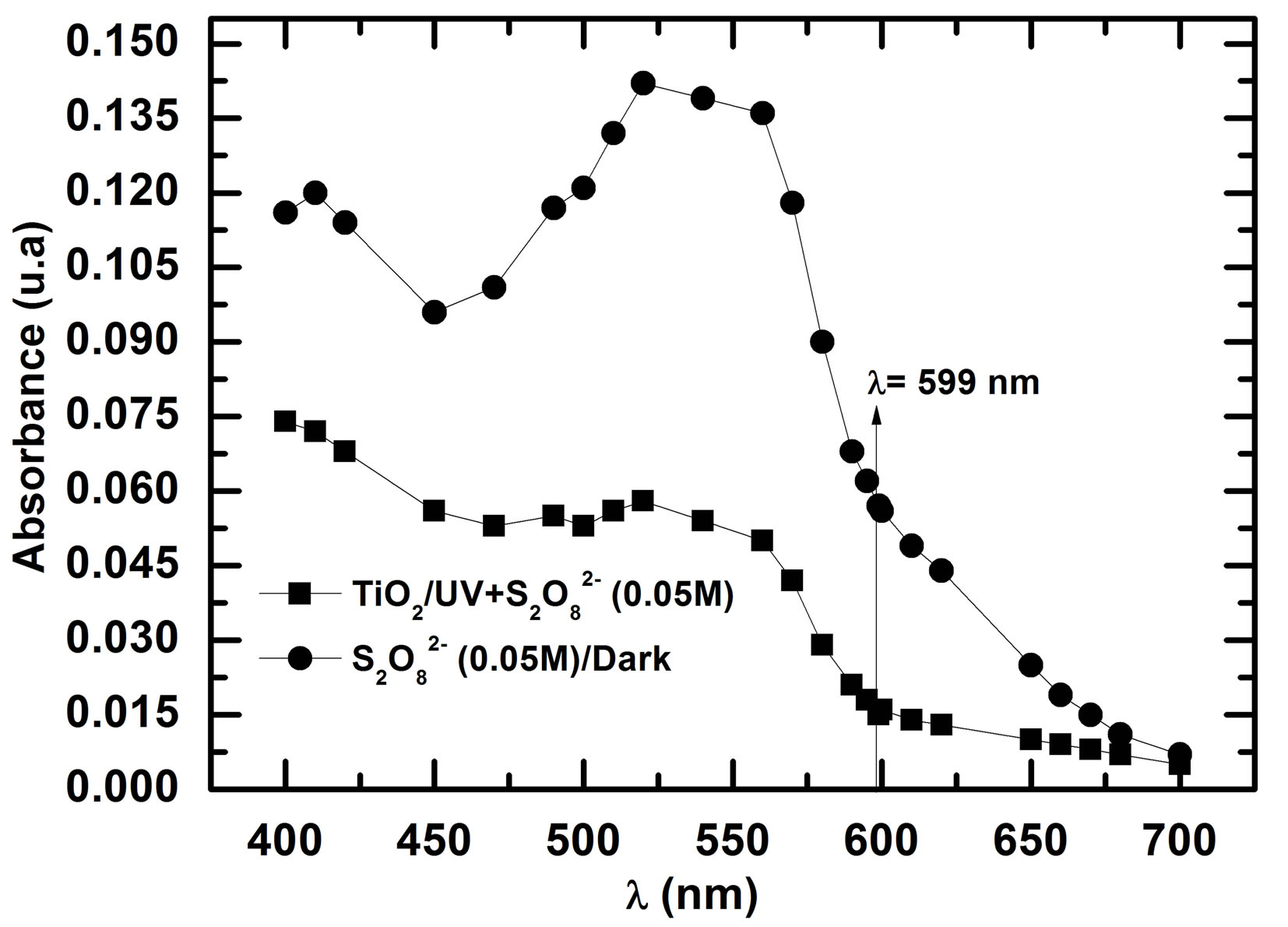
Figure 11. Absorbance evolution of solutions of RB5 + 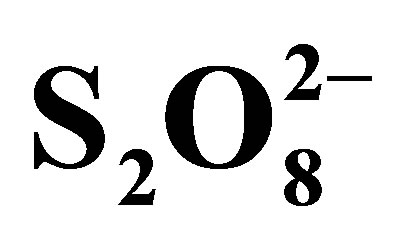 (0.05 M) after 20 min of contact.
