Food and Nutrition Sciences
Vol. 2 No. 5 (2011) , Article ID: 5796 , 5 pages DOI:10.4236/fns.2011.25067
Survival of Listeria monocytogenes during Frying of Chicken Burger Patties
![]()
1Center of Excellence for Food Safety Research, Faculty of Food Science and Technology, Universiti Putra Malaysia, Serdang, Malaysia; 2Faculty of Food Technology, Universiti Sultan Zainal Abidin, Terengganu, Malaysia; 3Center for Southeast Asian Studies, Kyoto University, Kyoto, Japan.
Email: *son@food.upm.edu.my
Received April 26th, 2011; revised May 19th, 2011; accepted May 26th, 2011.
Keywords: Listeria monocytogenes, Survival, Frying, Chicken Burger Patties
ABSTRACT
This study was aimed to determine sufficient frying time to reduce the number of Listeria monocytogenes present in chicken burger patties to non-detectable level which is fit for human consumption. Commercially available chicken burger patties were artificially contaminated with L. monocytogenes at level of approximately 9 log CFU/ml. The contaminated chicken burger patties were cooked for 0, 2, 4, 5, 8 and 10 minutes to determine survival of L. monocytogenes. Results demonstrated a linear correlation between mean log reduction of L. monocytogenes and frying time. L. monocytogenes was not detected in chicken burger patties that were cooked for 6 minutes and above. As a result from this study, it is suggested that a minimum frying time for burger patties is 6 minutes. This can be treated as a safety measure to avoid consequences of consumption of undercooked burger patties.
1. Background
L. monocytogenes has emerged as a significant foodborne pathogen in recent decade. Most human foodborne infections are associated with high incidence rate but low morbidity and mortality rate. However, this is opposite for human listeriosis, which is a relatively rare but potentially severe infection, associated with a fatality rate of up to 30% [1].
Outbreaks of L. monocytogenes have been related to the consumption of contaminated processed meat products, as well as frozen meat products. Previous studies found high microbial counts in frozen hamburgers [2-4]. Although the pathogens can be killed by pasteurization or other heating procedures, the possibilities for crosscontamination or undercooking are reasons for concern. Epidemiologic data show that, normally the food involved in listeriosis outbreaks are those in which the organism had multiplied and exceed the level of 100 CFU/g [5]. Since consumption of L. monocytogenes which exceed allowance level can cause serious adverse health effects to human, the bacterium survived upon inadequate cooking is an important factor to emphasize.
There are various types of frozen food products available at retail market nowadays. Consumers nowadays shift towards convenience foods to cope with their increasingly busy lifestyle. Growing preference for convenience, choice and ease of preparation of this type of food products has warranted their popularity among consumers. Hence it is very common to find frozen burger patties in domestic refrigerator. Most of the handling practices involved in preparation of burger patties are refrigerated storage and cooking. Therefore, this study was designed to examine survival of L. monocytogenes in chicken burger patties upon cooking process which aim to determine sufficient cooking time to reduce the number of L. monocytogenes present in chicken patties to a safe and acceptable level for human consumption.
2. Materials and Methods
2.1. Preparation of L. monocytogenes Inoculum
Pure culture of L. moncoytogenes ATCC 19155 was inoculated onto PALCAM agar (Merck, Germany) and incubated for 48 hours at 30˚C. The strain of L. monocytogenes was prepared by individually inoculating a single colony from the plate into 10 ml Tryptic Soy Broth (TSB; Merck, Germany), incubated with shaking at 37˚C and harvested exactly after 24 hours of incubation. The overnight cultures were centrifuged at 13,400 × g for 5 min and the supernatant was discarded. The cell pellets were then resuspended with 0.85% saline solution (NaCl, Merck, Germany). The absorbance of the cell suspension at 600 nm was adjusted to a reading of 0.393 for L. monocytogenes, which corresponded to about 1.35 × 109 CFU/ml.
