Pharmacology & Pharmacy
Vol.5 No.7(2014), Article
ID:47370,11
pages
DOI:10.4236/pp.2014.57083
Radix Polygoni Multiflori Praeparata and Dioscorea Bulbifera Rhizome Decoctions Display Combined Effects Detected by a Three-Probe Drug Cocktail with Substrates of Rat Hepatic Cytochrome P450 Enzymes
Li Jiang, Pingping Shan, Hui Yu, Jiayuan Tao, Chunyan Gong, Guoqing Shen*
Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, China
Email: *shenguoqing2012@aliyun.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 7 April 2014; revised 19 May 2014; accepted 17 June 2014
ABSTRACT
Objectives: Radix Polygoni Multiflori Praeparata (RPMP) and Dioscorea Bulbifera Rhizomes (DBR) are used in Chinese herbal medicine and have been frequently reported for adverse reactions on liver. In this research, we aimed to evaluate in vivo effects of RPMP and DBR on rat cytochrome P450 enzymes (CYP1A2, CYP2E1 and CYP3A2) with their respective substrates as probes. Methods: Rats were orally administered RPMP, DBR and RPMP/DBR combination at 12, 10 and (12 + 10) g/kg, respectively, or saline as a control, once daily for 7 days. Thereafter, a cocktail containing 10 mg/kg caffeine, 20 mg/kg chlorzoxazone and 10 mg/kg dapsone was tail vein injected to rats. At defined time points, plasma drug concentrations were simultaneously evaluated by HPLC. Pharmacokinetic parameters simulated by DAS software were used to assess RPMP and/or DBR effects on cytochrome P450 enzymes activity. ANOVA and Dunnett’s test were used for data analysis. Results: Caffeine metabolism was enhanced in RPMP animals and reduced after pretreatment with DBR, but no effect was observed in RPMP/DBR combination group. Chlorzoxazone and dapsone metabolism was enhanced in both RPMP and DBR groups and consequently in combination group. The data suggested that RPMP independently induces rat CYP1A2, CYP2E1 and CYP3A2 activity, while DBR independently inhibits activity of rat CYP1A2 and induces that of CYP2E1 and CYP3A2. RPMP/DBR combination showed no significant benefit compared with the two drugs alone and even showed a neutralized effect in CYP1A2 activity. Conclusions: Caution is needed when RPMP and/or DBR are co-administered with drugs metabolized by human CYP1A2, CYP2E1 and CYP3A4.
Keywords:Radix Polygoni Multiflori Praeparata, Dioscorea Bulbifera Rhizomes, Cytochrome P450, Herb-Drug Interactions, Three-Probe Drug Cocktail

1. Introduction
Plant products are increasingly popular as alternative medicines in the western world, and it is estimated that herbal medicines are now used by approximately 20% of the US population [1] . Radix Polygoni Multiflori (RPM, Heshouwu in Chinese) and Radix Polygoni Multiflori Praeparata (RPMP, Zhiheshouwu in Chinese) are root extracts of Polygonum multiflorum Thunb (Polygonaceae) used in oriental counties for centuries. Varieties of methods are used in RPM processing, with steaming with or without black soybean decoction being the most frequently used as reported by the Pharmacopoeia of the People’s Republic of China, 2010 edition [2] . RPMP, primarily used to tonify kidney and liver, has been also utilized in the treatment of premature hair graying, vitiligo and hyperlipemia. Dioscorea Bulbifera Rhizomes (DBR) constitutes a Chinese traditional medicine first described in “Qianjin Yueling” written in Tang dynasty [3] . Studies have revealed that DBR possess a wide range of pharmacological properties, including anti-tumor [4] -[8] , anti-inflammatory [9] [10] , anti-viral [11] and goiter inhibitory [12] effects. Indeed, DBR has been clinically used to treat struma and many tumor types in China for several decades [13] [14] . Historically, RPMP and DBR were first used in combination in Bai-Shi Pill, an ancient traditional Chinese medicine described in “Synopsis of the Golden Chamber” in the beginning of the third century. The Bai-Shi Pill has gained prestige for treating vitiligo for many years.
Many consumers have the misconception that herbal medicines are natural and therefore safe. However, recent findings have reported that long-term and excessive use of RPMP and DBR could result in liver damage, and even death [15] [16] . Recently, it was shown that processing of Radix Polygoni Multiflori and Radix Polygoni Multiflori Praeparata could result in hepatotoxic effects although the crude extracts did not induce liver injury [17] .
Multiple drug therapy is a common therapeutic practice, particularly in patients with several diseases or in a complex state. Herb-drug interactions (HDIs) may be significantly underreported and underestimated, and could be actually more frequent than drug-drug interactions. Indeed, a recent survey demonstrated that 15% of patients used herbal medicines while potential adverse HDIs were reported in 40% of herbal medicine users [18] . Potential interaction between herbal medicines and conventional drugs is a major safety concern, especially, for drugs with narrow therapeutic indexes (e.g. warfarin and digoxin), and may lead to severe adverse reactions possibly life-threatening. Therefore, there is a strong need to characterize, analyze and predict these interactions.
