American Journal of Plant Sciences
Vol.3 No.8(2012), Article ID:22192,4 pages DOI:10.4236/ajps.2012.38137
An Investigation of the Vegetative Anatomy of Piper sarmentosum, and a Comparison with the Anatomy of Piper betle (Piperaceae)
![]()
1National Center for Natural Products Research, School of Pharmacy, University of Mississippi, University, MS 38677, USA; 2Department of Pharmacognosy, School of Pharmacy, University of Mississippi, University, MS 38677, USA.
Email: ikhan@olemiss.edu
Received June 21st, 2012; revised July 18th, 2012; accepted July 26th, 2012
Keywords: Piper sarmentosum; Piper lolot; Piper betle; Piperaceae; Anatomy; Light and Scanning Electron Microscopy
ABSTRACT
This paper describes the detailed anatomy and micromorphology of the vegetative parts of Piper sarmentosum Roxb. (synonym, P. lolot C.DC.), which is a southeast Asian medicinal plant valued for its medicinal and culinary uses. The study also compares the anatomy of the leaf, petiole, stem and root of P. sarmentosum with those of the well-characterized P. betle L. The leaves of both the species are morphologically comparable and have similar culinary uses. The present study showed that the leaves of both the species possess more or less comparable anatomical features with a few differences; whereas, the microscopic features of the petiole, stem, and root of the two species are distinctive.
1. Introduction
Plants of the genus Piper L. (Piperaceae) are economically important because of their medicinal and culinary uses. The genus is represented by about 1050 species distributed mainly in the tropics [1]. Piper sarmentosum occurs in the tropical forests in Cambodia, southern China, India (NE India and Andaman Islands), Indonesia, Laos, Malaysia, Philippines and Vietnam [2,3].
Piper sarmentosum Roxb. is a dioecious, erect shrublet, up to 1.5 m tall, often stoloniferous (sarmentose); young parts minutely puberulent. Stems swollen at nodes; short aerial roots present on lower nodes. Leaves variable in shape and size; lower leaves broadly cordiform, up to 15 × 14 cm, base cordate, apex acuminate, long˗stalked; upper ones ovate˗oblong, truncate to cuneate at base, acute at apex, shortly stalked; primary veins 7, palmate, apical pair of veins usually arise 0.5 - 2 cm above from the base, prominently raised below, slightly so above. Spike oblongoid, 2 - 5 cm long and 0.8 - 1 cm thick; peduncle as long as spike [3,4].
P. sarmentosum is commonly called as Lolot pepper, La lot, Wild betel etc. Its botanical synonyms include Chavica hainana C. DC.; C. sarmentosa (Roxb.) Miq.; Piper albispicum C. DC.; P. brevicaule C. DC.; P. gymnostachyum C. DC.; P. lolot C. DC.; P. pierrei C. DC.; P. saigonense C. DC. [4,5].
The leaves of P. sarmentosum are traditionally used as a condiment and also in many South Asian cuisines. In traditional medicine, the leaves are consumed for their carminative properties and the whole plant possesses anti-inflammatory, expectorant and anodyne properties. The plant is also used to cure skin diseases, rheumatism, headache, diarrhea, fever, indigestion and toothache. In addition, the roots can be administered for the treatment of cough, asthma and toothache [6]. The dried fruits (infructescence) are occasionally used as spices and medicines [3].
