Food and Nutrition Sciences
Vol.4 No.1(2013), Article ID:27351,8 pages DOI:10.4236/fns.2013.41014
Assessment of Microbial Changes and Nutritional Qualities of Extruded White Yam (Dioscorea rotundata) and Bambara Groundnut (Vigna subterranean) Blends
![]()
1Food and Analytical Services Department, Federal Institute of Industrial Research, Oshodi, Nigeria; 2Department of Food Science and Technology, Federal University of Agriculture, Abeokuta, Nigeria; 3Department of Biochemistry, University of Lagos, Lagos, Nigeria.
Email: *toyinoluwole2@yahoo.com
Received September 16th, 2012; revised November 12th, 2012; accepted November 19th, 2012
Keywords: Co-Extrude; Yam Grits; Bambara Flour; Total Plate Count; Nutritional Evaluation; Weight Gain; Growth
ABSTRACT
Two flavoured extruded products were developed by co-extruding yam grits (750 µm) obtained from white yam (Dioscorea rotundata) and bambara groundnut flour (250 µm) in 160:40 respectively obtained from white bambara groundnut with added flavouring agents of salt (1% - 3%) and sugar (4% - 6%) in the feed blends at screw speed of 70 rpm, 17.5%, feed moisture and at the barrel temperature of 145˚C. The extruded products were packaged in low density polyethylene bag (0.02 µm gauge size) and stored at room temperature (28˚C ± 2˚C) and at refrigeration temperature (9˚C ± 2˚C) for a period of twenty weeks. The microbiological changes in the extruded products as determined by the total plate under both storage conditions showed that maximum total plate counts were 0.5 × 104 and 5.4 × 104 cfu/g at 9˚C ± 2˚C and 28˚C ± 2˚C respectively. Nutritional evaluation studies of extrudates were comparable (p ≥ 0.05) with standard casein diet with minimum crude protein content of 13.51% providing 1707.2 KJ energy per 100g of diet and supported weight gain and growth of laboratory animals.
1. Introduction
Extrusion cooking has been described as an important technique for modification and manufacture of a wide variety of traditional and novel foods and food blends [1-4]. Expanded snack foods ready to eat cereals and dry pet foods are manufactured from cereals and starches by high temperature short time extrusion cooking [5,6].
Yam (Discorea rotundata) is a good source of carbohydrates, vitamins and minerals [7], as well as some essential amino acids. It is traditionally eaten in Nigeria as fried yam slices, cooked and boiled which can be eaten like that or pounded into stiff dough “iyan”. It can also be eaten in roasted form and as well processed into flour used to prepare brown stiff dough called “Amala” [8].
Bambara groundnut is of immense nutritional value to Africans [9] as detailed composition study of the beans showed that they contain up to 24% protein with a good balance of the essential amino acids and relatively high proportions of lysine (6% - 8%) and methionine (1% - 3%). The other major component of the beans is carbohydrate which is mainly starch as high as 30%. This crop is often processed into flour, salted with oil added to it, mixed together and steamed in banana leaves and eaten as a snack food popularly known as “Okpa” in the Eastern parts of Nigeria. However, like most other leguminous crops the presence of bioactive compounds with toxic and/or anti-nutritional properties that can alter the body metabolism of consumers and exert a negative impact on the nutritional quality of the seed protein.
Generally, all these forms of traditional utilization and processing comes with one form of problem or the other ranging from non-uniformity of product quality, drudgery of processing techniques, low product shelf life [10], these couple with the need to develop a new and novel product from yam and Bambara groundnut form the basis for this research work.
Hence, in this work, yam being a rich source of carbohydrate was fortified with Bambara groundnut. The fortified yam products were flavoured with salt and sugar, packaged and stored for storage studies. Also the flavoured fortified extruded yam products were subjected to nutritional evaluation studies using experimental animals in order to determine the nutritive value of the extruded products when they are eventually consumed.
The results obtained at this stage, however, will reveal the potential utilization of the flavoured extruded yambambara extrudates as potential food products for consumers.
2. Materials and Methods
2.1. Materials Used
White yam (Discorea rotundata) was obtained from National Root Crop Research Institute, Umudike, Abia State, Nigeria; while white Bambara groundnut was obtained from a local market in Lagos, Nigeria. Common salt and granulated sugar were also obtained from a local market in Lagos, Nigeria.
A laboratory sized Single screw extruder (Komet Extruder, 1993 Model Made in Germany) was used in this study requiring a minimum input of 0.2 kg as feed raw materials for extrusion cooking.
