Agricultural Sciences
Vol.4 No.12(2013), Article ID:41160,6 pages DOI:10.4236/as.2013.412096
Enhancement of defense responses by Clonostachys rosea against Botrytis cinerea in tomatoes
![]()
1School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin, China; *Corresponding Author: dalcantara2007@yahoo.fr
2Colleges of Life Sciences, Northeast Agricultural University, Harbin, China
Copyright © 2013 Liana Dalcantara Ongouya Mouekouba et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 22 October 2013; revised 23 November 2013; accepted 4 December 2013
Keywords: Botrytis cinerea; Clonostachys rosea; Defense Enzymes; Glutathione S-Transferases; Lipoxygenases; Peroxidases; Pathogenesis Related
ABSTRACT
Clonostachys rosea (C. rosea) is a biocontrol agent that is used to combat and prevent phytopathogenic fungi attacks because of its ability to involve many factors and diverse modes of action. The reactions of C. rosea on the control of gray mold disease in tomato leaves were investigated in this study. To investigate the reactions of C. rosea in inducing resistance to tomato plants, three treatments, including Botrytis cinerea treatment (treatment B), C. rosea treatment (treatment C), C. rosea and B. cinerea treatment (treatment C + B) and water (control), to be applied on tomato leaves were set up. Disease severity was subsequently evaluated and compared with the control. The treatment of tomato leaves with C. rosea (15 μg/ml) significantly reduced the disease index after inoculation and severity of gray mold caused by Botrytis cinerea. The results indicated that the C. rosea treatment stimulated the activity of the defense related enzymes: Peroxidases (POX), lipoxygenases (LOX) and glutathione S-transferases (GST), and the treatment C + B reduced the incidence and severity of the gray mold. Furthermore, C. rosea treatment increased the activity of pathogenesis related proteins PR1. Therefore, our results suggest that C. rosea could enhance the resistance of tomato plants to gray mold through the activation of defense genes and via the enhancement of defense-related enzymatic activities.
1. INTRODUCTION
Tomato (Solanum lycopersicum Mill) is one of the important vegetable fruits grown and consumed worldwide. In China, tomato is grown on 1455 thousand hectares of lands, with the production capacity of 33,645 thousand tonnes, and with the monthly productivity of 23, 1 mth−1 [1]. Tomato is an important and popular vegetable fruit because of its high nutritive value, and as a significant source of vitamin A and C. However, tomato fruits are prone to a number of bacterial disease infections among which is gray mold, a very destructive disease caused by fungus Botrytis cinerea (B. cinerea), which results in the complete loss of the crop.
The fungus B. cinerea is a necrotrophic pathogen that colonizes host tissues and induces plant cell death. It is capable of infecting more than 200 plant species [2]. The infection process of B. cinerea is usually described by the following stages: penetration of the host surface, killing of host tissue/primary lesion formation, lesion expansion/tissue maceration and sporulation [3]. Plant diseases can be controlled using fungicides applied inundatively as sprays, but the use of fungicides can cause high and acute residual toxicity, long degradation period, environmental pollution and possible side-effects on human health when it finds its way into the food. For this reason, new alternatives have been explored to reduce the use of synthetic fungicides. The use of biological measures to control diseases has become an inevitable pursuit in disease prevention and treatment, especially in the agricultural production process, through testing and use of microorganisms antagonistic to B. cinerea.
As a mycoparasite, Clonostachys rosea (C. rosea) has been tested successfully as a biological control agent against divergent fungal plant pathogens [4,5]. It is an antagonistic fungus plant pathogens widely present in the soil for the growth of plants, and can produce a series of antibacterial metabolites. Many isolates of C. rosea are highly efficient antagonists against several plant pathogenic funguses, as previous reports have shown in the controls of B. cinerea in strawberry, raspberry, and tomato [4].
Many defense enzymes are involved in the proper functioning of any living systems. It gets more affected during the plant—pathogen interaction. These include oxidative enzymes such as peroxidases (POX), lipoxygenases (LOX) and glutathione S-transferases (GST). Therefore, these enzymes were used as the criteria for studying the effect of C. rosea on tomato leaves infected with B. cinerea. We also analyzed the expression of the pathogenesis related proteins PR1 genes after treatment with C. rosea.