Several Chinese herbal medicines were reported with potential CYP inhibitory activity resulting in HDIs with conventional prescription drugs. For example, Sanqi Panax Notoginseng injection alters rat liver microsome CYP3A activity in vitro, as a mixed inhibitor [19] ; Salvia tablet, taken for a week, was shown to significantly increase CYP1A2 activity in a clinical study [20] ; Sanqi Panax Notoginseng injection significantly induces CYP2E1 activity in vivo, but has no effect on CYP3A4 and CYP1A2 [21] .
Cytochrome P450 (CYP) is a mixed-function oxygenase system, which was first discovered in 1954 [22] . In humans, CYP enzymes are involved in the metabolism of exogenous substances (drugs, alcohols, anti-oxidants, organic solvents, anesthetic agents, dyes, environmental pollutants and chemicals) producing compounds which may be inactive, toxic or carcinogenic [23] [24] . They are also important in the metabolism of endogenous physiological compounds such as steroids, bile acids, fatty acids, prostaglandins, biogenic amines and retinoids [25] [26] . Among numerous CYPs identified to date, the three human CYP enzymes (CYP1A2, CYP2E1 and CYP3A4) are responsible for approximately 70% of CYP-mediated drug metabolism [27] -[29] . These enzymes were chosen for this study.
However, no comprehensive study has been reported emphasizing the impact of RPMP and DBR combination on the activity of liver CYP enzymes. Therefore, we aimed in this work at exploring how RPMP and DBR affect the regulatory mechanisms of rat hepatic CYP enzymes, with caffeine, chlorzoxazone, and dapsone respectively, as probe drug of CYP 1A2, 2E1 and 3A2 [30] [31] . Though humans and animals differ in isoform composition, expression and catalytic activities of drug-metabolizing enzymes, animal models are commonly used in the preclinical development of new drugs to predict the metabolic behavior in humans [32] . Interestingly, human CYP1A2, CYP2E1 and CYP3A4 are homologs to rat CYP1A2, CYP2E1 and CYP3A2, respectively.
2. Materials and Methods
2.1. Chemicals and Reagents
Caffeine, chlorzoxazone, dapsone, and antipyrine (internal standard) were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). HPLC-grade methanol was supplied by Tedia Company Inc. (OH, USA). All other reagents were of analytical grade and purchased from the Nanjing Chemical Reagent Company (Nanjing, China). Double distilled water was used in these experiments.
2.2. Authentication of Radix Polyoni Multiflori Preparata (RPMP) and Dioscoreae Bulbiferae Rhizomes (DBR)
The raw herbs of RPMP and DBR were purchased from Jiangsu Province Pharmaceutical Company and authenticated by morphological characterizations and thin layer chromatography in accordance with the Chinese Pharmacopoeia.
2.3. Preparation of RPMP and DBR Extracts
For extract preparation, raw RPMP and DBR were cut into small pieces, soaked in 10 volumes of distilled water for 30 min, and boiled twice for 1 h. The extracts were concentrated to 2.5 and 2 g/ml for RPMP and DBR, respectively. RPMP/DBR combination was made of a 1:1 volume ratio mixture of the extracts, resulting in a concentration of (1.25 + 1) g/ml mixture.
2.4. Animals and Treatment
Sprague-Dawley rats (200 ± 20 g) were obtained from the Department of Laboratory Animal Science, Nanjing Medical University. Rats were allowed one week acclimation period before experiment start, in a temperatureand humidity-controlled room with an alternating light/dark cycle of 12 h, with standard rodent chow and water ad libitum. All experimental procedures were in compliance with the Guide for Care and Use of Laboratory Animals (NIH version, revised 1996).
96 rats were randomly divided into 4 groups (n = 24) set to receive 0.9% sodium chloride solution (blank control, BCG), 12 g/kg RPMP (RPMP), 10 g/kg DBR (DBR) and (12 + 10) g/kg RPMP/DBR combination (MIX), daily for 7 days. 24 rats in each group were randomly divided into 4 sub-groups (n = 6) by blood collection points (see Table 1), and blood samples were collected from 6 rats in sub-group at three blood collection points.
Table 1. Schedule for blood sample collection.
Δ: sampling point.
2.5. Application to Pharmacokinetic Study
On the eighth day, a cocktail solution at a dose of 5 ml/kg containing caffeine (10 mg/kg), chlorzoxazone (20 mg/kg) and dapsone (10 mg/kg) was injected by caudal vein to all rats. Blood samples were collected into heparinized centrifuge tubes from the femoral vein pre-dosing, and 10 min, 20 min, 40 min, 1 h, 1.5 h, 2 h, 3 h, 5 h, 8 h, 12 h and 24 h post-dosing (see Table 1). After collection, blood samples were immediately centrifuged for 10 min at 3500 rcf and the resulting plasma samples frozen in polypropylene tubes at −20˚C until use.