2.2. Extrusion Cooking
Yam grits (<750 µm) obtained by processing white yam through various operations of washing peeling, pregelatinizing, drying and milling into grits and Bambara groundnut flour obtained by processing White Bambara groundnuts through processing operations of manual destining, cleaning, milling into flour (<250 µm) were co-extruded in a single screw extruder at ratio 4:1 yam grits and bambara flour respectively with added flavouring agents of salt (<1%) and sugar (2% - 3%) respectively. The extrusion cooking process occurred at the screw speed of 70 rpm, barrel temperature of 145˚C and 17.5% feed moisture. The resulting extrudates were allowed to cool after exiting the extruder die, packaged (0.002 µm gauge size) in high density polyethylene bag and stored at room temperature (28˚C ± 2˚C) and at refrigeration temperature (9˚C ± 2˚C) for storage and nutritional evaluation studies.
2.3. Storage Studies and Microbiological Analysis of Extrudates
The total plate count of the flavoured extrudates was determined using established procedure of Harrigan and Mclance [11].
1 gm of each sample already milled into flour was weighed into 9 ml of sterile normal saline solution. The mixture was thoroughly homogenized and diluted serially from 10−1 to 10−5. About 0.1 ml of 10−3 diluted sample was spread evenly with sterile horse stick on each media used, namely Malt extract (MA), Maconkey agar (MAC) and Nutrient agar (NA) and each incubated at 37˚C for a period of 24 hr. The colonies that grew on each plate were then counted manually. The extrudates were stored at room temperature (28˚C ± 2˚C) and in a refrigerator (9˚C ± 2˚C) and the microbiological test was done on each sample monthly for a period of 4 - 5 months.
2.4. Chemical Composition of Raw Materials and Flavoured Extrudates
The chemical composition of the extrudates determined includes moisture content, crude protein, crude fibre, ash, and carbohydrate by difference. These were carried out using established procedures of AOAC [12]. The energy content of the flavoured extrudates (DF1 and DF2) as well as the protein free diet was determined by calculation using Egan et al. [13] and James et al. [14] method respectively.
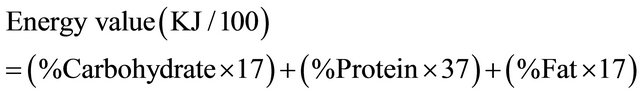
Total mineral content in the extrudates and faeces were determined by ashing in a furnace at 550˚C and individual minerals (calcium, iron and phosphorous) were determined in the ash solution on 10% HNO3 as follows: Phosphorous by colorimetry using the ammonium phosphate vanadium molybdate method. Calcium and Iron by the ortho-pheno-throline method of Pearson [15].
2.5. Determination of Trypsin Inhibitor Content of Extrudates
This was determined in the extrudates using established procedure of Kakade et al., [16]. One gram of sample was weighed and 50 ml of 0.01 N NaOH was added to each sample. The pH was adjusted to 8.8 using 1 NHCl to reduce pH to the required level. The sample was allowed to stay for 3hours and stirred continuously to maintain the sample in suspension. One ml extract, 2 ml of the diluted sample was taken and poured into three (3) test tubes while no trypsin solution was added to the third test tube. Also, 2 ml of distilled water was withdrawn in three (3) test tubes and 2 ml of trypsin solution was added to the test tube and was not added to the test tube, the sample in the test tube were returned to the water bath and warmed for 10 minutes. After 10 min, 5 ml of BAPA solution (Benzoyl-DL-arginine-p-nitroanalide hydrochloride) was added to all the test tubes, vortexed and warmed again for 10 min. After 10 min, 1 ml of glacial acetic acid was added to all the test tube except the third test tube that did not contain trypsin initially. The samples were filtered using Whatman No. 2 filter paper and the absorbance read at 420 nm using a spectrophotometer.