2.2. Sample Preparation and Inoculation
Chicken patties were purchased from a local grocery store. The patty was 60 g in weight, approximately 8.3 ± 0.3 cm in diameter and 1.0 ± 0.2 cm in thickness. To initiate the experiments, all of the chicken patties were disintegrated, grinded together and mixed thoroughly to enhance homogeneity of natural background microflora that might present originally in the sample. Thereafter, each portion of 60 g was then inoculated with 1 ml of inoculum so that the final concentration of cells was approximately 2.25 × 107 CFU/g. Inoculated sample was mixed well in a mould using sterile spatula to ensure even distribution of the inoculum within the patties, and reshaped into patty form using a patty former. A set of triplicate sample was left uninoculated and regarded as control sample. Finally, all prepared chicken patties were stored at 4˚C until the cooking process to simulate the storage condition in domestic kitchen.
2.3. Cooking of Burger Patties
Cooking oil was heated in a frying pan on a heater and the temperature of cooking oil was monitored at 140˚C ± 5˚C using a thermometer (TEL-TRU, Rochester, New York, USA) throughout the cooking process. Burger patties were cooked for 0, 2, 4, 6, 8 and 10 minutes. During the cooking process, the burger patties were turned over when half of the time had elapsed. After cooking, burger patties were sliced from the side for visual inspection
2.4. Enumeration of L. monocytogenes
After cooling, whole piece of each fried burger patties was homogenized with 0.85% saline water (1:3, w/v) using a stomacher [6]. Successive dilutions were prepared in same diluents and plated onto the surface of Palcam agar (Merck). The plates were incubated at 30˚C for 48 hours. All typical Listeria colonies observed were considered to be L. monocytogenes and the quantitative data were expressed as CFU/g.
2.5. Data Analysis
The resulting data were subjected to linear regression analysis using SPSS software (version 16.0). The regression analysis of the survival curve was performed using Excel Software (version 2007). Correlation between mean log of survivors and cooking time for burger patties was being evaluated.
3. Results
Table 1 showed the range and mean concentration of L. monocytogenes being detected in the sample after each treatment. L. monocytogenes was not detected in the uninoculated control samples. The concentration of L. monocytogenes in inoculated sample was ranged from 8.38 - 8.45 log CFU/g, with mean concentration of 8.42 log CFU/g. This number will be regarded as initial concentration of L. monocytogenes for all inoculated samples. A gradual reduction in concentration of L. monocytogenes in chicken patties was being observed for the first two minutes of cooking. The reduction ranged from 0.58 to 1.01 log CFU/g. However, the number of L. monocytogenes was decreased rapidly after the second minute of cooking (from 0.76 log reduction to 4.15 log reduction at the fourth minute). For cooking time of six minutes onwards, viable L. monocytogenes was not detected in the heat-treated samples. The regression analysis of the survival curve was shown in Figure 1.
4. Discussion
In this study, the negative control samples showed absence of viable L. monocytogenes after incubation for 48
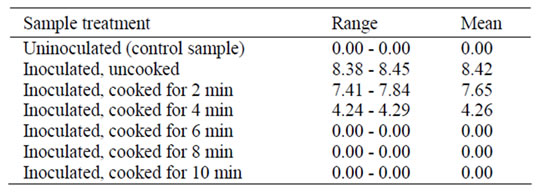
Table 1. The concentration of L. monocytogenes (log CFU/g) detected on chicken burger patties after each treatment.