For pharmacokinetic assays, 200 μl plasma were added to 20 μl internal standard antipyrine and mixed by vortex for 30 s. The mixture was extracted with 3.0 ml dichloromethane, mixed for 3 min, and centrifuged for 10 min at 3500 rcf. Then, exactly 2.6 ml of the organic phase were transferred to a clean tube and evaporated to dryness under nitrogen. The resulting residue was dissolved in 200 μl of the mobile phase, and 20 μl were injected into the HPLC instrument.
2.6. Instrument and Chromatographic Conditions
Chromatography analysis was performed using a Waters 2695 HPLC system (Waters, USA) with a binary pump, an online vacuum degasser, a thermo-regulated column compartment, a diode-array detector, and an autosampler. Chromatographic data were recorded and processed with the ChemStation10.02 browser. Three probe drugs were analyzed by a simplified HPLC method. The separation was carried out at 30˚C using a Phenomenox Luna-C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase consisted of a mixture of acetonitrile (A) and 0.1% phosphonic acid (B). Gradient elution was performed as follows: 1) mobile phase A was at 20% at 0 min; 2) a linear gradient was increased to 35% A from 0.0 to 10.0 min; 3) a linear gradient was increased to 40% A from 10.0 to 15.0 min; 4) a linear gradient was decreased to 20% A from 15.0 to 20.0 min; 5) an isocratic elution was maintained at 20% A from 20.0 to 25.0 min. Samples were run at a flow rate of 1.0 ml/min and absorbance was read at 280 nm with antipyrine used as internal standard. The concentrations of the three probe drugs were evaluated from peak area ratio.
2.7. Data Analysis
Data were presented as mean ± S.D. Pharmacokinetic parameter calculations were carried out using the DAS 2.0 pharmacokinetic program (Chinese Pharmacological Society, Beijing, China), and generated by a non-compartmental model (statistical moment). Statistically significant differences in pharmacokinetic parameters between treatment groups were assessed by one-way analysis of variance (ANOVA) followed by Dunnett’s test with the level of statistical significance setting at 0.05.
3. Results
After intravenous injection, the concentrations of three probe drugs were simultaneously determined by HPLC. The effect of RPMP and DBR on the three rat CYP enzymes was evaluated through the pharmacokinetic parameters of probe substrates.
3.1. Effects of RPMP and DBR on Rat Hepatic CYP1A2
The effects of the different Chinese herbal decoctions on pharmacokinetic parameters including the areas under the curve, AUC(0 − t) and AUC(0 − ∞); mean residence time, MRT(0 − t) and MRT(0 − ∞); half-life time (t1/2); drug clearance (CL) and apparent volume of distribution (Vz) for caffeine in rats are presented in Table2 Mean negative logarithm plasma ratio of concentration (C) to initial concentration (C0)-time curves of caffeine in different groups are presented in Figure 1(a). After pretreatment with RPMP, AUC(0 − t) and AUC(0 − ∞) values of caffeine were reduced by 40.7% and 42.2% in comparison with those of BCG (P < 0.01). Still for caffeine, MRT(0 − t) and t1/2 were also decreased by 27.2% and 41.0%, respectively, (P < 0.05), while CL increased significantly by 66.7% (P < 0.01). These data demonstrate that RPMP increased CYP1A2 metabolism of caffeine.
After pretreatment with DBR, AUC(0 − t) and AUC(0 − ∞) of caffeine increased significantly by 31.8% (P < 0.05) and 59.7% (P < 0.01) compared with values obtained in BCG. In addition MRT(0 − t), Vz and t1/2 increased significantly by 36.7%, 37.3% (P < 0.05) and 119.8% (P < 0.01), respectively, while CL of caffeine was significantly reduced by 38.9% (P < 0.05), demonstrating that DBR decreased CYP1A2 metabolism of caffeine.
After pretreatment with MIX, which represents the combination of RPMP and DBR, no apparent effects were observed. These results indicated that RPMP and DBR had contrary effect on CYP1A2 activity in vivo and the effects of the two herbs would be neutralized in a mix recipe.
3.2. Effects of RPMP and DBR on Rat Hepatic CYP2E1
The effects of Chinese herbal decoctions on pharmacokinetic parameters (AUC(0 − t), AUC(0 − ∞), MRT(0 − t), MRT(0 − ∞), t1/2, CL and Vz) of chlorzoxazone in rats are presented in Table3 Mean −logC/C0-time curves of chlorzoxazone in different groups are presented in Figure 1(b). After pretreatment with RPMP, AUC(0 − t) and AUC(0 − ∞) of chlorzoxazone decreased significantly by 55.5% and 43.2% in comparison with BCG (P < 0.01). MRT(0 − t) and t1/2 were also significantly reduced by 44.3% and 72.3% (P < 0.01), while CL of chlorzoxazone was enhanced by 187.0% (P < 0.01). These data demonstrate that RPMP increased CYP2E1 metabolism of chlorzoxazone.
Table 2. Main pharmacokinetic parameters of caffeine (10 mg/kg) in untreated and Chinese herbal decoction pretreated rats.