(0.05 M) after 20 min of contact.
disappearance is due to additional effect 1) decrease in pH (0.05 M of 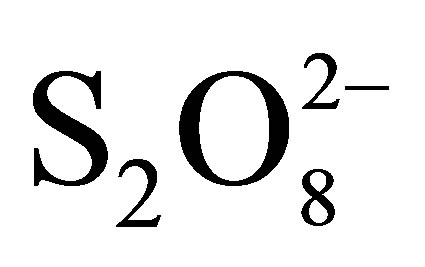 → pH0 = 3.2; 0.1 M of
→ pH0 = 3.2; 0.1 M of 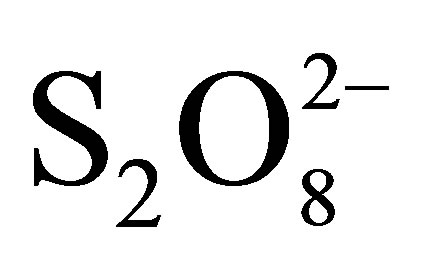 → pH0 = 2.7 and 0.2 M of
→ pH0 = 2.7 and 0.2 M of 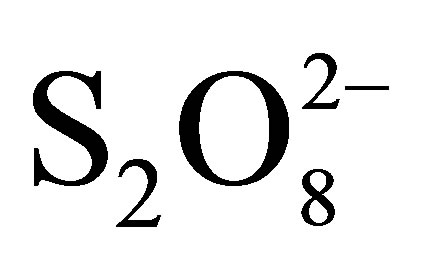 → pH0 = 2.5) involving the increase of RB5 adsorption even in dark and under illumination, 2) inhibition of the electron-hole recombination at the semiconductor surface illuminated according to the following equations [19,20]:
→ pH0 = 2.5) involving the increase of RB5 adsorption even in dark and under illumination, 2) inhibition of the electron-hole recombination at the semiconductor surface illuminated according to the following equations [19,20]:
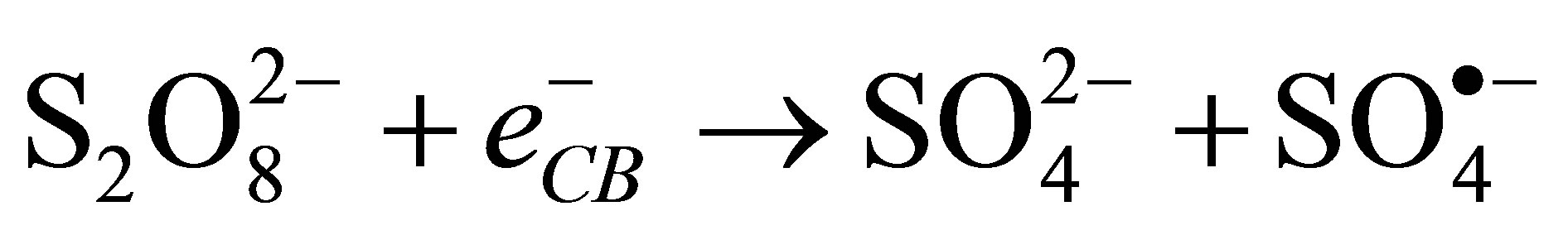 (7)
(7)
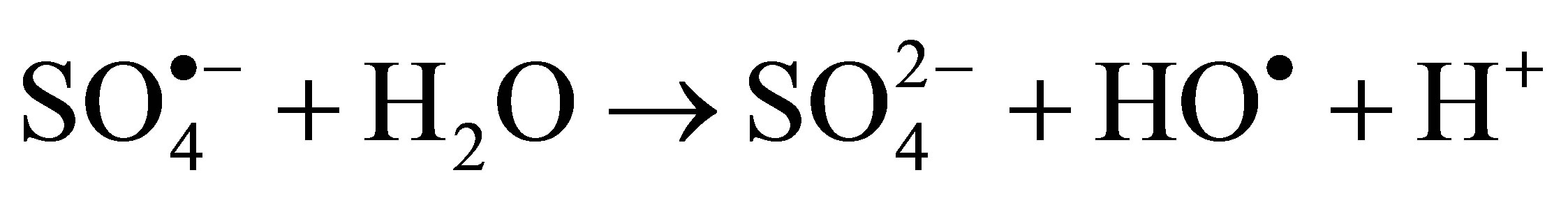 (8)
(8)
The additional production of hydroxyl radicals under illumination would lead to the best activity in these conditions.