Calculation:
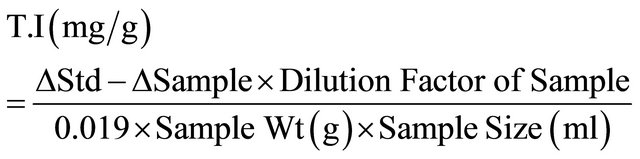
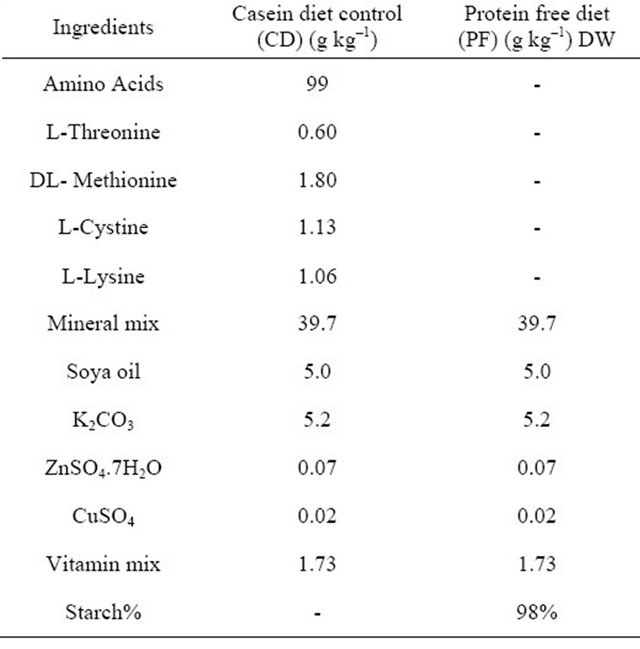
2.6. Formulation of Rat Diets
The rat diets casein control and protein free diet were formulated according to method of Lape [17] and Fasuyi et al. [18]. The various ingredients used include some amino acids such as L-Threorine, DL-Methionine, LCystine and Lysine, mineral mix, soya oil, potassium carbonate, magnesium citrate, zinc sulphate, copper sulphate, vitamin mix and starch. The detail of the formulation is as shown in Table 1.
Notations of experimental samples
DF1—Extrudate made from feed containing 160:40 yam grits and bambara flour, espectively with 4 g sugar and 3 g salt.
DF2—Extrudates made from feed containing 160:40 yam grits and bambara flour, respectively with 4 g sugar and 1 g salt.
CD—Casein diet.
PF—Protein free diet.
T9—Extrudates stored under refrigeration condition.
T28—Extrudate stored under ambient condition.
2.7. Nutritional Evaluation of Products
Determination of Protein Quality
The biological value (BV), protein digestibility (PD), net protein utilization (NPU), protein efficiency ratio (PER)
Table 1. Formulation of rat diets.
*Modified method of Lape (1984).
of the flavoured yam-bambara extrudates DF1 and DF2 as well as the casein diet (CD) and protein free diet (PF) were determined using established procedure [17,18]. The method employed in determining the mineral digestibility of the extrudates and other diets was as described by Obizoba, [19]. Each extrudate/diet was prepared in bulk and stored at 4˚C in the dark. Some weighed quantities were drawn daily and mixed with distilled water into a paste containing 40% - 50% dry matter before feeding to rats.
The nutritional values of the flavoured extruded products (diets) were evaluated using various parameters as stated below:
For proteins, the following parameters were computed below:
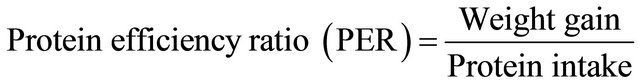
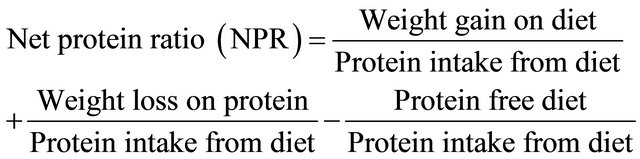
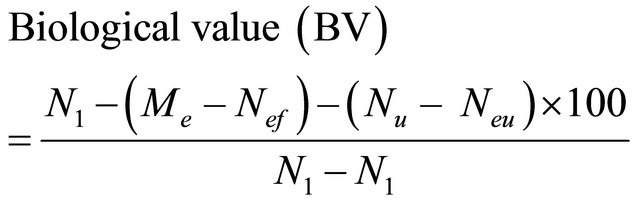
where:
N1 = Nitrogen intake; Nf = Faecal Nitrogen Nu = Uninary nitrogen; Neu = Endogenous uninary nitrogen Nef = Endogenous faecal nitrogen from rats fed with diet
2.8. Animal and Experimental Design
Weanling albino rats of the Wister strain were used. Five rats (3 males and 2 females) were assigned to the casein-based diet and each of the yam-bambara extrudates/diet under investigation. Rats were divided into groups with similar average weights (70 g ± 1 g) and growth rates and were housed in individual polyethylene metabolic cages in a room at about 28˚C and 60% relative humidity and with a 12 hour light/dark cycle.
Rats were fed with the diets (i.e. flavoured extrudates DF1, DF2 casein diet and protein free diet) for 7 days (adaptation period) and then for further 10 days (experimental period) during which feed intake were measured. They also received distilled water ad libitum and were weighed every three (3) days of the experimental period to determine average daily weight gain or loss.
During the last five (5) days of the experimental period, daily collections of the total urine and faeces of each rat were made separately, posted together and frozen until analysis. A 0.5 ml sample of 4.8 M HCI was added to recipients before urine collection to prevent loss in the form of ammonia. The care for all the animals was in accordance with the National Law on Animal care and use [20,21].