2. MATERIALS AND METHOD
2.1. Varieties Tested
Tomato homozygous line 704f was used for this study; seeds were propagated in the horticultural experimental station of the Northeast Agricultural University, Harbin, China.
2.2. Microbial Culture
B. cinerea was isolated from infected tomato plants growing in a greenhouse and maintained on potato dextrose agar (PDA) for 15 days at 28˚C. The spores were suspended in distilled water added to 0.01% Tween 80 and 0.01 mol/L KH2PO4 (pH = 5) 6.7 mmol/L and the concentration of spores was adjusted to 107 spores∙ml−1.
Strain antagonist C. rosea Gliocladium, isolated from soil taken from a lawn in the suburbs of Jilin (North East China), and gray mold of tomato, C. rosea, were cultured on potato dextrose agar (PDA) for 21 days at 21˚C.
2.3. Fungal Treatment and Infection
The fungal treatment was conducted by spraying (1.0 × 105 spores/ml). Tomato leaves were washed with sterile distilled water, dried on filter paper before been treated with the sterilized spores. The plants were assigned to three treatment groups and one control group: treatment B, tomato leaves treated only with B. cinerea, treatment C, tomato leaves treated only with C. rosea, and treatment (C + B), tomato leaves treated first with C. rosea, and 4 hours after the same plants were treated for a second time with conidia and mycelia of B. cinerea, and the control plants were treated with distilled water only. Every experiment was subjected to three replications.
2.4. Disease Assessment
After spraying, tomato plants were immediately transferred to an air-tight plastic bag to maintain a high relative humidity, and were incubated at 25˚C. The determination of the activity related to the defence was made by sampling tomato leaves after treatment administration for 32 hours at an interval of 4 hours. Treated leaf samples were taken to study the enzymatic activity.
The severity of the symptoms of gray mold disease was evaluated by calculation of the index on a scale of 0 to 5 on the basis of the Murray method with modifications [6], where 0 indicates no necrosis, here the leaves were completely healthy; 1 = less than 5% of the leaves were with Botrytis symptoms; 2 = less than 15% of the leaves were with Botrytis symptoms; 3 = less than 25% of the leaves were with Botrytis symptoms; 4 = less than 50% of leaves were with Botrytis symptoms; 5 = more than 50% of leaves were covered with Botrytis symptoms. The formula used to calculate the index of the disease is as follows:
 . (1)
. (1)
In which i = infection class, j = the number of leaves scored for that infection class, n = the total number of plants in the replicate, and k = the highest infection class.
2.5. Determination of the Activities Related to Defense
After spraying, tomato plants were immediately transferred to an air-tight plastic bag to maintain a high relative humidity and were incubated at 25˚C. The determination of the activity related to the defense was made by sampling of tomato leaves with after treatment administration for 32 hr at an interval of 4 hr. Treated leaf samples were taken for the enzymatic activity. The effect of C. rosea on tomato leaves as control of gray mold was examined by enzymatic extraction of enzymes defense. Every experiment was repeated three times.
2.6. Enzyme Activity Assay POX, LOX and GST
For enzyme assays, fresh leaves tissues were collected at different times after treatment application.