Identification of the leaves of Piper sarmentosum is often difficult because of the possible confusion with the leaves of betel (P. betle L.) due to morphological resemblances as well as their similar usage as condiments. The anatomy of P. betle has been well studied previously [7-12], whereas, anatomical study of P. sarmentosum has been neglected. P. sarmentosum is also morphologically similar to and has been in the past confused with another species, viz., P. longum L. [2,3]. Even though Gilbert [5] merged P. lolot C. DC. as a synonym under P. sarmentosum Roxb., many publications [1] and some online databases like www.ars-grin.gov; www.plantnames.unimelb. edu.au, www.wikipedia.org, etc. still treat the two species as distinct ones.
In spite of the prevailing confusions with other morphologically similar species, the anatomy of Piper sarmentosum has been neglected. Therefore, the present work was undertaken to provide detailed anatomy and micromorphology of the species, by light and scanning electron microscopy (Figures 1-3). In addition, the anatomy of leaf, petiole, stem and root of P. sarmentosum was compared with those of P. betle (Figure 4 & Table 1), for proper identification.
2. Materials and Methods
Authentic samples of the fruiting plant of Piper sarmentosum (MPG # C004) and the male plant of P. betle (MPG # C006) grown in the green house at the University of Mississippi (UM)’s medicinal plants garden (MPG) were used for this study. Specimens of P. sarmentosum from live plants (NCNPR # 9860) as well as fresh twigs (NCNPR # 9861), purchased online from www.toptropicals.com and www.importfood.com, respectively, were also used for the study. Herbarium specimens of all the samples were deposited in the Repository of Botanicals at the National Center for Natural
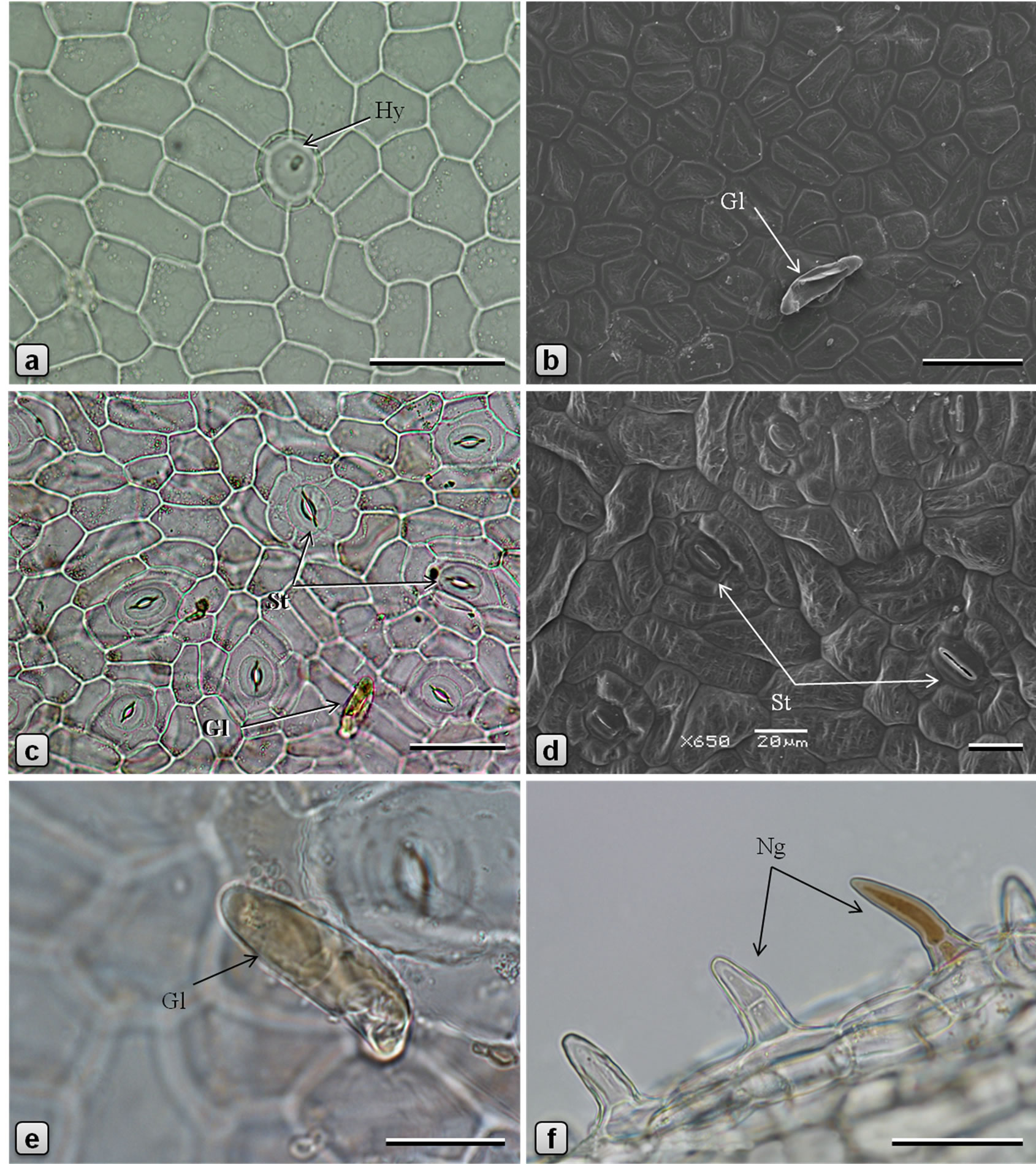
Figure 1. Leaf epidermis of Piper sarmentosum. [a, c, e, f: light microscopy (LM); b, d: scanning electron microscopy (SEM)]. a, b: adaxial epidermis; c, d: abaxial epidermis; e: glandular trichome on abaxial surface of lamina; and f: non-glandular trichomes on abaxial surface of midrib. (Gl: glandular trichome; Hy: hydathode; Ng: nonglandular trichome; St: stomata). Scale bars: a, b, c, f = 50 µm; d, e = 20 µm.
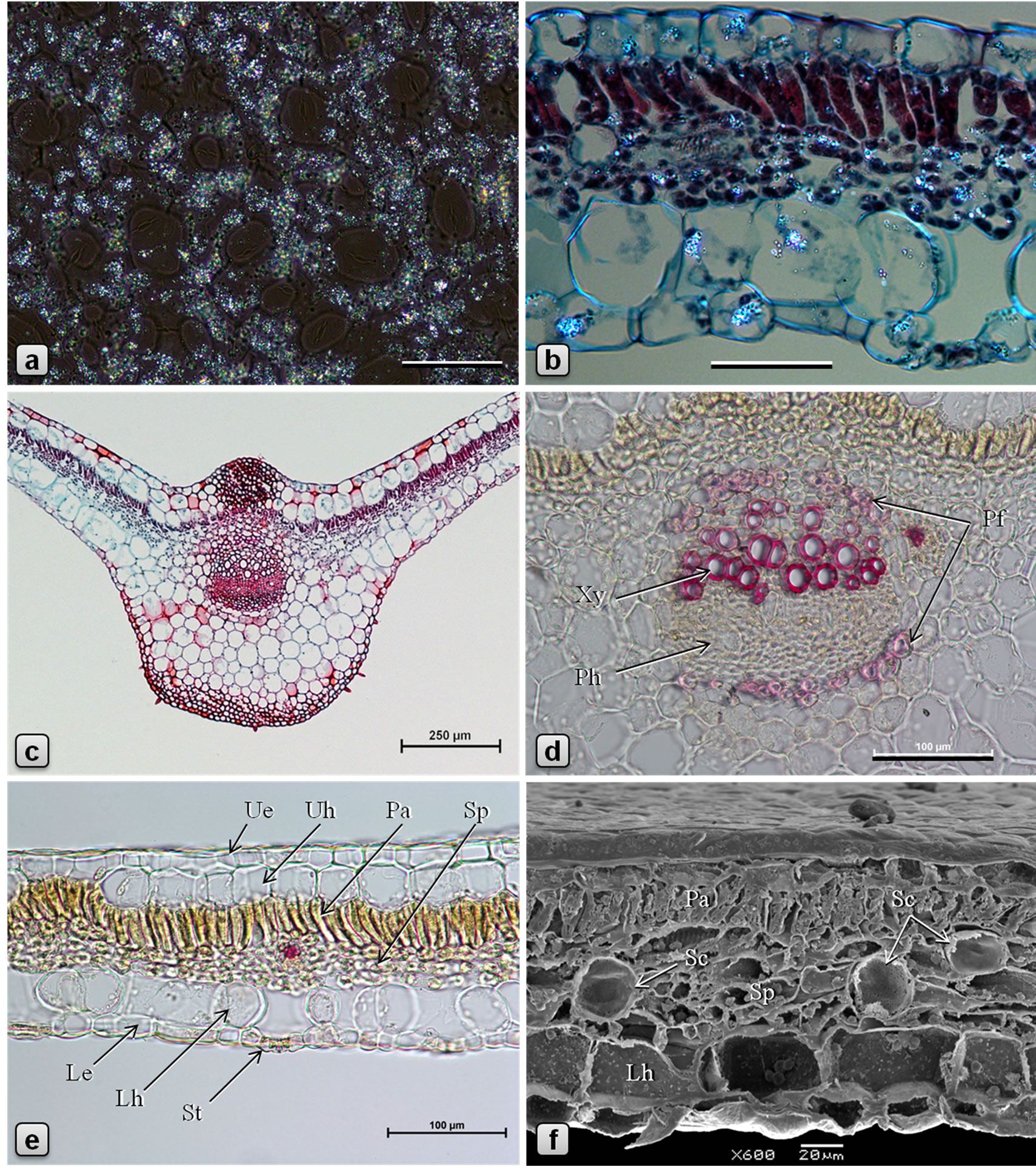
Figure 2. Leaf anatomy of Piper sarmentosum. [a, b: polarized light, partially crossed polarizers (PM); c, d, e: LM; f: SEM; b, c: stained in safranin/fast green; d, e: stained in phloroglucinol/HCl]. a: abaxial epidermis showing abundant sand crystals of calcium oxalate; b: a portion of TS of lamina showing distribution of calcium oxalate crystals in various tissues; c: TS of leaf through midrib; d: enlarged view of midrib vascular bundle in TS showing arcs of pericyclic fibers above xylem and below phloem; e, f: TS of leaf through lamina. (Le: lower/abaxial epidermis, Lh: lower hypodermis, Pa: palisade tissue, Pfpericyclic fibers, Ph: phloem, Sc: secretory cell, Sp: spongy tissue, St: stomata, Ue: upper/adaxial epidermis, Uh: upper hypodermis, Xy: xylem). Scale bars: a, b = 50 µm; c = 250 µm; d, e = 100 µm; f = 20 µm.