2.9. Statistical Analysis
Means, standard deviation of the data were calculated using Microsoft Excel package while the test and separation of means among the samples were carried out using Analysis of variance (ANOVA) and Duncan’s New Multiple Range Tests (DNMRT) with SPSS package version 15.
3. Results and Discussion
3.1. Microbiological Changes in Stored Extrudates
Figure 1 shows the microbiological changes as measured by the total plate count in the flavoured extrudates stored at different conditions of temperature. The microbiological load as measured by the total plate count per gram of sample was generally low in all the samples stored under refrigeration conditions. This shows that temperature has a significant effect on the growth of micro-organisms. Generally as the storage time increased, the measured total plate count at room temperature steadily increased while for samples stored in the refrigerator, there was a much lower rate of increase in the total plate count. The total plate count of the extrudates on week 0 showed that the extrudates had an initial total plate count of 0.2 × 104
cfu/g. On storage in high density polyethylene bag (gauge size 0.03 µm), there was a steady increase in the total plate count among the extrudates both at room temperature and at refrigeration temperatures. As the storage time further increases to 8 weeks, there was a continuous increase in the total plate count particularly at the room temperature. A similar trend was observed when the extrudates were stored at 12, 16 and at 20 weeks.
Based on the above result (Figure 1), extrudate DF3 was discarded because of the relatively higher microbial load when stored under both conditions investigated in this study as compared with the other extrudates (i.e. DF1 and DF2).
The maximum permissible level of total aerobic colony of ready-to-eat foods as given by Fylde Borough Council extracted from manual of PHLSG [22] was 104 to less than 106 cfu/g of ready-to-eat food products. This therefore shows that the extrudates were stable under the different storage conditions investigated in this study. The microbial loads of the extrudates stored under refrigeration conditions were generally low which showed that temperature had a significant effect on the growth of micro-organisms [23].
Also micro-organisms have been reported to be inhibited by the inclusion of salt, sugar and spices [24] in food.
The microbial load of any food material is however a useful index of quality in the extrudate as well as revealing the potential safety status of the extruded food products from human consumption point of view and storage of the products. Total counts have been reported to be
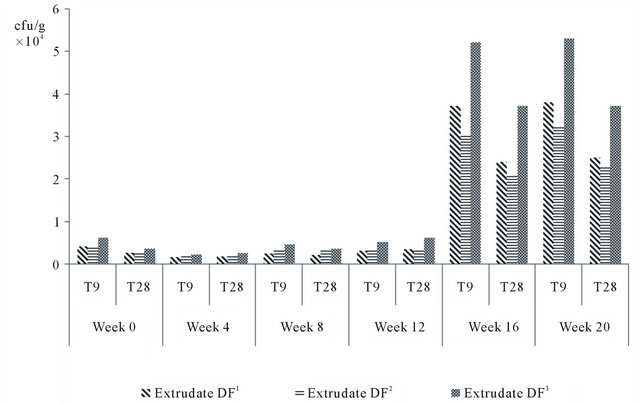
Figure 1. Total plate count of extrudates with respect to storage period and conditions.
useful monitor in processing of food products and may therefore reflect poor handling or storage at retail level [25]. Bacillus cereus and coliforms have however been identified as the major organisms causing spoilage in extruded food products and the total plate count must also be very low in order to rule out the possibility of formation of preformed toxins in the extruded food products [22].
3.2. Chemical Composition of Raw Materials and Flavoured Extrudates
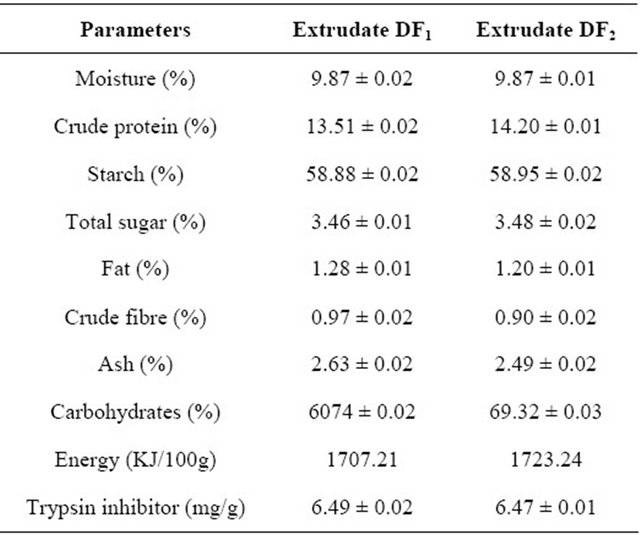
The chemical composition of the flavoured extrudates ((DF1 and DF2) as shown in Table 2 revealed that they contained 13.31% and 14.20% crude protein respectively. The starch content were 60.83%, 58.95% and 98% in DF1, DF2 and PF diets respectively while the fat content was 1% and 1.28% in DF1 and DF2 respectively. The crude fibre was 2.04% in the flavoured extrudates, DF1 and 1.09% in the flavoured extrudate DF2, 2.63% and 2.90% ash in DF1 and DF2 respectively, while the energy value was 1707.21 KJ/100g in the flavoured extrudate DF1, 1724.24 KJ/100g in the flavoured extrudate, DF2 and1671.4 KJ/100g in the protein free diet. The high values of crude protein in the flavoured extrudates were expected as Bambara groundnut have been reported to be a very good source of protein which could be as high as 22% - 25% in the raw beans [26].