BCG (control group): 0.9% sodium chloride solution, 5 ml/kg for 7 days. RPMP: Radix Polygoni Multiflori Praeparata decoction, 12 g/kg, P.O., once daily for 7 consecutive days. DBR: Dioscorea Bulbifera Rhizome decoction, 10 g/kg, P.O., once daily for 7 consecutive days. MIX: RPMP, 12 g/kg and DBR, 10 g/kg, P.O., once daily for 7 consecutive days. *P < 0.05 compared with related parameters in BCG rats. **P < 0.01 compared with related parameters in BCG rats.
Table 3. Main pharmacokinetic parameters of chlorzoxazone (20 mg/kg) in untreated and Chinese herbal decoction pretreated rats.
BCG (control group), RPMP, DBR, MIX: the same as Table2 *P < 0.05 compared with related parameters in BCG rats. **P < 0.01 compared with related parameters in BCG rats. ▲P < 0.05 compared with related parameters in MIX rats. ▲▲P < 0.01 compared with related parameters in MIX rats.
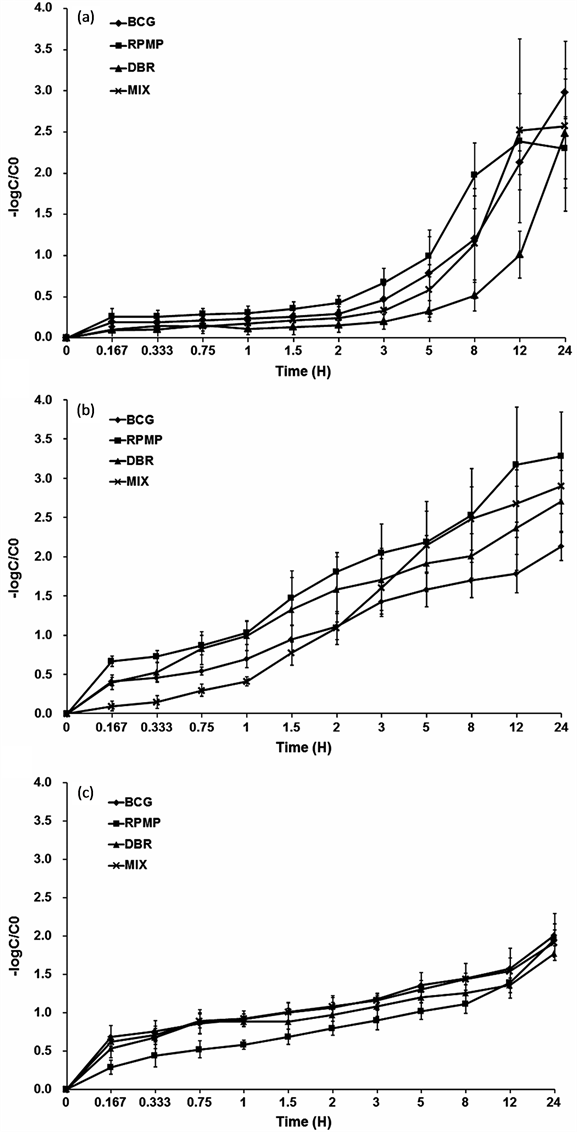
Figure 1. Negative logarithm plasma ratio of concentration (C) to initial concentration (C0)-time curves of (a) Caffeine (10 mg/kg); (b) chlorzoxazone (20 mg/kg); (c) dapsone (10 mg/kg) in untreated, and RPMP, DBR, and RPMP/ DBR pretreated rats (n = 6). BCG (0.9% sodium chloride solution for 7 days). RPMP (RPMP, 12 g/kg, P.O., once daily for 7 consecutive days). DBR (DBR, 10 g/kg, P.O., once daily for 7 consecutive days). MIX (RPMP, 12 g/kg and DBR, 10 g/kg, P.O., once daily for 7 consecutive days). Error bars represent S.D.
After pretreatment with DBR, AUC(0 − t) and AUC(0 − ∞) of chlorzoxazone were reduced by 50.9% (P < 0.01) and 57.7% (P < 0.05) compared with those obtained for BCG. The t1/2 decreased significantly by 36.4% (P < 0.05) while CL of chlorzoxazone increased by 119.6% (P < 0.01) in the DBR group, demonstrating that DBR has the same effect on CYP2E1 metabolism of chlorzoxazone.
After pretreatment with MIX, AUC(0 − t) and AUC(0 − ∞) of chlorzoxazone were significantly reduced by 39.2% and 56.4% (P < 0.01) compared with those of BCG. MRT(0 − t) and t1/2 also decreased by 62.9% and 60.2% (P < 0.01), with CL of chlorzoxazone significantly increasing by 62.5% and 49.3% (P < 0.01). Vz values were decreased by 78.3% in MIX animals (P < 0.01). The data indicated that MIX treatment increased the CYP2E1 metabolism of chlorzoxazone but was not significantly different from RPMP or DBR alone.