For POX assay 1 g of tomato leaves was homogenized in 1 ml of 10 mM phosphate buffer (pH. 6.0) in a prechilled mortar and pestle on ice. The homogenate was centrifuged at 12,000 rpm for 20 min at 4˚C and the supernatant served as enzyme source. All the experiments were carried out at 4˚C. POX enzyme assay was carried out as described by [7] with minor modifications. The reaction mixture (3 ml) consisted of 0.25% (v/v) guaiacol in 10 mM potassium phosphate buffer (pH 6.0) containing 10 mM hydrogen peroxide. Addition of 100 ml of crude enzyme extract initiated the reaction, which was measured spectrophotometrically at 470 nm for 1 min. One unit of POX enzyme activity is defined as change in absorbance min−1∙mg−1 protein. For LOX assay 1 g of tomato leaves was homogenized in 1 ml of 200 mM sodium phosphate buffer (pH. 6.5) in a pre-chilled mortar and pestle on ice. The homogenate was centrifuged at 12,000 rpm for 20 min at 4˚C and the supernatant served as enzyme source. All the experiments were carried out at 4˚C. LOX activity was estimated by following the procedure of [8]. The activity was determined spectrophotometrically by monitoring the appearance of conjugated diene hydroperoxide, absorbance was measured at 234 nm. The reaction mixture contained 2.7 ml of 0.2 M sodium phosphate buffer (pH 6.5), 0.3 ml of 10 mM linoleic acid in Tween 20 and 50 ml of the enzyme extract. The enzyme activity was expressed as a change in the absorbance min−1∙mg−1 protein. For GST assay 1 g of tomato leaves was grounded with 10 ml of the buffer solution [0.1 mol/L Tris-HCl (pH 7.8), 0.5 mmol/L EDTA, 0.5 mmol/L and 1 mercaptoethanol % polyvinyl pyrrolidone (PVP)]. The extracts were centrifuged at 2000 rpm for 10 min at 4˚C, and then take the supernatant centrifuged at 12,000 rpm for 10 min at 4˚C, and the supernatant was collected as enzyme source. For the GST assay, 1-chloro-2,4-dinitrobenzene (CDNB) was used as the substrate, 30 µl of enzyme extracts were incubated with 0.9 mL of 3.3 mmol/L GSH 1.97 ml 100 mmol/L potassium phosphate buffer (pH 6.5) and 100 μl of 30 mmol/L CDNB (dissolved in ethanol) [9]. The absorbance was recorded between 90 s and 120 s at 340 nm. Reaction mixture without enzyme was used as a control. The GST activity is expressed in Umg−1 protein.
2.7. cDNA Preparation
The samples of the leaves were collected from different treatments. Total RNA was extracted using TRIzol Reagent (Invitrogen, USA) according to the manufacturer’s instructions. Isolated RNA was dissolved in 20 μL of RNase free H2O2, quantified by spectrophotometry and stored at −80˚C. 8 μL of total RNA extracted from tomato leaves were reverse transcribed with Easy Script First-Strand cDNA Synthesis SuperMix according to the manufacturer’s protocol and stored at −80˚C.
2.8. Real-Time PCR
Bio-Rad Super SYBR Green mix was used for the reaction. Each PCR reaction (20 μL) contained Bio-Rad Super SYBR 10 μL Green mix, 2 μL cDNA (25 ng), 0.6 μL each primer (10 μM) and 6.8 μL dd H2O. The PCR reactions were dispensed into ABI optical reaction tubes (Applied Biosystems, Foster City, CA, USA). The reaction tubes were centrifuged at 2500 rpm for 10 s to settle the reaction mixtures to the bottom of the wells. The PCR was carried out with an icycler real-time quantity PCR system (BIO-RAD). The RT-PCR was performed as follows: 94˚C for 3 min, 1 cycle, 95˚C for 45 s, 52˚C for 45 s, 72˚C for 60 s, 35 cycles and 72˚C for 10 min. After each run, a dissociation curve was designed to confirm specificity of the product and avoid production of primers-dimers. All statistical analyses were performed with 2-ΔΔCt methods [10]. The sequences of β-actin amplification reverse primer and forward primer were: CCACCTTAATCTTCATGCTGCT and ACATTGTGCTCAGTGGTGGTACT. The sequences of PR1 amplification reverse primer and forward primer were: AATGAACCACCATCCGTTGTTG and TGTCCGAGAGGCCAAGCTATAAC. Primers used for β-actin are as published by [11]. Primers used for pathogenesis related proteins PR1 were found on the NCBI site.
2.9. Statistical Analysis
Statistical analysis was performed with a statistical program from social sciences (SPSS) 10.0. Data was analyzed by using one-way ANOVA. Duncan multiple range tests were used to separate between data. Differences at P < 0.05 were considered to be significant.