Products Research (NCNPR), in the School of Pharmacy, University of Mississippi, MS, USA.
2.1. Sample Preparation for Light Microscopy
Freshly collected samples were fixed in FAA for two days and sections were prepared using razor blades. The sections were treated with chloral hydrate solution and stained with phloroglucinol/HCl, and photomicrographs were prepared using Nikon E600 and Nikon E600 POL microscopes equipped with Nikon DS˗Fiv camera systems and Nikon Elements imaging software (Nikon Inc., Tokyo, Japan).
The fixed specimens were also used for the preparation of permanent slides. The specimens were dehydrated

Figure 3. Micromorphology and anatomy of Piper sarmentosum. (a: SEM; b-f: LM; b, d: stained in safranin/fast green; c, e, f: stained in phloroglucinol/HCl). a: surface of petiole showing parallely elongated epidermal cells and abundant nonglandular trichomes; b: TS of petiole showing U-shaped arrangement of vascular bundles and a central mucilage canal; c: TS of stem showing discontinuous ring of collenchymatous outer cortex, cortical and medullary whorls of vascular bundles and a large central mucilage canal; d: TS of a collateral medullary vascular bundle of stem surrounded by pith parenchyma; e: TS of aerial root showing wide cortex and pith separated by a narrow ring of vascular tissue; f: TS of root showing collenchymatous ring of outer cortex with sporadic fibers, wide parenchymatous inner cortex, wide ring of excentric xylem and a central pith. (Ca: cambium, Cb: cortical bundle, Cf: cortical fibers, Ck: cork, Co: collenchyma, Cx: cortex, Mb: medullary bundle, Mc: Mucilage canal, Pa: parenchyma, Ph: phloem, Pi: pith, Vb: vascular bundle, Xy: xylem). Scale bars: a, d = 100 µm; b, e, f = 500 µm; c = 250 µm.
using a series of alcohol solutions (10%, 20%, 30%, 50%, 70%, 95% and 100%) and passed through graded series of xylene: alcohol solutions up to 100% xylene. The specimens were then transferred to molten paraffin and specimen blocks were prepared using molds. Tissues embedded in blocks of paraffin wax were sectioned at thickness of 6 - 15 µm using a Leica RM2255 fully automatic rotary microtome (Leica Microsystems, Wetzlar, Germany) and the sections were stained by safranin and counter stained with Fast Green using routine protocols [13,14].
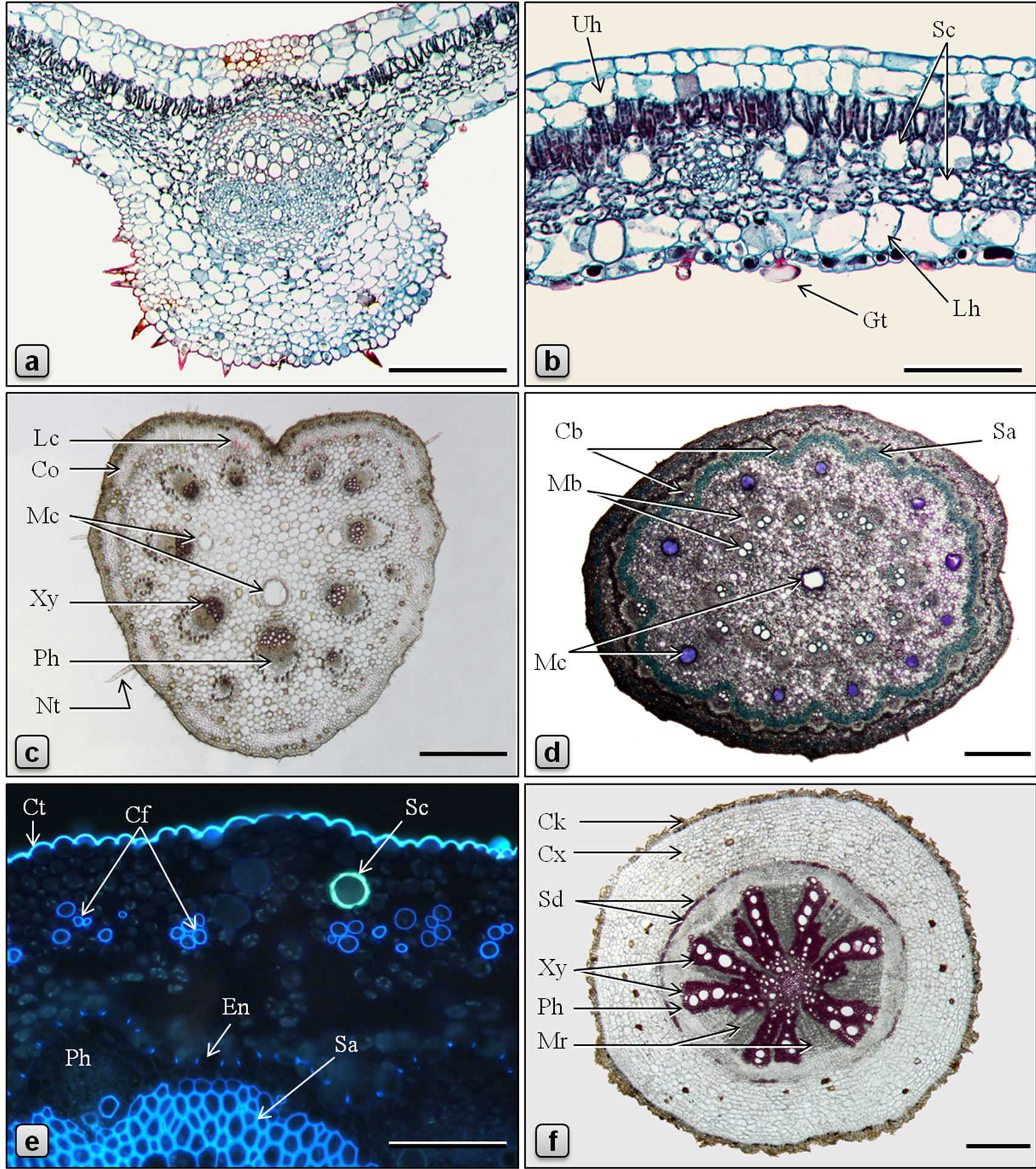