4. Conclusion
The results of our study showed that TiO2 PC500 coated on non woven paper illuminated with UV light could be efficiently used to degrade the Reactive Black 5 (RB5). Photodegradation efficiency of dye was neglected when photolysis was carried out in the absence of TiO2 and small in the absence of the UV light. The kinetic curves of the photocatalytic degradation follow a pseudo-first kinetics order with respect to dye concentration. The results indicated that the degree of degradation of RB5 was obviously affected by volumetric flow rate, step of the reuse, pH, and addition of 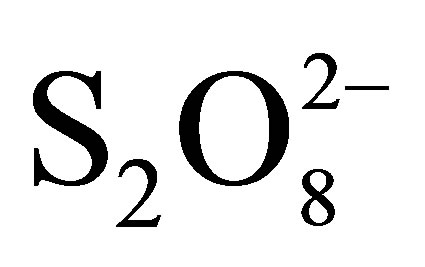 as electron scavenger. The high rate constant, kapp, was obtained with volumetric flow rate of 108 L/h. This flow permits the best adsorption-diffusion-reaction process in flow loop reactor used in this work. Successive experiments of RB5 removal at natural pH with the same paper show the gain in photocatalytic efficiency until height use follows by loss of efficiency probably due to saturation of the paper by byproducts. However, the use of TiO2 coated on Ahlstrom non-woven fibers could be a promising method as it can avoid the tedious final filtration of Titania in slurries and permit the photocatalyst recycling. The zero point charge determined was about 5.4. Hence, at more acidic pH values, the particle surface is positively charged, while at pH values above 5.4, it is negatively charged. In this study, it has been shown that the degradation rate for the model compound under investigation is strongly influenced by the reaction pH, where the efficiency of degradation rate for the decomposition of the dye was better at pH 3, whereas it was lower in alkaline media. The persulphate ions can efficiently oxidize the dye in the absence of TiO2 and ligh. Under illumination, the efficiency was maximized due to additional production of hydroxyl radicals further to peroxydisulphate reaction with photogenerated electron in conducting band of TiO2. These results suggest that photocatalysis may be envisaged as a method for the treatment of diluted colored wastewaters, in particular, in textile industries or loincloth factories in West-Africa countries.
as electron scavenger. The high rate constant, kapp, was obtained with volumetric flow rate of 108 L/h. This flow permits the best adsorption-diffusion-reaction process in flow loop reactor used in this work. Successive experiments of RB5 removal at natural pH with the same paper show the gain in photocatalytic efficiency until height use follows by loss of efficiency probably due to saturation of the paper by byproducts. However, the use of TiO2 coated on Ahlstrom non-woven fibers could be a promising method as it can avoid the tedious final filtration of Titania in slurries and permit the photocatalyst recycling. The zero point charge determined was about 5.4. Hence, at more acidic pH values, the particle surface is positively charged, while at pH values above 5.4, it is negatively charged. In this study, it has been shown that the degradation rate for the model compound under investigation is strongly influenced by the reaction pH, where the efficiency of degradation rate for the decomposition of the dye was better at pH 3, whereas it was lower in alkaline media. The persulphate ions can efficiently oxidize the dye in the absence of TiO2 and ligh. Under illumination, the efficiency was maximized due to additional production of hydroxyl radicals further to peroxydisulphate reaction with photogenerated electron in conducting band of TiO2. These results suggest that photocatalysis may be envisaged as a method for the treatment of diluted colored wastewaters, in particular, in textile industries or loincloth factories in West-Africa countries.