3. RESULTS
3.1. Effects of C. rosea on Tomato Gray Mold
In this experiment, the treatment with C. rosea (15 μg/ml) for 4 h before inoculation with B. cinerea had significantly protective and curative effect against gray mold in the tomato leaves. We studied the effect of C. rosea on the control of gray mold inoculation with B. cinerea. As shown in Table 1, the combination of 15 μg/ml C. rosea significantly inhibited the gray mold inoculation with B. cinerea. C. rosea treatment can enhanced the resistance of tomato plants to B. cinerea infection.
The data are the average of three replicates. The different letters in the same column indicate a significant difference at 5% level.
3.2. Effect of C. rosea Treatment on Activities of POX, LOX and GST
The control leaves did not show a large amount of POX. Four hours after treatment application, increase in activity was observed for the three experimental setups, but this increase was followed by a gradual decrease. The POX activity accumulation was detected at 20 h after treatment application in the three setups but the leaves treated with C. rosea and then inoculated with pathogen attained a maximum peak (Figure 1).
The control leaves did not show a large amount of LOX, but after 20 h a significant increase in LOX was

Table 1. Effects of C. rosea treatment on tomato leaves.
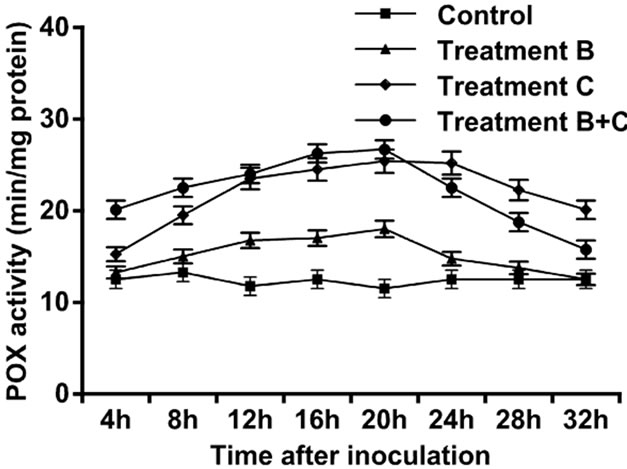
Figure 1. Activity of peroxidase enzyme in leaves of tomato treated with B. cinerea, C. rosea, C. rosea + B. cinerea, and distilled water.
observed, and peak was attained at 28 h. Increase in activity was observed four hours after treatments were applied in the three setups. Tomato leaves treated only with B. cinerea showed an increase in LOX activity, followed by an exponential growth that attained maximum level at 32 h. Tomato leaves treated only with C. rosea showed an increase in the LOX activity followed by an exponential growth that reached maximum level at 32 h. Tomato leaves treated with C. rosea and inoculated with B. cinerea showed an increase in the LOX activity with maximum value attained at 20 h, afterwards followed by a decline. Thus, of the three treatments, treatment C had the highest contribution to LOX activity (Figure 2). The control leaves did not show a large amount of GST. Tomato leaves treated only with B.cinerea showed an increase in GST activity within the first four hours after inoculation but followed by gradual decline to 20 h. The GST activity in leaves inoculated with C. rosea only, and in the leaves treated with C. rosea and inoculated with B. cinerea showed a gradual increase and each reached maximum level at 32 h (Figure 3).
3.3. C. rosea Promoted the Expression of PR1 Genes
Leaf treatment with C. rosea (15 µg/ml) increased the expression of PR1 gene. The expression of the PR1 gene reached its maximum level at 32 h in C. rosea treated tomato plants, about 4-fold higher when compared with the control plants at the different time points (Figure 4).
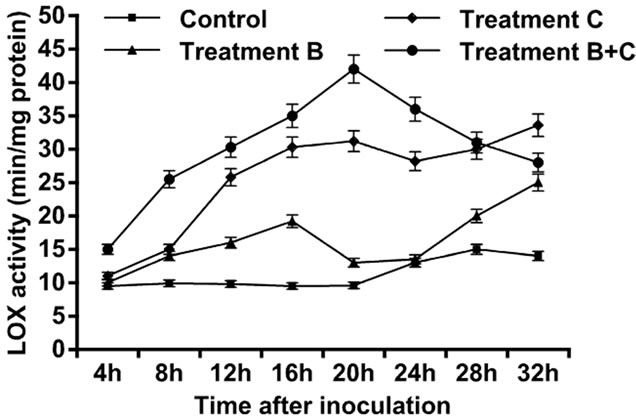
Figure 2. Activity of lipoxygenases enzyme in leaves of tomato with B. cinerea, C. rosea, C. rosea + B. cinerea and distilled water.