Also the starch content values in extrudates DF1 and DF2 were also expected as both raw yam and bambara groundnut contain appreciable level of starch [9,26].
Both flavoured extrudates contained appreciable levels of calcium, iron and phosphorous as shown in Figure 2 below. Raw yam and bambara groundnut have been reported to contain appreciable levels of calcium, Iron and phosphorous [7,24]. Both crops contain appreciable levels of calcium, Iron and Phosphorous.
3.3. Protein Quality and Animal Feeding
The potential value of a food for supplying a particular nutrient can be determined by chemical analysis but the actual value of the food to the animal can be arrived at only after making allowances for the inevitable losses that occur during digestion, absorption and metabolism [18,27].
The digestibility of a food is defined as that portion of the food which is not excreted in the faeces and which is therefore assumed to be absorbed by the animal.
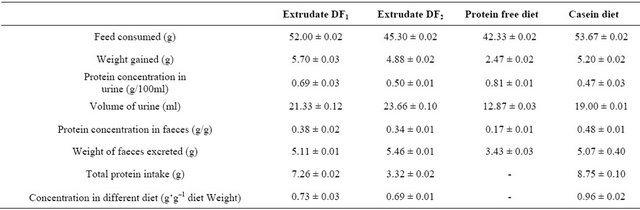
The rat feeding experiment conducted in this study showed that there was significant difference among the experimental diets at (p ≤ 0.05) in terms of the protein concentration in the different diets fed to the experimental animals with the casein diet (CD) containing the highest value of protein followed by the extrudate/diet DF1 as shown in Table 3.
Table 2. Chemical composition of flavoured extrudates.
Values are mean of three determinations ± SD values.
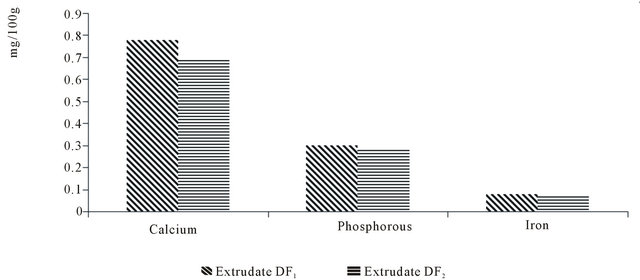
Figure 2. Level of some minerals in the experimental diets.
Also there were significant differences among the yam-bambara extrudates in terms of the protein concentration in faeces of rate fed with different flavoured extrudates (p ≤ 0.05) with the casein diet recording the highest value protein. This was followed by the protein concentration in the faeces of rats fed with extrudate DF2 (0.38 g·g–1) followed by extrudate DF1 (0.34 g·g–1) and lastly by the protein free diet, PF which had a protein concentration in the faeces of the rats of 0.17 g·g–1.
The study also revealed that in terms of the total protein intake by each experimental rat, there were significant differences (p ≤ 0.05) between the protein intake in group DF1, DF2 and casein diet. However, there were significant differences (p ≤ 0.05) among the total protein intake by each rat fed with extrudate/diet DF1, DF2 CD and PF, which was supposed to be an almost protein free diet. However, the casein diet remained the best diet in terms of the total protein intake by the rats as it had the highest mean protein intake value of DF1 with a mean protein intake value of 7.26 g in the fed rats. A mean protein intake of 3.99 g was obtained for extrudates/diet DF2 while the least protein intake of 0.11 was obtained free diet. A maximum protein intake value of 8.97 g was recorded in the casein diet used in this study.
Generally, there were significant differences (p ≤ 0.05) among the extrudate and diet fed to the experimental animals in term of feed consumed, faeces excreted, urine excreted and weight gain in the rats. Flavoured extrudate DF1 was better consumed by the animals than flavoured extrudate DF2.