To sum up, chlorzoxazone metabolism was obviously enhanced in RPMP and DBR groups, suggesting that RPMP and DBR had the potential to increase rat hepatic CYP2E1 activity. Nevertheless, chlorzoxazone metabolism in the MIX group showed no superimposed effect.
3.3. Effects of RPMP and DBR on Rat Hepatic CYP3A2
The effects of Chinese herbal decoctions on pharmacokinetic parameters (AUC(0 − t), AUC(0 − ∞), MRT(0 − t), MRT(0 − ∞), t1/2, CL and Vz) of dapsone in rats are presented in Table4 Mean −logC/C0-time curves of dapsone in different groups are presented in Figure 1(c). After pretreatment with RPMP, AUC(0 − t) and AUC(0 − ∞) of dapsone were significantly reduced by 74.4% and 84.0% (P < 0.01) compared with those of BCG. MRT(0 − t) and t1/2 decreased significantly by 63.6% and 70.8% (P < 0.01), while CL and Vz of dapsone were enhanced by 100.0% and 139.9% (P < 0.01). These data demonstrate that RPMP increased CYP3A2 metabolism of dapsone.
After pretreatment with DBR, AUC(0 − t) and AUC(0 − ∞) values of dapsone were significantly reduced by 44.7% (P < 0.01) and 36.8% (P < 0.05) compared with those of BCG. The t1/2 decreased by 33.0% (P < 0.05) while CL and Vz of dapsone were enhanced by 50.0% and 137.8%, respectively (P < 0.01), demonstrating that DBR increased CYP3A2 metabolism of dapsone.
After pretreatment with MIX, AUC(0 − t) and AUC(0 − ∞) of dapsone decreased significantly by 31.8% and 47.3% (P < 0.05) compared with the values obtained for BCG. The t1/2 values were decreased by 26.4% (P < 0.05), while CL and Vz of dapsone were significantly increased by 62.5% and 49.3%, respectively (P < 0.01). The data indicated that MIX treatment increased the CYP3A2 metabolism of dapsone but was not significantly different from RPMP or DBR alone.
To sum up, dapsone metabolism was obviously enhanced in RPMP and DBR groups, suggesting that RPMP and DBR had the potential to induce rat hepatic CYP3A2 activity. Nevertheless, dapsone metabolism in the MIX group showed no superimposed effect.
Table 4. Main pharmacokinetic parameters of dapsone (10 mg/kg) in untreated and Chinese herbal decoction pretreated rats.
BCG (control group), RPMP, DBR, MIX: the same as Table2 *P < 0.05 compared with related parameters in BCG rats. **P < 0.01 compared with related parameters in BCG rats. ▲P < 0.05 compared with related parameters in MIX rats. ▲▲P < 0.01 compared with related parameters in MIX rats.
4. Discussion
Vitiligo is an acquired pigmentary disorder of unknown etiology that is clinically characterized by the development of white macules related to selective loss of melanocytes. The prevalence of this disease is around 1% in the United States and in Europe [33] , around 0.7% in China, but ranges from less than 0.1% to greater than 8% worldwide [34] . Common therapies include topical corticosteroids, calcineurin inhibitors and phototherapy.
To date, RPMP and DBR are among the leading herbal drugs used by vitiligo patients in China, often concomitantly with synthetic drugs. Therefore there is an increasing need to elucidate/identify potential interactions between herbs and synthetic drugs to allow safe and effective use of RPMP and DBR. Hence, we investigated the effect of these herbal drugs on rat CYP1A2, CYP2E1 and CYP3A2 activity.
CYP1A2 is one of the major P450 enzymes in human liver which accounting for approximately 13% of total content of this enzyme group [35] . CYP1A2 plays an important role in the metabolism of several clinically used drugs including caffeine, theophylline, clozapine, and tacrine, and foodborne procarcinogens such as polycyclic aromatic hydrocarbons or imidazoquinoline derivatives [36] [37] . Using caffeine as probe, we found that RPMP and DBR independently induced and inhibited rat CYP1A2 activity, respectively. However, treatment with RPMP/DBR combination (i.e. 12 g/kg RPMP and 10 g/kg DBR) resulted in no effects on rat CYP1A2.
CYP2E1 is another major member of the cytochrome P450 enzyme family and is involved in the metabolism of a number of low molecular mass xenobiotics, including ethanol, long chain fatty acids, chlorzoxazone, and anaesthetics such as enflurane, sevoflurane, methoxyflurane and isoflurane. Drugs such as paracetamol and isoniazid also count among CYP2E1 substrates that exhibit toxicological and clinical significance. Using chlorzoxazone, it was shown that phenobarbital induces monkey brain CYP2E1 protein but not hepatic CYP2E1, in vitro or in vivo [38] . The hydrophobic active site of CYP2E1 is described as the smallest in all CYP enzymes, enabling the enzyme to accommodate low molecular mass substrates [39] . Our data showed that RPMP and DBR induced rat CYP2E1 independently, with RPMP/DBR combination showing the same induction as RPMP alone.