5. Acknowledgements
The authors thank the International Foundation for Science (IFS) and the World Academy of Science for Advancement in Developing Countries (TWAS) for their precious financial support for this research.
The authors also wish to thank Ahlstrom Research and Services for supplying the photocatalytic nonwoven paper via Dr. Ing. Bertrand Gombert of Universty of Poitiers.
REFERENCES
- Aguedach, Abdelkahhar, S. Brosillon, J. Morvan and El K. Lhadi, “Influence of Ionic Strength in the Adsorption and during Photocatalysis of Reactive Black 5 Azo Dye on TiO2 Coated on Non Woven Paper with SiO2 as a Binder,” Journal of Hazardous Materials, Vol. 150, No. 2, 2008, pp. 250-256.
- T. Kodom, E. Amouzou, G. Djaneye-Boundjou and L. M. Bawa, “Photocatalytic Discoloration of Methyl Orange and Indigo Carmine on TiO2 (P25) Deposited on Conducting Substrates. Effect of H2O2 and
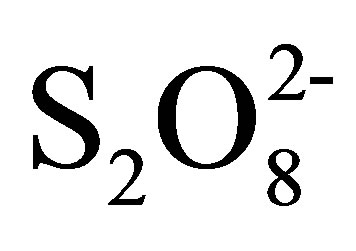 ,” International Journal of Chemical Technology, Vol. 4, No. 3, 2012, pp. 51-59.
,” International Journal of Chemical Technology, Vol. 4, No. 3, 2012, pp. 51-59. - Gbandi Djaneye-Boundjou, Etsri Amouzou, Tomkouani Kodom, Ibrahim Tchakala and Limam Moctar Bawa Kiliouféyi Anodi, “Photocatalytic Degradation of Orange II Using Mesoporous TiO2 (P25) and Fenton Reactive (Fe2+/H2O2),” International Journal of Environmental Science, Management and Engineering Research, Vol. 1, No. 2, 2012, pp. 91-96.
- T. Kodom, G. Djaneye-Boundjou, L. M. Bawa, B. Gombert and N. Alonso-Vante, “Etude de la Photodégradation du Reactive Black 5 et du Reactive Orange 16 en Solution Aqueuse en Utilisant des Couches Minces de TiO2,” International Journal of Biological and Chemical Sciences, No. 357, 2011.
- H. Gulyas, C. F. L. Jorge, M. Reich and R. Otterpohl, “Reclaiming Biologically Pretreated Greywater for Reuse by Photocatalytic Oxidation: Qualitative Study on the Removal of Trace Organics,” Journal of Water Resouce and Protection, Vol. 5, 2013, pp. 568-584. http://dx.doi.org/10.4236/jwarp.2013.56058
- N. Barka, A. Assabbane, A. Nounahb and Y. Aît Ichou, “Photocatalytic Degradation of Indigo Carmine in Aqueous Solution by TiO2-Coated Non-Woven Fibres,” Journal of Hazardous Materials, Vol. 152, 2008, pp. 1054- 1059. http://dx.doi.org/10.1016/j.jhazmat.2007.07.080
- M. Mullet, P. Fievet, A. Szymczyk, A. Foissy, J.-C. Reggiani and J. Pagetti, “A Simple and Accurate Determination of the Point of Zero Charge of Ceraminc Membranes,” Desalination, Vol. 121, 1999, pp. 41-48. http://dx.doi.org/10.1016/S0011-9164(99)00006-5
- Chatterjee, Debabrata, V. R. Patnam, A. Sikdar, P. Joshi, R. Misra and N. N. Rao, “Kinetics of the Decoloration of Reactive Dyes over Visible Light-Irradiated TiO2 Semiconductor Photocatalyst,” Journal of Hazardous Materials, Vol. 156, No. 1-3, 2008, pp. 435-441.