In terms of faeces excreted, rats fed with extrudate/diet DF2 had the highest excreted faeces, followed by extrudate DF1. In terms of urine excreted, rats fed with extrudate DF2 produced the highest level of urine followed by the rats fed with flavoured extrudate DF1. It appears that flavoured extrudates/diet (DF1) is better than flavoured extrudates/diet (DF2) from the standpoint of feed consumed, faeces excreted and weight gained by the laboratory animals during the study as shown in Table 3.
The protein efficiency ratio (PER) normally uses growth of the rat as a measure of the nutritive value of dietary proteins. It is defined as the weight gain per unit weight of protein eaten. The extrudates produced supported the growth of the laboratory animals used with flavoured extrudate DF1 giving a better growth in the experimental rats used then the flavoured extrudate or diet DF2.
Biological value shows a direct measure of the food protein which can be utilized by the animal for synthesizing body tissue and compounds and may be defined as the percentage of the nitrogen absorbed which is retained by the animal. The biological value in both flavoured extrudates was very high showing that most of the nitrogen was absorbed by the animals. Part of the nitrogen of the faeces, the metabolic faecal nitrogen, is not directly derived from food. Urinary nitrogen also contains a proportion of nitrogen known as endogenous urinary nitrogen which is not directly produced from the ingested food by the test animals.
Both extrudates (DF1 and DF2) produced moderate quantities of urinary protein and faecal protein. Urinary nitrogen/protein and faecal nitrogen protein have been reported to be totally directly produced from ingested food nitrogen by the animals, but it is nitrogen derived from expendable tissues and secretions together with waste nitrogen arising during normal catabolism and re-synthesis of tissue proteins.
The existence in both faeces and urine of nitrogen fractions whose excretion is independent of food nitrogen is most convincingly demonstrated by the fact that some nitrogen is excreted when the animal is given a nitrogen free diet [22,28] as revealed by the urinary nitrogen/ protein and faecal nitrogen/protein produced by the experimental animals fed with non-protein diet in this study.
The study also indicated that extrudate DF1 was better
Table 3. Nutritional qualities of yam-bambara extrudates.
consumed than extrudate DF2 by the experimental animals employed in this study. This might be due to the salt to sugar ratio in the feed blend. Sugar and salt have been reported to enhance flavour and sensory acceptability of certain foods [4,6].
Rats fed with the flavoured extrudate DF2, however, had the highest excreted faeces lower levels of protein in urine excreted than the flavoured extrudate DF1 implying that rats were fed with the flavoured extrudate DF2 did not make use of the extrudate properly in the digestive system thereby getting rid of them as faeces. This, in turn, affected the weight gain of the experimental rats fed with extrudate/diet DF2 resulting into lower weight gained by the rats than in the rats fed with extrudate/diet DF1.
Similar studies on extruded products have shown similar observations [29-31]. The study has shown that extrudate DF1 was better than extrudate DF2 based on the positive feed consumption, faeces excreted and weight gain pattern obtained in this study. High protein efficiency ratios of 0.74 and 0.79 have been reported in the extrudates DF1 and DF2 with high biological values of 0.99 and 0.96, respectively. A similar observation in extruded corn-soya mixtures had been documented [29]. It has been reported that extrusion cooking improves the protein digestibility of extruded food products [28,30, 31].
The challenging performance of rats fed with extruded diets to those fed casein diet was attributed to the effect of extrusion cooking as a form of heat treatment to the mixtures. Heat is known to improve the availability of some nutrients, inactivate enzymes that speed up nutrient damage and destroy undesirable microorganisms and food contaminant [23,28,29,31]. It also favourably changes the physical attributes of food such as colour, texture and flavour [32] and improves palatability and digestibility due possibly to higher starch damage [28, 29,32,33].
The urinary output of rats fed with yam-bamabara extrudates DF1 and DF2 were higher than those from casein diets. Rats with lower urinary nitrogen output showed higher nitrogen retention [27]. Low urinary and faecal nitrogen indicate high protein quality of fed diets/extrudates [31]. Also the biological values of 0.99 and 0.96 obtained for extrudates DF1 and DF2 were however comparable to that obtained in extruded Bread fruit-soya blend of 0.94 as reported by Nwabueze [28] while the standard casein diet had a biological value of 1.0, lower biological values of diets fed to rats may be due to lower nitrogen intake by the fed rats and higher urinary output, which could be indicative of poor utilization of digested/retained nitrogen [30].
The protein efficiency ratio (PER), is an index of protein quality in the extrudates which shows the relationship between weight gain by the test animals and the corresponding protein as nitrogen intake [18,23,25].