CYP3A enzyme, one of the dominant CYP enzymes in both liver and extra-hepatic tissues such as intestine, plays an important role in the oxidation of xenobiotics and contributes to the metabolism of about 60% of drugs [40] [41] . Human CYP3A4 is one of the most abundant drug-metabolizing P450 isoforms in human liver microsomes, accounting for approximately 40% of total P450. Therefore, characterization of the CYP3A4 isoform responsible for the metabolism of drugs and herbal constituents is important for identification of potential drug-drug or herb-drug interactions in humans [42] [43] , especially since CYP3A4 significantly contributes to drug metabolism in the small intestine [40] . Our data showed that RPMP and DBR induced rat CYP3A2 independently, while RPMP/DBR combination showed the same induction as DBR. These findings provide a fundamental explanation for HDIs experienced in the clinic.
In these experiments, RPMP/DBR mix were used to treat the animals but showed no significant benefit compared with the two drugs alone. As for CYP1A2, the mixed drugs even showed a set-off effect. By the way, it is an open question as to whether lower doses of these drugs used in combination would achieve similar efficacy while exhibiting less side effects.
Furthermore, our data suggested that RPMP may be a potent inducer of rat CYP1A2, CYP2E1 and CYP3A2. According to the existing data and the National Commission of Chinese Pharmacopoeia (2010), anthraquinones (such as emodin) and stilbene glycosides are generally considered RPMP active compounds or ingredients. Conversely, DBR might be composed of potent inhibitors of rat CYP1A2, and some inducers of CYP2E1 and CYP3A2. Diosbulbine A is generally considered the active compound of DBR. However, no studies have explored the effect of purified active compounds of RPMP or DBR on CYP enzymes. Therefore, future studies should fill this gap.
From the data presented here, we cannot exclude that co-administration of these herbs may influence metabolism of the drugs metabolized by human CYP1A2, CYP2E1 and CYP3A4 and change their plasma concentrations. Clinical studies are required to fully understand the safety of RPMP and DBR in terms of regulation of the activity of CYP enzymes.
5. Conclusion
Our data suggested that RPMP independently induces rat CYP1A2, CYP2E1 and CYP3A2 activity, while DBR independently inhibits activity of rat CYP1A2 and induces that of CYP2E1 and CYP3A2. RPMP/DBR combination showed no effects on CYP1A2 activity, although demonstrating potential induction of that of CYP2E1 and CYP3A2. Therefore, caution is needed when RPMP and/or DBR are co-administered with drugs metabolized by human CYP1A2, CYP2E1 and CYP3A4.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by AoSaiKang Pharmaceutical Science Foundation of the Jiangsu Pharmaceutical Association (201014).
References
- Bent, S. (2008) Herbal Medicine in the United States: Review of Efficacy, Safety, and Regulation: Grand Rounds at University of California, San Francisco Medical Center. Journal of General Internal Medicine, 23, 854-859.http://dx.doi.org/10.1007/s11606-008-0632-y
- Commission of Chinese Pharmacopoeia (2010) Pharmacopoeia of the People’s Republic of China. China Medico-Pharmaceutical Science & Technology Publishing House, Beijing, 164-165.
- Du, L.X., Luo, M.M. and Liu, S.M. (2007) Advances in Modern Toxicology Studies on Dioscorea bulbifera. Liaoning University of Traditional Chinese Medicine, 9, 71-72.
- Li, J.H., Zhang, X.H. and Chi, H.H. (2000) Study on Anti-Tumor Effect of Different Bulbifera Extracts. Journal of Hebei Jiaotong Vocational and Technical College, 17, 5-7.
- Gao, H.Y., Wu, L.J. and Kuroyanagi, M. (2001) Seven Compounds from Dioscorea bulbifera L. (Natural Medicine Note). Nature Medicine, 277-281.
- Xu, Y., Chen, C.C., Yang, L., Wang, J.M., Ji, L.L., et al. (2011) Evaluation on Hepatotoxicity Caused by Dioscorea bulbifera Based on Analysis of Bile Acids. Acta pharmaceutica Sinica, 46, 39-44.
- Chen, X.L., Wu, S.H. and Zhao, J.B. (1998) Suppression Effect of the Bulbifera Alcohol Extract on Mice Xenografts Tumor. Journal of the Fourth Military Medical University, 19, 354-355.
- Suo, Q. and Cui, L.R. (2008) Study on Anti-Tumor Effect of Serum Containing Bulbifera and Angelica. China Medical Technologies, 15, 113-114.
- Tan, X.Q., Yuan, J.L., Chen, H.S., Wang, J.Y. and Wang, J.S. (2003) Study on Antiinflamtory Components in Dioscorea bulbifera Rhizome. Second Military Medical University, 24, 677-679.
- Rao, Y.Z., Wen, T.L. and Zhao, H.M. (2010) Study on Anti-Inflammatory Effect of Methanol Extracts from Dioscorea bulbifera L. Anhui Agricultural Science Bulletin, 16, 64-65.