- Zielinska, Beata, J. Grzechulska, B. Grzmil and A. W. Morawski, “Photocatalytic Degradation of Reactive Black 5: A Comparison between TiO2-Tytanpol A11 and TiO2- Degussa P25 Photocatalysts,” Applied Catalysis B: Environmental, Vol. 35, No. 1, 2001, pp. L1-L7.
- Ling, C. Mei, A. R. Mohamed and S. Bhatia, “Performance of Photocatalytic Reactors Using Immobilized TiO2 Film for the Degradation of Phenol and Methylene Blue Dye Present in Water Stream,” Chemosphere, Vol. 57, No. 7, 2004, pp. 547-554.
- W. H. Leng, H. Liu, S. A. Cheng, J. Q. Zhang and C. N. Cao, “Kinetics of Photocatalytic Degradation of Aniline in Water over TiO2 Supported on Porous Nickel,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 131, No. 1-3, 2000, pp. 125-132.
- M. A. Behnajady and N. Modirshahla, “Kinetic Modeling on Photooxidative Degradation of C.I. Acid Orange 7 in a Tubular Continuous-Flow Photoreactor,” Chemosphere, Vol. 62, No. 9, 2006, pp. 1543-1548. http://dx.doi.org/10.1016/j.chemosphere.2005.05.027
- C. So, M. Cheng, J. Yu and P. Wong, “Degradation of Azo Dye Procion Red MX-5B by Photocatalytic Oxidation,” Chemosphere, Vol. 46, No. 6, 2002, pp. 905-912. http://dx.doi.org/10.1016/S0045-6535(01)00153-9
- M. A. Fox and M. T. Dulay, “Heterogeneous Photocatalysis—Chemical Reviews,” Chemical Reviews, Vol. 93, No. 1, 1993, pp. 341-357. http://dx.doi.org/10.1021/cr00017a016
- Y. J. Li, X. M. Zhou, C. Wei, L. Y. Li, M. X. Zen, S. D. Qin and S. G. Sun, “Photodecolorization of Rhodamine B on Tungsten-Doped TiO2/Activated Carbon under Visible-Light Irradiation,” Journal of Hazardous Materials, Vol. 227-228, 2012, pp. 25-33.
- F. Thevenet, O. Guaïtella, J. M. Herrmann, A. Rousseau and C. Guillard, “Photocatalytic Degradation of Acetylene over Various Titanium Dioxide-Based Photocatalysts,” Applied Catalysis B: Environmental, Vol. 61, No. 1-2, 2005, pp. 58-68.
- W. Tang and A. Huren, “UV/TiO2 Photocatalytic Oxidation of Commercial Dyes in Aqueous Solutions,” Chemosphere, Vol. 31, No. 9, 1995, pp. 4157-4170.
- Mozia, Sylwia, M. Tomaszewska and A. W. Morawski, “Photodegradation of Azo Dye Acid Red 18 in a Quartz Labyrinth Flow Reactor with Immobilized TiO2 Bed,” Dyes and Pigments, Vol. 75, No. 1, 2007, pp. 60-66.
- Augugliaro, Vincenzo, A. B. P. C. Baiocchi, V. L. E. Garcıa-Lopez, S. Malato, G. Marcı and M. P. L. Palmisano and E. Pramauro, “Azo-Dyes Photocatalytic Degradation in Aqueous Suspension of TiO2 under Solar Irradiation,” Chemosphere, Vol. 49, No. 10, 2002, pp. 1223- 1230. http://dx.doi.org/10.1016/S0045-6535(02)00489-7
- Konstantinou, K. Ioannis and T. A. Albanis, “TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations: A Review,” Applied Catalysis B: Environmental, Vol. 49, No. 1, 2004, pp. 1-14.