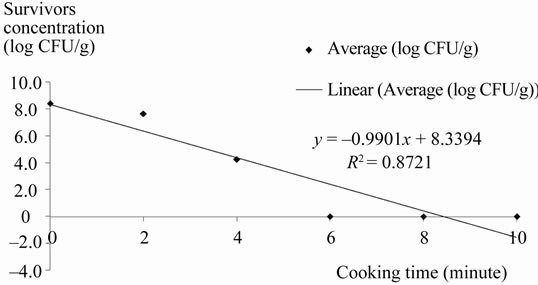
Figure 1. Regression analysis of the survival curve showing the linear correlation (R2 = 0.87) between log number of survivors and the cooking time for the chicken patties.
hours. Therefore, all samples in this study are assumed to be free from interference of originally present L. monocytogenes. However, it is worthy to note the difference in number of L. monocytogenes between the original spiked concentration and actual plating count. As high as 8.42 log CFU/g of L. monocytogenes had been detected after inoculation and incubation, instead of the number of L. monocytogenes being inoculated originally (7.35 log CFU/g). There is an increment of approximately 1.07 log L. monocytogenes in chicken patties after overnight storage in refrigerator. Implication from this observation can be due to psychrotrophic nature of L. monocytogenes.
Psychrotrophic nature pampers the survival and growth of L. monocytogenes in refrigerated conditions, as well as in refrigerated food products. To date, there are numerous reports on growth of L. monocytogenes at refrigerated condition [7,8]. Glass and Doyle [9] reported 2 - 5 log of increment in L. monocytogenes population on vacuum-packaged artificially contaminated frankfurters after four weeks of storage at refrigerator. Besides, this bacterium was found to survive brining and smoking process used to treat smoked salmon, and demonstrated a 4 log cycle increase in pathogen numbers after one month refrigerated storage [10]. At the same time, Rorvik et al. [11] also detected an increase of 3 - 4 log cycles of L. monocytogenes in vacuum-packaged smoked salmon over a five weeks storage period at 4˚C. It is quite surprising because the initial concentration of L. monocytogenes in the particular case was only 6 CFU/g. Nonetheless, there are contradictions on the argument that L. monocytogenes can grow at refrigerated temperature. MacRae and Gibson [12] had demonstrated negligible growth of this bacterium in refrigerated products at 5˚C. In fact, the survival and growth of L. monocytogenes in refrigerated foods is most probably influenced by other intrinsic factors, such as presence of lactic acid bacteria and initial pH. The presence of intrinsic factors can be either pampering the pathogens to promote their growth, or inhibiting the pathogens to suppress their growth.
The purpose of artificially inoculated the sample with high level of L. monocytogenes was to imitate the worst situation, in which the burger patties are becoming heavily contaminated due to proliferation of the bacteria after prolonged storage of foods at refrigerated temperature or other temperature abuse condition [13,14]. Tompkin [5] asserted that, during extended refrigerated storage for more than 35 days for example, the bacterium could multiply to potential hazardous levels in the food products, especially when the product is temperature-abused. This statement is further supported by Swaminathan and Gerner-Smidt [15], by stating that L. monocytogenes is able to multiply itself to unsafe levels during refrigerated storage of food products during distribution and at the consumer’s home. Furthermore, a survey conducted in Slovenia in year 2008 regarding consumers’ awareness of food safety showed that, only 50% of the respondents ever thought of using cooling bag for transport of refrigerated or frozen foods. And there was 44% of respondents did not aware of proper refrigerator temperature [16]. Therefore, for the prediction in worst case, this study could render the minimal sufficient cooking time of burger patties, in order to warrant the safe consumable level regardless original concentration of L. monocytogenes in the products.
Cooking time of two minutes and four minutes promoted some reduction in the population of L. monocytogenes, due to inactivation which occur on or near the outer surface of the patties. As observed from the result, a cooking time of six minutes or more is sufficient to inactivate approximately 8.42 log of L. monocytogenes cells in chicken patties. This suggests that, at the sixth minute of frying, the applied heat is able to reach the geometric center of the patties with thickness of 1 cm and deactivated all L. monocytogenes present in it. This finding is actually in parallel to a study done by Passos and Kuaye [6], in which they also discovered that, the effect of cooking time on destruction of L. monocytogenes in hamburgers become more intense as from six minutes onwards. In addition, other researchers also reported similar findings, where they found that, increased lethality on pathogens was demonstrated with increased processing time [17,18]. The outcome from this study is in agreement with their standpoint, where the regression analysis of the survival curve from this study demonstrated that there is a linear correlation (R2 = 0.87) between the mean log concentration of survivors and the cooking time.