- Xu, Y.Z., Bai, X.Q. and Zhou, Q. (1998) Study on Virus Inhibitory Effect of Ethanol Extract of Dioscorea bulbifera. Chemical and Pharmaceutical Bulletin, 23, 535-537.
- Zhu, F.L. and Jia, L. (2006) Research Progress of Dioscorea bulbifera. Shizhen Med Mat Med Res, 17, 851-853.
- Chen, Y., Cheng, M. and Liu, C.X. (2004) Present Development and Prospect of Hepatotoxic Chinese Materia Medica. Chinese Traditional and Herbal Drugs, 35, 1315-1317.
- Zhao, Y., Piao, H.Y. and Chu, X.J. (2010) Based on Efficacy and Material Base of Toxicity Research Progress. Heilongjiang Medical Journal, 34, 821-824.
- Wang, J.M., Cui, D.P. and Cui, Y. (2011) Research Progress in Chemical Components, Pharmacological Actions and Toxicity of Diterpene Lactones Isolated from Dioscoreae bulbifera L. Rhizome. Journal of Chinese Materia Medica, 26, 1319-1321.
- Wang, J.M., Lei, J.F., Ji, L.L., Liu, H., Wang, Z.T., et al. (2011) Research Progress and Strategy in Toxicity of Dioscorea bulbifera Rhizome with Main Bioactivity of Antitumor Action. Chinese Journal of Experimental Traditional Medical Formulae, 12, 256-259.
- Yu, J., Xie, J., Mao, X.J., Wang, M.J., Li, N., et al. (2011) Hepatoxicity of Major Constituents and Extractions of Radix Polygoni Multiflori and Radix Polygoni Multiflori Praeparata. Journal of Ethnopharmacology, 137, 1291-1299. http://dx.doi.org/10.1016/j.jep.2011.07.055
- Bush, T.M., Rayburn, K.S., Holloway, S.W., Sanchez-Yamamoto, D.S., Allen, B.L., et al. (2007) Adverse Interactions between Herbal and Dietary Substances and Prescription Medications: A Clinical Survey. Alternative Therapies in Health and Medicine, 13, 30-35.
- Han, Y.L., Yu, Q., Meng, X.L., Li, D., Bian, K., et al. (2009) Inhibition of Dengzhan-Xi-Xin Injection on Rat Liver Microsomal CYP3A. Chinese Journal of Clinical Pharmacology and Therapeutics, 14, 891-895
- Yang, Q.H., Meng, X.Q. and Li, Y.L. (1997) Observation on the Effect of Compound Danshen Inducing Human CYP 450 1A2. Journal of Harbin Medical University, 31, 295-296.
- Liu, S.J., Ju, W.Z., Chen, W.K., Xu, L.J., Xiong, N.N., et al. (2010) Influence of Xue-Shuan-Tong Injection on P450 Activities Using a Cocktail Method. Chinese Pharmaceutical Journal, 45, 115-118.
- Klingenberg, M. (1958) Pigments of Rat Liver Microsomes. Archives of Biochemistry and Biophysics, 75, 376-386. http://dx.doi.org/10.1016/0003-9861(58)90436-3
- Guengerich, F.P. (1992) Characterization of Human Cytochrome P450 Enzymes. The FASEB Journal, 6, 745-748.
- Isin, E.M. and Guengerich, F.P. (2007) Complex Reactions Catalyzed by Cytochrome P450 Enzymes. Biochimica et Biophysica Acta (BBA)-General Subjects, 1770, 314-329. http://dx.doi.org/10.1016/j.bbagen.2006.07.003
- Slaughter, R.L. and Edwards, D.J. (1995) Recent Advances: The Cytochrome P450 Enzymes. The Annals of Pharmacotherapy, 29, 619-624.
- Kerremans, A.L. (1996) Cytochrome P450 Isoenzymes—Importance for the Internist. The Netherlands Journal of Medicine, 48, 237-243. http://dx.doi.org/10.1016/0300-2977(96)00002-2
- Lewis, D.F. (2003) P450 Structures and Oxidative Metabolism of Xenobiotics. Pharmacogenomics, 4, 387-395. http://dx.doi.org/10.1517/phgs.4.4.387.22752
- Daly, A.K. (2004) Pharmacogenetics of the Cytochromes P450. Current Topics in Medicinal Chemistry, 4, 1733-1744. http://dx.doi.org/10.2174/1568026043387070
- Ingelman-Sundberg, M. (2004) Pharmacogenetics of Cytochrome P450 and Its Applications in Drug Therapy: The Past, Present and Future. Trends in Pharmacological Sciences, 25, 193-200. http://dx.doi.org/10.1016/j.tips.2004.02.007
- Fan, H.R., He, F., Li, Q.S., Huang, Y.R., Wei, G.L., et al. (2004) Study on Influence of Ginsenoside Re on Cytochrome P450 Isoforms by Cocktail Approach Using Probe Drugs, Caffeine, Chlorzoxazone, Omeprazole And dapsone in Rats. Asian Journal of Drug Metabolic and Pharmacokinetics, 4, 91-98.