4. Conclusions
It is possible to produce nutritious and shelf stable extruded food product using appropriate combinations of pre processed white yam and bambara groundnut under the extrusion conditions investigated in this study. The developed extruded Food products are however suitable for human consumption, promote weight gain with high protein qualities in terms of high biological value and protein efficiency ratio in the experimental rats used in this study.
Extrusion cooking of yam-bambara mixtures has therefore proved to support growth and weight gain in weaning rats. The implication of this research therefore is that a careful selection of indigenous plant protein sources could be of nutritional benefit in terms of consumer weight gain and nitrogen utilization that may be comparable to casein diet when correctly blended and processed using a single screw extruder.
5. Acknowledgements
The authors wish to acknowledge the Federal Institute of Industrial Research, Oshodi, Lagos, Nigeria for providing facilities for preliminary aspect of this project. Tallon Industries, Igbogila, Ogun state, Nigeria is also acknowledged for providing the single screw extruder used for this study and Nigerian Medical Research Institute, Yaba, Lagos, Nigeria is also acknowledged for providing facilities for the animal feeding experiment.
REFERENCES
- P. Colona and C. Mercier, “Macromolecular Modifications of Manioc Starch Components by Extrusion Cooking with and without Lipids,” Carbohydrate Polymer, Vol. 3, No. 2, 1983, pp. 87-128. doi:10.1016/0144-8617(83)90001-2
- F. Hsieh, I. C. Peng and H. E. Huff, “Effect of Salt, Sugar and Screw Speed on Processing and Product Variable of Cornmeal Extruded with a Twin Screw Extruder,” Journal of Food Science, Vol. 55, No. 1, 2006, pp. 224-227. doi:10.1111/j.1365-2621.1990.tb06057.x
- I. Nkama and K. B. Filli “Development and Characteristics of Extruded Fura from Mixtures of Pearl Millet and Grains Legume Flours,” International Journal of Food Properties, Vol. 9, No. 2, pp. 157-165. doi:10.1080/10942910600596605
- K. B. Filli, I. Nkama, V. A. Jideani and M. U. Abubakar, “Influence of Extrusion Variables on Some Functional Properties of Extruded Millet-Soybean for the Manufacture of ‘Fura’,” African Journal of Food Science, Vol. 46, No. 6, 2010, pp. 342-352.
- R. Chinnaswamy and M. A. Hanna, “Optimum Extrusion Cooking Conditions for Maximum Expansion of Corn Starch,” Journal of Food Science, Vol. 53, No. 3, 1988, pp. 834-836. doi:10.1111/j.1365-2621.1988.tb08965.x
- A. Olapade and D. O. Adetuyi, “Comparison of Different Methods of Producing Bambara (Voandzeia subterranean L. Thou) Flours for Preparation of ‘Moin-Moin’,” Nigerian Food Journal, Vol. 25, No. 2, 2007, pp. 150-157.
- G. Osuji, “Advances in Yam Research. The Biochemistry and Technology of the Yam Tuber,” University Press Nigeria, Anambra State, 1990, p. 50.
- S. E. Omonigho and M. J. Ikenebomeh, “Effect of Temperature Treatment on the Chemical Composition of Pounded White Yam during Storage,” Food Chemistry, Vol. 71, No. 2, 2000, pp. 215-220. doi:10.1016/S0308-8146(00)00158-8
- T. S. Barimala, S. C. Achineuhu, L. Yibatama and E. N. Amadi, “Studies on the Solid Substrate Fermentation of Bamabara Groundnut,” Journal of Science Food and Agriculture, Vol. 66, No. 4, 1994, pp. 443-446. doi:10.1002/jsfa.2740660404
- A. O. Odior1 and E. S. Orsarh, “Design and Construction of a Yam Pounding Machine,” International Journal of Natural and Applied Sciences, Vol. 4, No. 3, 2008, pp. 319-323.
- W. F. Harrigan and M. E. Mclance, “Laboratory Methods in Microbiology,” Academic Press, New York, 1976, pp. 8-40, 106-112, 128, 199-204.
- AOAC, “Official Methods of Association of Analytical Chemist,” 18th Edition, Washington DC, 2005.
- H. Egan, R. S. Kirk and R. Sawyer, “Pearson’s Chemical Analysis of Foods,” 8th Edition, Churchhill Living Stone, London, 1991.
- C. S. James, “Analytical Chemistry of Food,” Aspen Publishers Incorporated, Gaithersburg, 1998, pp. 64-65, 71- 135.
- D. Pearson, “The Chemical Analysis of Foods,” 7th Edition, Churchill Living Stone, London, 1976, pp. 19, 23- 24.