Figure 3. Activity of glutathione Stransferases enzyme in leaves of tomato treated with B. cinerea, C. rosea, C. rosea + B. cinerea, and distilled water.
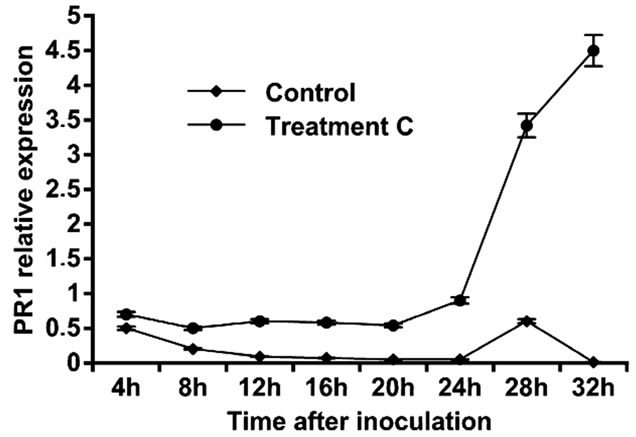
Figure 4. Expression of pathogenesis related proteins PR1 in leaves of tomato treated with C. rosea.
4. DISCUSSION
In the present study, we examined the ability of C. rosea, a biocontrol agent, to induce tomato defense reactions and resistance against gray mold when applied to tomato leaves. Results indicated that C. rosea can protect tomato crops from B. cinerea invasion. The protective effect was comparatively better if inoculation of B. cinerea occurred at 4 h after treatment with C. rosea, compared with inoculation at 20 h and 32 h (Table 1). Our results showed that treatment of tomato leaves with C. rosea significantly increased the POX, LOX and GST enzyme activities and effectively inhibited gray mold in the tomato (Figures 1-3). These results are consistent with previous findings that C. rosea improves resistance to wheat plants infected by Fusarium culmorum [12].
Peroxidases (E.C.I.ll.I.7) are believed to be one of the most important factors of the plant’s biochemical defense against pathogenic microorganisms, and is actively involved in the self-regulation of plant metabolism after infection [13]. LOX are defense-related enzymes, with broad spectrum action activity. They play key roles in plant-pathogen interactions. In this study we found that the infected leaves triggered an increase in POX and LOX activity. These results correspond with those reported by Vanitha and Umesha [14] which showed that inoculation of Ralstonia solanacearum on some different tomato cultivars presented resistance against R. solanacearum causing increase in peroxidases (POX) and lipoxygenases (LOX) activities. Glutathione S-transferases (GSTs) play a role both in normal cellular metabolism as well as in the detoxification of a wide variety of xenobiotic compounds. GST has been implicated in numerous stress responses, including those arising from pathogen attack and oxidative stress [15]. In this study the inoculation of tomato leaves by the pathogen alone caused an increase in the value of GST activity, which is the same for tomato leaves treated with the agent antagonist alone producing a large amount of the GST activity, thereby strengthening resistance to the pathogenic invasion, but the value of GST activity in leaves inoculated with the pathogen and treated with the antagonistic agent was larger compared with the other two treatments. Pathogenesis-related protein (PRs) can be induced by different stress stimuli and plays an important role in plant defense against pathogenic constraints, and in general adaptation to stressful environments [16]. In this study the tomato leaves treated with C. rosea caused a rapid induction of the pathogenesis related proteins PR1 gene. These substances are associated with the process of local disease defense. Comparing the expression level of defense enzymes activity in this study among the three different types of treatments, it was revealed that the application of treatment C + B performed best. In conclusion the application of C. rosea can increase the activity of pathogenesis related proteins PR1 and can be able to evoke enzyme defense activities and PR1 in tomato plants infected by B. cinerea, but also it can be successful in mounting defense response on tomato plants from B. cinerea invasion. Therefore, it could be a potential candidate for plant vaccine preparation to combat gray mold in tomato plants.