Figure 4. Anatomy of Piper betle. (a-d, f: LM; e: fluorescence microscopy, UV Ex = 330 - 380 nm; a, b: stained in safranin/fast green; d, e: stained in toluidine blue; c, f: stained in phloroglucinol/HCl). a: TS of leaf through the midrib; b: TS of leaf through lamina; c: TS of petiole; d: TS of stem showing a ring of mucilage canals between cortical and medullary whorls of vascular bundles and a central mucilage canal; e: a portion of TS of stem showing a secretory cell in the cortex, cortical fibers and endodermis with casparian thickening; f: TS of root. (Cb: cortical bundle, Cf: cortical fibers, Ck: cork, Co: collenchyma, Ct: cuticle, Cx: cortex, En: endodermis, Gt: glandular trichome, Lc: lignified cells, Lh: abaxial hypodermis, Mb: medullary bundle, Mc: mucilage canal, Mr: medullary ray, Nt: nonglandular trichome, Ph: phloem, Sa: sclerenchyma, Sc: secretory cell, Sd: sclereid, Uh: adaxial hypodermis, Xy: xylem, Ph: phloem). Scale bars: a, b, e = 100 µm; c, d, f = 500 µm.
2.2. Sample Preparation for Scanning Electron Microscopy (SEM)
Fresh samples of various parts of the plant were fixed overnight in gluteraldehyde (2%) solution, washed with water and dehydrated by passing through increasing concentrations of acetone in water according to a standard method [15]. The dehydrated specimens were then critical point dried in a Denton Vacuum critical point drier (Denton Vacuum, Moorestown, NJ, USA) using liquid CO2 as a cryogenic fluid. The fully dried samples were mounted on aluminum stubs using glued carbon tapes and then coated with gold using a Hummer 6.2 sputter
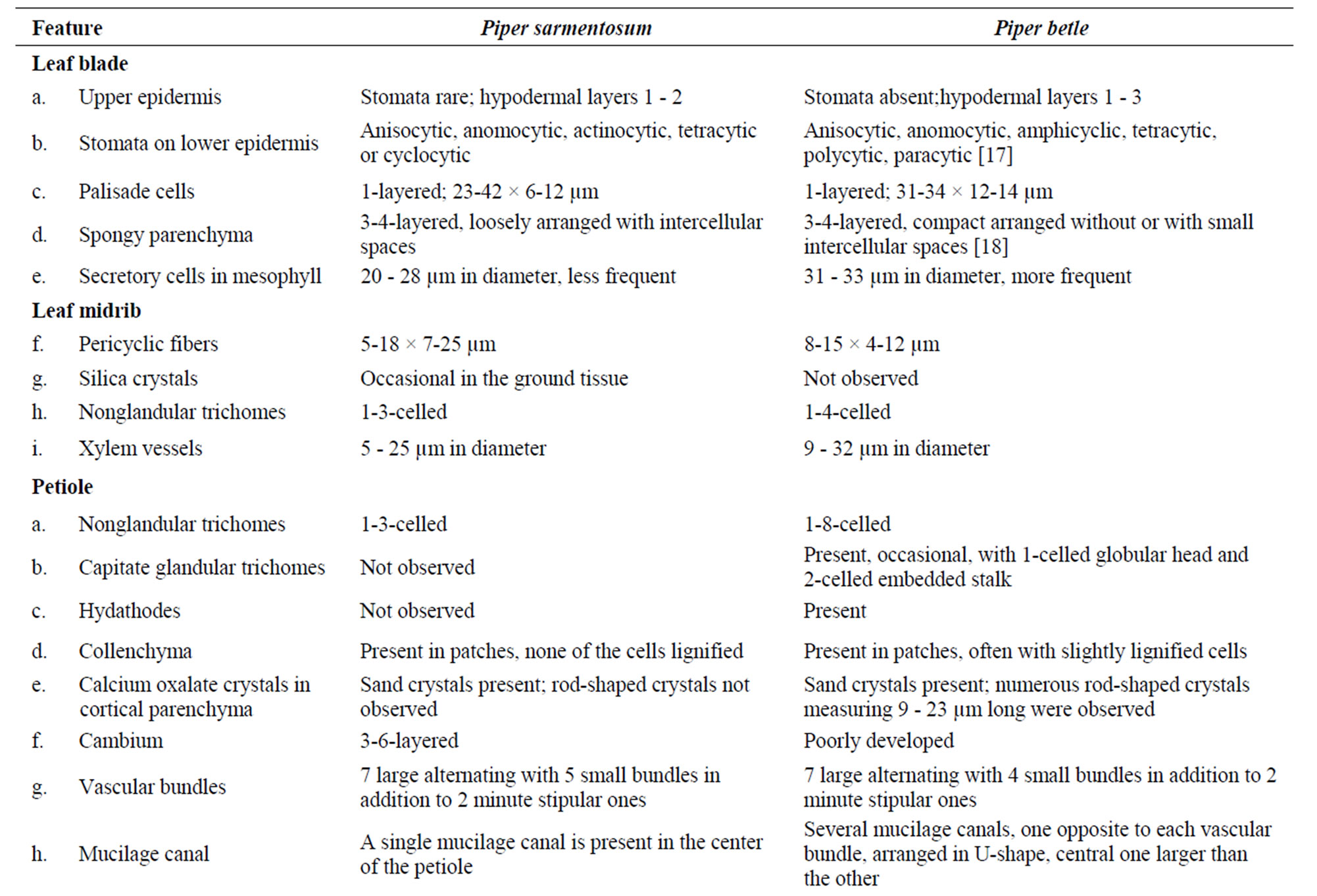
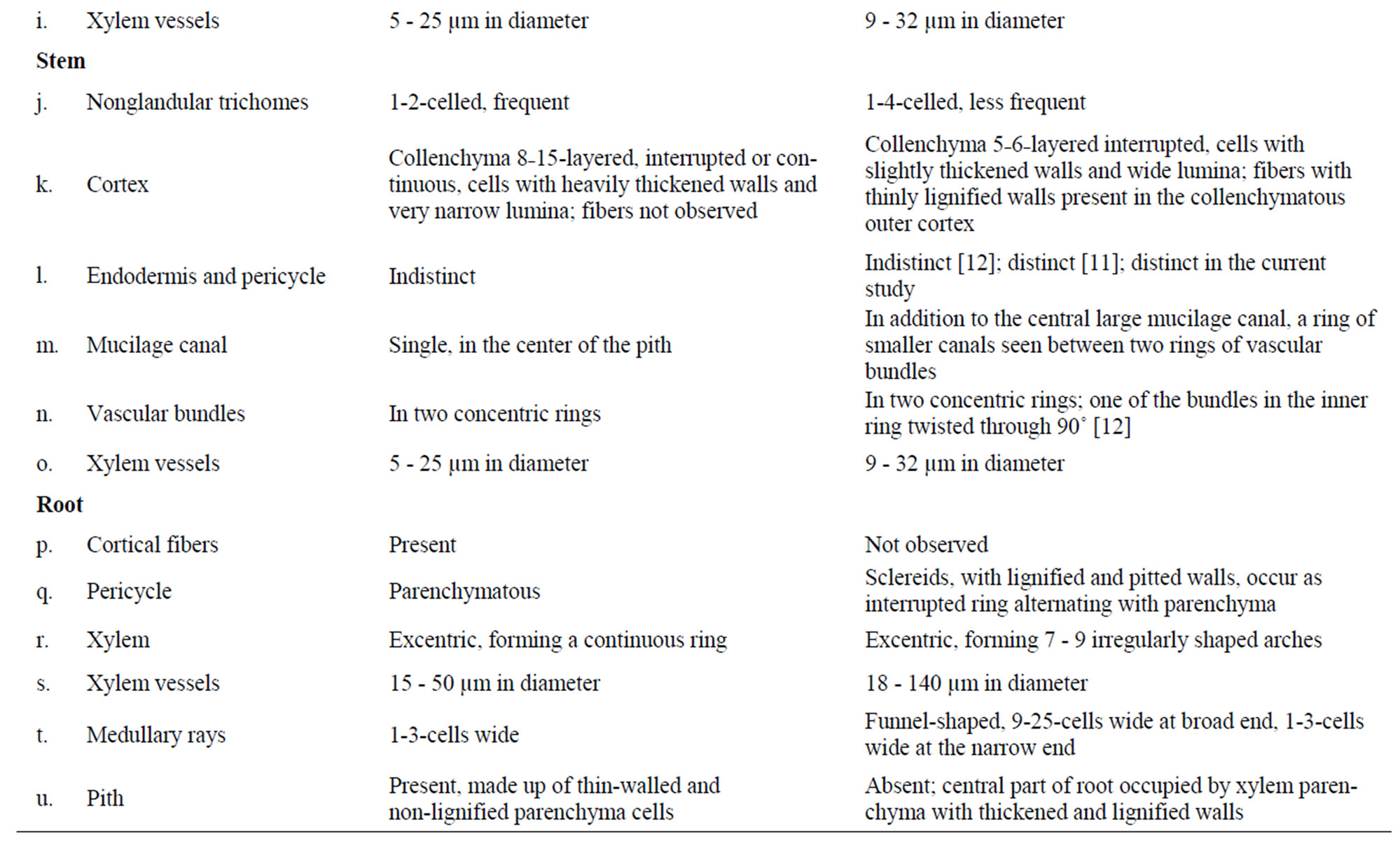
Table 1. Comparative anatomy of Piper sarmentosum and P. betle.
coater (Anatech USA, Union City, CA, USA) supplied with argon gas. Photomicrographs of the specimens were prepared using a model JSM-5600 SEM (JEOL Ltd., Tokyo, Japan).
3. Results and Discussion
3.1. Leaf (Figures 1-2)
Leaf dorsiventral in cross section (Figures 2(c)-(f)), 140 - 180 µm thick. Adaxial epidermis consists of an outer epidermal layer and 1-2 inner hypodermal layers; cells of epidermal layer square or tangentially elongated rectangular shaped and measuring 14 - 40 µm high and 12 - 53 µm long, in surface view (Figures 1(a), (b)), cells polygonal, measuring 23 - 57 µm long and 14 - 36 µm wide, with thin and