- Sharma, A., Pilote, S., Bélanger, P.M., Arsenault, M. and Hamelin, B.A. (2004) A Convenient Five-Drug Cocktail for the Assessment of Major Drug Metabolizing Enzymes: A Pilot Study. British Journal of Clinical Pharmacology, 58, 288-297. http://dx.doi.org/10.1111/j.1365-2125.2004.02162.x
- Martignoni, M., Groothuis, G.M. and de Kanter, R. (2006) Species Differences between Mouse, Rat, Dog, Monkey and Human CYP-Mediated Drug Metabolism, Inhibition and Induction. Expert Opinion on Drug Metabolism & Toxicology, 6, 875-894. http://dx.doi.org/10.1517/17425255.2.6.875
- Alikhan, A., Felsten, L.M., Daly, M. and Petronic-Rosic, V. (2011) Vitiligo: A Comprehensive Overview. Part I. Introduction, Epidemiology, Quality of Life, Diagnosis, Differential Diagnosis, Associations, Histopathology, Etiology, and Work-Up. Journal of the American Academy of Dermatology, 65, 473-491. http://dx.doi.org/10.1016/j.jaad.2010.11.061
- Li, W.Y., Yu, W.D., Dong, Q., Wang, P. and Chen, Q.X. (2004) A Complex Prescription for Vitiligo Activates Mitochondrial ATP Synthase-6 Expression in B-16 Murine Melanoma Cells. Journal of Ethnopharmacology, 92, 193-196. http://dx.doi.org/10.1016/j.jep.2004.03.021
- Shimada, T., Mimura, M., Inoue, K., Nakamura, S.I., Oda, H., et al. (1997) Cytochrome P450-Dependent Drug Oxidation Activities in Liver Microsomes of Various Animal Species Including Rats, Guinea Pigs, Dogs, Monkeys, and Humans. Archives of Toxicology, 71, 401-408. http://dx.doi.org/10.1007/s002040050403
- Carrillo, J.A., Christensen, M., Ramos, S.I., Alm, C., Dahl, M.L., et al. (2000) Evaluation of Caffeine as an in Vivo Probe for CYP1A2 Using Measurements in Plasma, Saliva, and Urine. Therapeutic Drug Monitoring, 22, 409-417. http://dx.doi.org/10.1097/00007691-200008000-00008
- Faber, M.S., Jetter, A. and Fuhr, U. (2005) Assessment of CYP1A2 Activity in Clinical Practice: Why, How, and When? Basic & Clinical Pharmacology & Toxicology, 97, 125-134. http://dx.doi.org/10.1111/j.1742-7843.2005.pto_973160.x
- Lee, A.M., Joshi, M., Yue, J. and Tyndale, R.F. (2006) Phenobarbital Induces Monkey Brain CYP2E1 Protein but Not Hepatic CYP2E1, in Vitro or in Vivo Chlorzoxazone Metabolism. European Journal of Pharmacology, 552, 151-158. http://dx.doi.org/10.1016/j.ejphar.2006.09.006
- Porubsky, P.R., Meneely, K.M. and Scott, E.E. (2008) Structures of Human Cytochrome P-450 2E1. Insights into the Binding of Inhibitors and Both Small Molecular Weight and Fatty Acid Substrates. The Journal of Biological Chemistry, 283, 33698-33707. http://dx.doi.org/10.1074/jbc.M805999200
- Obach, R.S., Zhang Q.Y., Dunbar, D. and Kaminsky, L.S. (2001) Metabolic Characterization of the Major Human Small Intestinal Cytochrome P450s. Drug Metabolism and Disposition, 29, 347-352.
- Kanazu, T., Yamaguchi, Y., Okamura, N., Baba, T. and Koike, M. (2004) Model for the Drug-Drug Interaction Responsible for CYP3A Enzyme Inhibition. II: Establishment and Evaluation of Dexamethasone-Pretreated Female Rats. Xenobiotica, 34, 403-413. http://dx.doi.org/10.1080/00498250410001685746
- Gurley, B.J., Gardner, S.F., Hubbard, M.A., Williams, D.K., Gentry, W.B., et al. (2002) Cytochrome P450 Phenotypic Ratios for Predicting Herb-Drug Interactions in Humans. Clinical Pharmacology & Therapeutics, 72, 276-287. http://dx.doi.org/10.1067/mcp.2002.126913
- Zhou, S.F., Lim, L.Y. and Chowbay, B. (2004) Herbal Modulation of P-Glycoprotein. Drug Metabolism Reviews, 36, 57-104. http://dx.doi.org/10.1081/DMR-120028427
Abbreviations
CYP: Cytochrome P450;
DDIs: Drug-Drug Interactions;
HDIs: Herb-Drug Interactions;
RPMP: Radix Polygoni Multiflori Praeparata;
DBR: Dioscorea Bulbifera Rhizomes;
NOTES

*Corresponding author.