5. ACKNOWLEDGEMENTS
This work was supported by Trans-Century Training Program Foundation for the Talents by Heilongjiang Provincial Education Department(1251-NCET-004)to A. X. Wang and Returned Oversea Scholar Foundation by Heilongjiang Provincial Education Department to X. L. Chen.
REFERENCES
- Food and Agriculture Organization of United Nations (2007) Agriculture data (FAO). http://fr.wikipedia.org/wiki/Tomate
- Jarvis, W.R. (1997) Botrytinia and Botrytis species: Taxonomy, physiology and pathogenicity. Canada Department of Agriculture, Ottawa.
- Van Kan, J.A. (2006) Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends in Plant Science, 11, 247-253. http://dx.doi.org/10.1016/j.tplants.2006.03.005
- Sutton, J.C., Li, D.W., Gang, P., Hai, Y. and Zhang, P. (1997) Gliocladium roseum a versatile adversary of a Botrytis cinerea in crops. Plant Disease, 81, 316-328. http://dx.doi.org/10.1094/PDIS.1997.81.4.316
- Xue, A.G. (2003) Biological control of pathogens causing root rot complex in weld pea using Clonostachys rosea strain ACM941. Phytopathology, 93, 329-335. http://dx.doi.org/10.1094/PHYTO.2003.93.3.329
- Murray, S.L., Thomson, C., Chini, A., Read, N.D. and Loake, G.J. (2002) Characterization of a novel, defenserelated Arabidopsis mutant, cir1, isolated by luciferase imagin. Molecular Plant―Microbe Interactions, 15, 557- 566. http://dx.doi.org/10.1094/MPMI.2002.15.6.557
- Hammerschmidt, R., Nuckles, E. and Kuc, J. (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiologia Plantarum, 20, 73-80.
- Borthakur, A.B., Bhat, B. and Ramasossm C.S. (1987) The positional specifications of the oxygenation of linoleic acid catalyzed two forms of lipoxygenase isolated from Bengal gram (Cicer arietinum). Journal of Biosciences, 11, 257-263. http://dx.doi.org/10.1007/BF02704676
- Habig, W.H., Pabst, M.J. and Jakoby, W.B. (1974) Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry, 249, 130-139.
- Yuan, J.S., Reed, A., Chen F. and Stewart Jr., C.N. (2006) Statistical analysis of real-time PCR data. BMC Bioinformatics, 7, 1186-1471. http://dx.doi.org/10.1186/1471-2105-7-85
- Wang, F.D., Feng, G.H. and Chen, K.S. (2009) Defense responses of harvested tomato fruit to burdock fructooligosacc haride, a novel potential elicitor. Postharvest Biology and Technology, 52, 110-116. http://dx.doi.org/10.1016/j.postharvbio.2008.09.002
- Robertia, R., Veronesia, A.R., Cesaria, A., Casconeb, A., Di Berardinob, I., Bertinib, L. and Caruso, C. (2008) Induction of PR proteins and resistance by the biocontrol agent Clonostachys rosea in wheat plants infected with Fusarium culmorum. Plant Science, 175, 339-347. http://dx.doi.org/10.1016/j.plantsci.2008.05.003
- Saravana, T., Bhaskaran, R. and Muthuswamy, M. (2004) Pseudomonasfluorescens induced enzymological changes in banana roots (Cv. Rasthali) against Fusarium wilt disease. Journal of Plant Pathology, 3, 72-80. http://dx.doi.org/10.3923/ppj.2004.72.80
- Vanitha, S.C. and Umesha, S. (2008) Variations in defense related enzyme activities in tomato during the infection with bacterial wilt pathogen. Journal of plant interactions, 3, 245-253. http://dx.doi.org/10.1080/17429140802032863
- Marrs, K.A. (1996) The functions and regulation of glutathione s-transferases in plants. Plant Physiology, 47, 127-158.
- Edreva, A. (2005) Pathogenesis related proteins: Research progress in the last 15 years. General and Applied Plant Physiology, 31, 105-124.