straight or slightly curved anticlinal walls; cells on the veins are parallely elongated; cuticle thin, smooth; stomata rarely observed; glandular trichomes present mainly in the laminar region; non-glandular trichomes found on the veins. Hypodermal layers 1 - 2 in the vein and veinlet regions but absent in laminar region between veins; cells larger than those of epidermal layer, polygonal, 12 - 60 µm high, 15 - 65 µm wide, compactly arranged without intercellular space. Palisade cells 1- layered, columnar or funnel-shaped with broader adaxial end, 23 - 42 µm high, 6 - 12 µm in diameter, loosely arranged, straight or slightly curved. Spongy cells 3 - 5 layered, nearly circular, 9 - 20 µm in diameter, loosely arranged with airspaces. Secretory cells of 20 - 28 µm in diameter are present in the mesophyll. Lamina is traversed by several small vascular bundles to veinlets, with few xylem elements adaxially and phloem abaxially and surrounded by a unilayered parenchymatous bundle sheath. Abaxial epidermis consists of an epidermal layer and 1 - 2 hypodermal layers; cells of hypodermal layers polyhedral, rectangular or circular, 33 - 55 µm high, 30 - 70 µm wide, discontinuous at stomata forming large air spaces opposite to each stomata; cells of the epidermal layer are squarish, 8 - 20 µm high and 10 - 35 µm long; in surface view cells polygonal, measuring 15-50 × 7-25 µm, with straight or slightly curved and slightly irregularly thickened anticlinal walls; some of the cells contain yellow secretions; stomata abundant (Figures 1(c), (d)), of anisocytic, actinocytic, anomocytic, tetracytic and cyclocytic type [16], with 3 to 7 subsidiary cells arranged in one or more whorls, measuring 24 - 30 µm long and 20 - 27 µm wide; stoma about 20 µm long and 5 µm wide. Almost every cell in the adaxial epidermis contains prisms (10-13 µm × 3-6 µm), small druces and rods (8-13 µm × 4-5 µm) of calcium oxalate. Sand crystals of calcium oxalate were seen in almost every cell in the leaf, except vascular tissues. Minute prismatic crystals of calcium oxalate are seen in palisade and spongy cells; large silica crystals of varying size and shape are occasionally seen in hypodermal cells (Figures 2(a), (b)). Numerous hydathodes (Figure 1(a)) of 17 - 22 µm in diameter, some with inclusions, are observed on both the epidermis (Figures 1(a)-(f)).