- M. L. Kakade, J. J. Rackis, J. E. Mcghee and G. Puski, “Determination of Trypsin Inhibitor Activity of Soy Products. A Collaborative Analysis of an Improved Procedure,” Journal of Cereal Chemistry, Vol. 51, 1974, pp. 376-382.
- M. I. Lape, “Nutritional Quality of Yam (Dioscorea dumetorwin and D. rotundata) Flour for Growing Rats,” Journal of Science of Food and Agriculture, Vol. 66, No. 4, 1994, pp. 447-455. doi:10.1002/jsfa.2740660405
- A. O. Fasuyi, “Nutritional Evaluation of Cassava (Manihot esculenta Crantz) leaf Protein Concentrate (CLPC): An Alternative Protein Sources in Rat Assay,” Pakistan Journal of Nutrition, Vol. 4, No. 1, 2005, pp. 50-56. doi:10.3923/pjn.2005.50.56
- I. C. Obizoba, “The Nutritive Value of Mixtures of Two Varieties of Bambara Groundnut and Sorghum,” Journal of Cereal chemistry, Vol. 66, No. 4, 1989, pp. 249-259.
- M. Zimmermon, “Ethical Guideline for Investigation of Experimental Pain in Conscious Animal,” Pain, Vol. 16, No. 2, 1983, pp. 109-110. doi:10.1016/0304-3959(83)90201-4
- O. A. T. Ebuehi and O. O. Aleshinloye, “Extracts of Cnestis ferruginea and Rawolfia vomitoria Affect Blood Chemistry and GABAergic Neurotransmission in Ketamine—Induced Psychotic Rats,” Nigerian Quarterly Journal of Hospital Medicine, Vol. 22, No. 4, 2010, pp. 171-176.
- PHLSG, “The Microbiological Quality of Ready-to-Eat Foods Sampled at the Point of Sale, (Public Health Laboratory Service Guidelines),” Brough Council, 2008.
- N. W. Destrosier and J. N. Destrosier, “The Technology of Food Preservation,” 4th Edition, CBS Publisher and Distributors, New Delhi, 2004, pp. 340-363.
- C. W. Bamforth, “Food, Fermentation and Micro-Organisms,” Blackwell Publishing Company, Oxford, 2005, pp. 17-18. doi:10.1002/9780470995273
- C. H. Collins, P. M. Lyne and J. M. Grange, “Microbiological Methods,” 6th Edition, Buttermorths, London, 1989, pp. 178, 198.
- S. H. Brough and S. N. Azam-Alli, “The Effect of Soil Moisture on the Proximate Composition of Bambara Groundnut,” Journal of Science Food and Agriculture, Vol. 60, No. 2, 1992, pp. 177-203. doi:10.1002/jsfa.2740600207
- P. McDonald, R. A. Edward and J. F. D. Greenhalgh, “Animal Nutrition,” 3rd Edition, Oliver and Boyd, 1979.
- T. U. Nwabueze, “Growth Performance of Rats Fed Raw and Extruded African Breadfruit Based Complementary Diet. A Response Surface Analysis,” Journal of Science of Food and Agriculture, Vol. 88, No. 3, 2008, pp. 522- 528. doi:10.1002/jsfa.3116
- V. Baskran and A. Bhattacharaya, “Nutritional Statics of the Protein of Corn-Soya Based Extruded Products Evaluated by Rat Bioassay,” Plant Foods for Human Nutrition, Vol. 59, No. 3, 2005, pp. 101-104. doi:10.1007/s11130-004-4309-3
- T. U. Nwabueze, “Bioavailability of Vitamins and Minerals in Adult Rats Fed Raw and Extruded African Bread Fruit (Treculia africana) Mixtures,” Journal of Food Agriculture and Environment, Vol. 5, No. 314, 2007, pp. 465-470.
- T. U. Nwabueze, “Weight Analysis and Nitrogen Balance Assay in Rat Fed Extruded African Bread Fruit Based Diets,” Nigerian Food Journal, Vol. 46, No. 1, 2008, pp. 27-41.
- K. Owusu-Domfen, “Trypsin Inhibitor Activity of Cowpea (Vigna unguiculata) and Bambara Beans (Voandzera subteranea),” Ghana Journal of Science, Vol. 5, 1972, pp. 99-102.
- G. R. Jansen, J. M. D Harper and L. Dean, “Nutritional Evaluation of Blended Foods Made with a low Cost Extruder Cooker,” Journal of Food Science, Vol. 44, No. 3, 1978, pp. 1322-1328.
NOTES
*Corresponding author.