Apart from cooking, other common practices regarding survivability of L. monocytogenes in chicken burger patties such as freezing and thawing were not emphasized in this study in parallel. This is because, no matter how bad is the storage condition or to which extent the L. monocytogenes can multiply during temperature abuse scenario, heat treatment is still the ultimate preventive measure taken to inactivate the L. monocytogenes present in burger patties. Moreover, all consumers understand that frozen burger patties need to be cooked before consumption. But they might not know the exact time and temperature of cooking. Therefore this study is important to provide information regarding the temperature and time needed to completely inactivate L. monocytogenes in burger patties.
Although the high level of L. monocytogenes present in foods can be fully deactivated after sufficient heat treatment, another safety issue to be concerned is cross contamination between contaminated products with other ready-to-eat foods. The survey done by Jevsnik et al. [16] showed that there was approximately 50% of the respondents carried out thawing process of meat products on working surfaces, and only one-third wash utensils with hot water and detergent before re-use of the utensils. Hence, half of the consumers are considered not aware to the potential hazards associate with cross contamination in kitchen. In fact, the exudates from meat products might contain higher level of microbial load to serve as a source of pathogens to other foods. L. monocytogenes can be spread by contact with infected surface or product during food preparation. Various researchers claimed that, poor hygiene practice during food handling in domestic environment is one of the major factors for the outbreak of foodborne illness [19,20]. Hence, consumers should be educated on these safety issues to prevent the occurrence of cross contamination between contaminated foods with cooked foods.
5. Conclusions
This is the first study conducted in Malaysia to examine the sufficient cooking time to deactivate the viable L. monocytogenes cells present in chicken burger patties. A cooking time of six minutes is sufficient to fully deactivate 8.42 log CFU/g of L. monocytogenes in 1 cm thick chicken patties. This can be treated as a safety measure to avoid consequences of consumption of undercooked burger patties. Outcome of this study is useful especially to fill in the data gap in quantitative microbial risk assessment of L. monocytogenes in burger patties.
6. Acknowledgements
This study was funded by Grant-in-Aid for Scientific Research (KAKENHI 191010) from the Japan Society for the Promotion of Sciences and in-part by Science Fund (Project No. 05-01-04-SF0379) from the Ministry of Science, Technology and Innovation (MOSTI), Malaysia.
REFERENCES
- B. Lorber, “Listeriosis,” Clinical Infectious Diseases, Vol. 24, No. 1, 1997, pp. 1-9. doi:10.1093/clinids/24.1.1
- M. S. L. Coelho and D. F. Barbosa, “Estudo Comparativo da Qualidade Microbiológica de Hambúrgueres Processados Artesanal e Industrialmente,” Revista Ceres, Vol. 40, No. 229, 1993, pp. 235-241.
- M. J. Torner, M. Castillo, S. Pla and M. J. Hernandorena, “Estudio de la Calidad Sanitaria de Productos cáRnicos Frescos Elaborados en Carnicerías-salchicherías del Área de Salud de Xátiva,” Alimentaria, Vol. 33, No. 262, 1995, pp. 27-31.
- C. O. Gill, K. Rahn, K. Sloan and L. M. McMullen, “Assessment of the Hygienic Performances of Hamburger Patty Production Processes,” International Journal of Food Microbiology, Vol. 36, No. 2-3, 1997, pp. 171-178. doi:10.1016/S0168-1605(97)01268-3
- R. B. Tompkin, “Microorganisms in Foods 7, International Commission on Microbiological Specifications for Foods,” Klumer Academics/Plenum Publishers, London, 2007, pp. 285-309.