Two types of trichomes are observed in P. sarmentosum: glandular (Figures 1(b), (e)) and non˗glandular (Figure 1(f)). Glandular trichomes [referred as “hydathode” by Chibber [11] and as “pearl-glands” by Vasuki et al. [9] in P. betle] present in the laminar region of the upper and lower surfaces; stalk 1-celled, narrow, sunken in the epidermis, surrounded by 5 - 7 radiating epidermal cells; body unicellular, ovate-oblong, up to 58 µm long and 14 µm in diameter, often containing yellow substance, curved or nearly parallel to the leaf surface, smooth and thin-walled, obtuse at apex and narrowed at base. Non-glandular trichomes present usually on the veins, on both surfaces of leaf but more densely on the abaxial surface, most commonly 1-celled but also frequently 2-3-celled, up to 37 µm long and 14 µm across, usually straight or rarely slightly curved, with broad base and more or less pointed apex, rarely containing yellowish brown content, outer wall warty.
Stomatal index = 3.8 - 4.5 - 6.7 Palisade ratio = 3 - 7 - 14 Vein islet number per mm2 = 1 - 2 - 3
3.2. Leaf Midrib
Midrib is prominently raised abaxially and slightly raised adaxially. Trichomes mostly confined to the abaxial surface. In transverse section, both adaxial and abaxial epidermal layers covered externally by thin cuticle and most cells with papillae of growing trichomes. Hypodermal layers of adaxial epidermis 2 - 3, but are replaced in the ridge by a patch of angular type of collenchyma, which is 10 - 15 cells wide and 10 - 15 cells high and the cells measuring 6 - 18 µm in height and 12 - 20 µm in width. Mesophyll continuous but undifferentiated in the midrib region, made up of circular chlorenchyma. Vascular bundle solitary, collateral; xylem adaxial, composed of vessels, tracheids, and xylem parenchyma; vessels 80 - 175 µm long and 5 - 26 µm in diameter, with spirally thickened and lignified walls; tracheids 360 µm long and 16 - 25 µm wide, with simple˗pitted and lignified walls; xylem parenchyma cells polygonal, 5 - 15 µm long and 5 - 23 µm wide; phloem abaxial, wide; cells polygonal and thin-walled, about 5 × 5 µm; phloem parenchyma 5 - 18 µm long and 5 - 15 µm wide; cambium usually indistinct, if present, then it is made up of 2 - 5 layers of radially arranged polygonal, tangentially elongated cells measuring 3 - 7 µm high and 4 - 10 µm wide. Arcs of pericyclic fibers are present above the xylem and below the phloem; fibers linear with pointed tips, 400 - 490 µm long, circular, oval or polygonal in cross section, having dimensions 5-18 µm × 7-25 µm, with thickened, lignified, and slit-shaped pitted walls. A few mucilage canals of circular or more or less rectangular shape in cross section, measuring 20 - 25 µm long and 10 - 15 µm wide, are seen in the phloem. Chlorenchyma cells of 1 - 2 cell wide are present on either sides of vascular bundle. Two additional very small vascular bundles, one on each side of the main vascular bundle, are sometimes present. Ground tissue is made up of loosely arranged, more or less circular parenchyma cells measuring 30 - 75 µm in diameter, some of them containing oil droplets. Most of the parenchyma cells in the ground tissue of the midrib are filled with numerous starch grains which are polyhedral or shell-shaped, usually solitary, measuring 2.3- 6.5 × 2.4-4.1 µm. Few secretory cells are scattered in the ground tissue. A patch of 3-5-layered angular collenchyma present adjacent to lower epidermis (Figures 2(c), (d)).
3.3. Petiole
Petiole grooved adaxially, heart˗shaped in cross section (Figure 3(b)). Epidermal layer made up of tabular or squarish cells measuring 15 - 23 µm long and 10 - 15 µm high, with dense cytoplasm, covered externally by cuticle of about 2 µm thick, most cells giving rise to 1-3-celled nonglandular trichomes (Figure 3(a)). Hypodermal layers 1 - 2, consisting of polygonal, circular or slightly tangentially elongated parenchyma cells. This is followed by 6 - 10 layers of angular collenchyma found as interrupted patches all around the petiole; cells polygonal with densely thickened walls and very narrow lumina. These collenchyma patches are separated from each other by thin-walled parenchyma of few to many cells wide. Vascular bundles collateral, few, arranged in a U-shaped arc in the ground tissue, larger and smaller bundles alternating with each other, and every bundle is positioned opposite to a collenchyma patch; phloem is facing outwards and the xylem facing the center of the petiole; xylem vessels 135 - 385 µm long and 14 - 31 µm wide, walls spiral or annular thickened, lignified, straight or obliquely pitted; some of the protoxylem elements slightly wavy; tracheids 165 - 240 µm long and 16 - 19 µm wide, with bluntly pointed tips, and spirally thickened and lignified walls. Vascular bundles are sheathed by 1-5-layered collenchyma with slightly thickened walls. Ground tissue made up of polygonal or circular parenchyma cells, most of them containing sand crystals of calcium oxalate, and abundant polyhedral or shell-shaped starch grains measuring 4.5 - 6.5 µm; few cells contain silica crystals of varying shape and size and few others contain oil droplets. A large lysigenous mucilage canal, 90 - 250 µm in diameter, is present at the center of the petiole.
3.4. Stem
Surface view of the epidermal layer of the stem exhibits polygonal cells with straight and slightly thickened anticlinal walls. Stomata present, similar to those of leaf. Nonglandular trichomes scattered, 1-2-celled. In transverse section (Figure 3(c)), the stem shows the following features: epidermis consists of an outer epidermal layer and an inner hypodermal layer; cells of epidermal layer tangentially elongated, measuring 10 - 17 µm high and 17 - 26 µm long, covered externally with thick cuticle, with scattered 1-2-celled nonglandular trichomes; cells of hypodermal layer are smaller, squarish. Outer cortex collenchymatous, 8 - 15 cells wide, forming a continuous ring beneath the hypodermis, if interrupted then the patches are separated by the extension of inner cortex; cells are of angular type, with heavily thickened walls and narrow lumina. Inner cortex parenchymatous, 5 - 10 cells wide; cells mostly circular, occasionally outer layers of cells polygonal and inner ones slightly tangentially elongated, 10 - 40 µm in diameter, most of the cells contain abundant starch grains and many contain oil droplets; circular secretory cells present in both outer and inner cortex. Vascular tissue is represented by twowhorls of cortical and medullary bundles; cortical bundles about 24, one-third of them are larger than the others, all embedded in a wavy ring of 3 - 6 cell wide lacunar collenchyma tissue made up of polygonal cells measuring 10 - 28 µm high and 5 - 20 µm wide, with heavily thickened walls; vascular bundles (Figure 3(d)) collateral with phloem above and xylem below, separated by cambium. Xylem composed of vessels, tracheids and xylem parenchyma; 2 - 4 mucilage ducts of 21 - 43 µm in diameter are found in xylem region; xylem vessels 17 - 35 µm in diameter, annular or spirally thickened; tracheids 120 - 190 µm long and 8 - 19 µm across, with pitted and lignified walls; xylem parenchyma polygonal, 5-18 × 4-12 µm, thin-walled. Phloem wide, cells polygonal, thin-walled, about 5 - 10 µm across. Cambium composed of 3 - 6 layers of tangentially elongated rectangular cells. Medullary vascular bundles about 5, similar to cortical bundles but larger in size, arranged in a ring in the middle region of pith; cambium cells tangentially elongated, rectangular, thin-walled, 7-12 × 3-6 µm, radially arranged; phloem parenchyma polygonal, 7-18 × 6-12 µm; sieve cells 5-15 × 3-10 µm. Secretory cells present in both phloem and xylem. Pith several-cells wide, parenchymatous, traversed by 5 large medullary vascular bundles in the middle; cells circular, 20 - 80 µm diameter, walls slightly thickened; starch grains abundant. The center of the stem is occupied by a large, lysigenous type mucilage duct. Minute prismatic crystals of calcium oxalate are rarely seen in the mucilage.
3.5. Aerial Root
Aerial roots are short, slender, cylindrical, brownish outgrowths from the lower nodes of stem. In transverse section (Figure 3(e)), the aerial root has the following features: Epidermis consists of 1-layer of squarish or tabular cells, externally with mycorrhiza, some cells give rise to short unicellular rhizoids. Occasionally, cork and cork cambium consisting of more or less tangentially elongated and radially arranged cells are seen. Cortex made up of 15 - 20 layers of polygonal or circular parenchyma cells, with fairly thickened walls. Cortex is followed by a layer of endodermis which consists of rectangular, tangentially slightly elongated cells, 12-25 × 10-17 µm, radial walls casparian-thickened. Pericycle made up of 1 - 2 layers of tabular parenchyma cells slightly tangentially elongated, 9-15 × 5-10 µm. Vascular tissue is arranged in a ring. Phloem occurs in patches; cells polygonal, thin-walled, 3 - 9 µm across; mucilage canals of 4 - 5 µm are rarely observed. Cambium partially distinct. Xylem composed of xylem vessels, xylem parenchyma, tracheids and fibers, all having lignified and thickened walls; xylem vessels usually circular or oval in cross sectional view, 14 - 40 µm long and 10 - 25 µm wide, having pitted and spirally thickened walls; tracheids with pitted walls; parenchyma cells polygonal, with pitted or reticulately thickened walls; and fibers with pitted, heavily thickened walls. Central part of root is occupied by parenchymatous pith with polygonal or circular cells measuring 14 - 35 µm in diameter. Occasional xylem vessels with spiral thickening, solitary or in groups of 2 - 3 are observed in the pith region. Most of cells in the cortex, pith and xylem parenchyma contain abundant starch grains measuring 1.5 - 2.5 µm in diameter, solitary or found in globular aggregations of 9 - 13 µm in diameter. Secretory cells with cell inclusions and parenchyma cells containing oil droplets are common in cortex and pith.
3.6. Root
The Transverse section of the primary main root is circular in outline (Figure 3(f)). Epidermis made up of 1-layer of tabular cells containing brown contents; some of the cells giving rise outwards to slender, unicellular rhizoids or root hairs. This is followed by 1 - 2 layers of meristematic parenchyma (phellogen). Outer cortex consists of a ring of 2 - 3 layers of thick-walled parenchyma; cells polygonal, 16 - 33 µm long, 10 - 26 µm high. Cortical fibers, solitary or less frequently in groups of 2 - 4, sporadically distributed in the outer cortex; cells with thickened, slightly to heavily lignified and striated walls, oval or circular in outline, 9 - 25 µm long, 9 - 17 µm wide. Inner cortex parenchymatous, 8 - 13 cell wide; with the inner most 5 - 6 layers arranged in radial rowscells are elliptical to semi-circular, tangentially slightly elongated, loosely arranged with relatively large intercellular spaces, thin-walled, 20 - 57 µm long, 9 - 43 µm high; almost every cell in the cortex is filled with starch grains, a few cells show brown contents. Endodermis unilayered, cells tabular, tangentially elongated, side walls with lignified casparian thickenings, 15 - 45 µm long, 5 - 20 µm high. Pericycle consists of 2-layers of tabular, tangentially elongated cells, measuring 14 - 27 µm long and 5 - 15 µm high; pericycle extends into the vascular tissue (up to mid level) as medullary rays which are 1-3-cells wide, made up of radially elongated thinwalled parenchyma cells. The stele is occasionally eccentric which imparts anomalous structure to the root anatomy. Phloem occurs as patches between medullary rays; cells polygonal, thin-walled, 3 - 12 µm long, 2 - 10 µm high. Cambium indistinct. Xylem elements forming a continuous ring of 190 - 335 µm wide, consisting of vessels, fibers and xylem parenchyma—all with lignified walls. Vessels 15 - 50 µm in diameter, with spirally thickened, pitted and lignified walls; xylem parenchyma consists of polygonal cells, with moderately thickened, pitted and lignified walls, radially arranged and somewhat elongated, measuring 6 - 31 µm in length, 6 - 27 µm in breadth. Pith occupies the central part of the root; cells polygonal or circular, 12 - 40 µm in diameter, filled with starch grains which are of two types: large, polyhedral grains measuring 8 - 10 µm in diameter and smaller shell-shaped grains measuring 2 - 5 µm in diameter. Mucilage canals absent.