- M. H. C. R. Passos and A. Y. Kuaye, “Influence of the Formulation, Cooking Time and Final Internal Temperature of Beef Hamburgers on the Destruction of Listeria monocytogenes,” Food Control, Vol. 13, No. 1, 2002, pp. 33-40. doi:10.1016/S0956-7135(01)00080-9
- S. Buncic, L. Paunovic and D. Radisic, “The Fate of L. monocytogenes in Fermented Sausages and in Vacuum Packaged Frankfurters,” Journal of Food Protection, Vol. 54, No. 6, 1991, pp. 413-417.
- R. C. McKellar, R. Moir and M. Kalab, “Factors Influencing the Survival and Growth of Listeria monocytogenes on the Surface of Canadian Retail Wieners,” Journal of Food Protection, Vol. 57, No. 5, 1994, 387-392.
- K. A. Glass and M. P. Doyle, “Fate of Listeria monocytogenes in Processed Meat Products during Refrigerated Storage,” Applied and Environmental Microbiology, Vol. 55, No. 6, 1989, pp. 1565-1569.
- S. Guyer and T. Jemmi, “Behaviour of Listeria monocytogenes during Fabrication and Storage of Experimentally Contaminated Smoked Salmon,” Applied and Environmental Microbiology, Vol. 57, No. 5, 1991, pp. 1523- 1527.
- L. M. Rorvik, M. Yndestad and E. Skjerve, “Growth of Listeria monocytogenes in Vacuum-Packed, Smoked Salmon during Storage at 4˚C,” International Journal of Food Microbiology, Vol. 14, No. 2, 1991, pp. 111-117. doi:10.1016/0168-1605(91)90097-9
- M. Macrae and D. M. Gibson, “Survival of Listeria monocytogenes in Smoked Salmon,” In: P. Zeuthen, J. C. Cheftel, C. Eriksson, T. R. Gormley, P. Linko and K. Paulus, Eds., Chilled Foods: The Revolution in Freshness, Elsevier, London, 1990, pp. 168-174.
- P. Rosset, M. Cornu, V. Noel, E. Morelli and G. Poumeyrol, “Time-Temperature Profiles of Chilled Readyto-Eat Foods in School Catering and Probabilistic Analysis of Listeria monocytogenes Growth,” International Journal of Food Microbiology, Vol. 96, No. 1, 2004, pp. 49-59. doi:10.1016/j.ijfoodmicro.2004.03.008
- M. J. Zhu, A. Mendonca and D. U. Ahn, “Temperature Abuse Affects the Quality of Irradiated Pork Loins,” Meat Science, Vol. 67, No. 4, 2004, pp. 643-649. doi:10.1016/j.meatsci.2004.01.005
- B. Swaminathan and P. Gerner-Smidt, “The Epidemiology of Human Listeriosis,” Microbes and Infection, Vol. 9, No. 10, 2007, pp. 1236-1243. doi:10.1016/j.micinf.2007.05.011
- M. Jevsnik, V. Hlebec and P. Raspor, “Consumers’ Awareness of Food Safety from Shopping to Eating,” Food Control, Vol. 19, No. 8, 2008, pp. 737-745. doi:10.1016/j.foodcont.2007.07.017
- A. W. Kotula, C. M. Chesnut, B. S. Emswiller and E. P. Young, “Destruction of Bacteria in Beef Patties by Cooking,” Journal of Animal Science, Vol. 45, No. 1, 1977, pp. 54-58.
- S. K. Tamminga, R. R. Beumer and E. H. Kampelmacher, “Microbiological Studies on Hamburgers,” Journal of Hygiene, Vol. 88, No. 1, 1982, pp. 125-142.
- doi:10.1017/S0022172400069989
- J. P. Speirs, A. Anderton and J. G. Anderson, “A Study of the Microbial Content of the Domestic Kitchen,” International Journal of Environmental Health Research, Vol. 5, No. 2, 1995, pp. 109-122. doi:10.1080/09603129509356839
- E. Scott, “Foodborne Disease and Other Hygienic Issues in the Home,” Journal of Applied Bacteriology, Vol. 80, No. 1, 1996, pp. 5-9.