4. Conclusion
In the current contribution, a detailed microscopy of the leaf, petiole, stem, aerial root, and root of Piper sarmentosum is provided (Figures 1-3). In addition, a comparative study of anatomy of leaf, petiole, stem and root of P. betle is provided (Table 1, Figure 4). The leaf anatomy of both the species are more or less comparable except that P. betle usually has more hypodermal layers in the adaxial epidermis and more of the secretory cells in the mesophyll region. Capitate glandular trichomes with stalk sunken in the epidermis; cortical fibers embedded in collenchyma; additional mucilage canals opposite to major vascular bundles; and numerous rod-shaped crystals of calcium oxalate in ground tissue are observed in the petiole only in P. betle. Of the two species, P. betle stem can easily be distinguished by the presence of cortical fibers as well as an additional ring of mucilage canals while these features are not observed in P. sarmentosum. The root anatomy in P. sarmentosum shows cortical fibers in outer cortex; parenchymatous pericycle; a continuous ring of xylem with 1-3-cell wide shallow medullary rays; and well defined parenchymatous pith. Whereas, in P. betle, cortical fibers are not observed; pericycle consists of incomplete ring of lignified and pitted sclereids; xylem forming 7 - 9 irregularly-shaped arches separated by 9-25-cells wide medullary rays; and the pith is replaced by lignified and thickened-walled xylem parenchyma.
5. Acknowledgements
This research was supported in part by Science Based Authentication of Dietary Supplements funded by the Food and Drug Administration grant no. 1U01FD 004246- 02; and the United States Department of Agriculture, Agricultural Research Service, Specific Cooperative Agreement No. 58-6408-2-0009. We thank Dr. Aruna Weerasooriya, Senior Research Scientist at the medicinal plants garden, National Center for Natural Products Research, School of Pharmacy, The University of Mississippi, USA for providing and authenticating plant samples for this study.
REFERENCES
- D. J. Mabberley, “Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses,” 3rd Edition, Cambridge University Press, Cambridge, 2008.
- S. P. Mathew, A. Mohandas and G. M. Nair, “Piper sarmentosum Roxb.—An Addition to the Flora of Andaman Islands,” Current Science, Vol. 87, No. 2, 2004, pp. 141-142.
- P. C. M. Jansen, “Piper sarmentosum Roxb. ex Hunter,” In: C. C. de Guzman and J. S. Siemonsma, Eds., Plant Resources of South-East Asia No. 13: Spices, Backhuys Publisher, Leiden, 1999.
- Y.-C. Tseng, N. Xia and M. G. Gilbert, “Piperaceae,” In: W. Zhengyi and P. H. Raven, Eds., Flora of China-Cycadaceae through Fagaceae, Vol. 4, Science Press, Beijing and Missouri Botanical Garden Press, St. Louis, 1999.
- M. G. Gilbert and N. H. Xia, “Notes on the Piperaceae of China,” Novon, Vol. 9, No. 2, 1999, pp. 190-198. doi:10.2307/3391797
- C. Wiart, “Medicinal Plants of Asia and the Pacific,” CRC Press, Boca Raton, 2006.
- H. Solereder, “Systematic Anatomy of the Dicotyledons —A Handbook for Laboratories of Pure and Applied Botany,” Vol. 2, The Clarendon Press, Oxford, 1908.
- B. Seetha Lakshmi and K. C. Naidu, “Comparative Morphoanatomy of Piper betle L. Cultivars in India,” Annals of Biological Research, Vol. 1, No. 2, 2010, pp. 128-134.
- K. Vasuki, R. Senthamarai, T. Shri Vijaya Kirubha, P. Balasubramanian and S. Selvadurai, “Pharmacognostical Studies on Leaf of Piper betle,” Der Pharmacia Lettre, Vol. 3, No. 5, 2011, pp. 232-235.
- C. R. Metcalfe and L. Chalk, “Anatomy of the Dicotyledons,” Vol. 2, Clarendon Press, Oxford, 1950.
- H. M. Chibber, “The Morphology and Histology of Piper betle, L. (the Betle Vine),” Journal of the Linnaean Society, Botany, Vol. 41, 1912, pp. 357-383. doi:10.1111/j.1095-8339.1913.tb02484.x
- Y. S. Murty, “Studies in the Order Piperales. IV. A Contribution to the Study of Vegetative Anatomy of Three Species of Piper,” Proceedings of the National Institute of Sciences in India, Vol. 25, Part B, 1973, pp. 31-38.
- C. J. Chamberlain, “Methods in Plant Histology,” University of Chicago Press, Chicago, 1901.
- S. E. Ruzin, “Plant Microtechnique and Microscopy,” Oxford University Press, New York, 1999.
- M. A. Hayat, “Principles and Techniques of Electron Microscopy: Biological Applications,” 4th Edition, Cambridge University Press, New York, 2000.
- W. R. J. Van Cotthem, “A Classification of Stomatal Types,” Botanical Journal of the Linnean Society, Vol. 63, No. 3, 1970, pp. 235-246. doi:10.1111/j.1095-8339.1970.tb02321.x
- D. D. Pant and R. Banerji, “Structure and Ontogeny of Stomata in Some Piperaceae,” Botanical Journal of the Linnean Society, Vol. 59, No. 378, 1965, pp. 223-228. doi:10.1111/j.1095-8339.1965.tb00059.x
- P. C. Datta and A. Dasgupta, “Comparison of Vegetative Anatomy of Piperales. II. Leaves,” Acta Biologica Academiae Scientiarum Hungaricae, Vol. 28, 1977, pp. 97-110.

