Pharmacology & Pharmacy
Vol.3 No.1(2012), Article ID:16946,6 pages DOI:10.4236/pp.2012.31014
Radioiodination of Cephalexin with 125I and Its Biological Behavior in Mice
![]()
1Faculty of Pharmacy, Cairo University, Cairo, Egypt; 2Labeled Compounds Department, Hot Labs Center, Atomic Energy Authority, Inshas, Egypt; 3Cyclotron Project, Nuclear Research Center, Atomic Energy Authority, Inshas, Egypt.
Email: *ab_amin@Hotmail.com
Received October 7th, 2011; revised November 24th, 2011; accepted December 26th, 2011
Keywords: Cephalexin; Na125I; Electrophilic Substitution Reaction; Radioiodination; Inflammation
ABSTRACT
A procedure for radioiodination of Cephalexin with iodine-125 was carried out via an electrophilic substitution reaction. Using 3.7 MBq of Na125I, 200 μl of cephalexin as substrate, 200 μl of iodogen as oxidizing agent in acetone at 40˚C for 20 min, a maximum radiochemical yield of 125I-Ceph (95%) was obtained. The labeled compound was separated and purified by means of high-pressure liquid chromatography (HPLC). The biological distribution in normal and inflamed (septic) mice indicates the suitability of radioiodinated cephalexin for imaging of inflammation
1. Introduction
Cephalexin is a first-generation cephalosporin antibiotic, introduced in 1967 by Eli Lilly and Company [1]. Cephalexin is used to treat urinary tract [2], respiratory tract, skin and soft tissue infections and sometimes to treat acne. In addition to being a rational first-line treatment for cellulitis, it is a useful alternative to penicillins in patients with penicillin hypersensitivity. Caution should always be taken when prescribing cephalosporins to those with strong history of true penicillin hypersensitivity [3]. However, cephalexin and other first-generation cephalosporins are known to have a modest cross-allergy in patients with penicillin hypersensitivity. Cephalexin fights bacteria in the body. It works by interfering with the bacteria’s cell wall formation. This weakens the cell wall, causing its rupture, and killing of the bacteria.
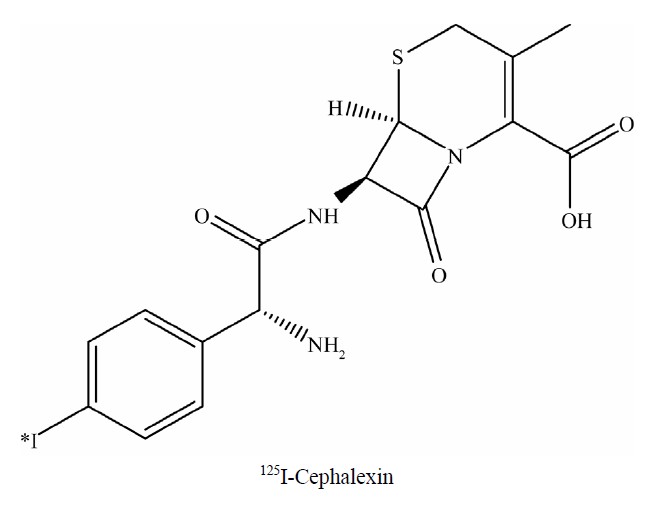
A studies of 125I-Labeled derivatives of the beta-lactam antibiotics cephalexin, cephradine, cefaclor and 6- alpha-aminopenicillanic acid have been obtained by reacting these compounds with (125I)-Bolton-Hunter reagent .The iodinated derivatives showed a very high affinity of binding to their target proteins with apparent half-saturating concentrations in the nano-molar range [4]. Other studies interested in the pharmacokinetics of cefaclor and cephalexin were characterized in patients with creatinine clearance ranging from 0 to 147 ml/min. Serum and urine levels of the antibiotics were measured by bioassay. For cephalexin, corresponding half-lives were 15.4 h and 58 min, that study did present a dosage nomogram for calculating the appropriate adjustments to the loading dose based on patient weight and maintenence dose based on corrected creatinine clearance [5]. The objective of this work was oriented to label of Cephalexin with 125I and the factors affecting the labeling yield were investigated. The suggested structure of 125ICephalexin which formed via an electrophilic substitution reaction in the presence of iodogen as oxidizing agent; where 125I+ for H+ exchange in the ring is in Paraposition.
2. Experimental
2.1. Materials and Methods
All chemicals used in the present work were of analytical grade. Cephalexin was obtained from Pharco Company, Egypt and was used without any purification. Na125I (185 MBq/5 mL) in diluted NaOH, was purchased from Institute of Isotopes, Budapest, Hungary. The statistical analysis was performed using SPSS ® software, USA.
2.2. Labeling of Cephalexin with Na125I
The labeling reaction was carried out by the precipitation of iodogen on the wall of glass tube using chloroformic solution of iodogen. The experiment was performed by taking (25 - 300 μg) of cephalexin followed by (25 - 300 μg) of iodogen. Then 10 mL (3.7 MBq Na125I) of NaOH solution were added and the reaction mixture was kept at different temperature for specific time. The different parameters that affect the radiochemical yield of 125I-Ceph were studied.
2.3. Radiochemical Yield and Purity
The radiochemical yield was determined by TLC and Electrophoresis while the radiochemical purity by HPLC:
2.3.1. Electrophoresis Analysis
Electrophoresis was done using cellulose acetate strips. These strips were moistened with 0.02 M phosphate buffer pH 7 and then were placed in the chamber. Samples of 5 µL were applied at a distance of 12 cm from cathode. Standing time and applied voltage were continued for 90 min. Developed strips were removed, dried and cut into 1cm segments. They were counted using a well-type NaI (Tl) detector connected with a singlechannel γ-counter.
2.3.2. HPLC Analysis
The radiochemical yield% and the purityof Cephalexin was determinedly injection of 10 mL of unlabeled drug into the column (Rp-18. 250 × 4 mm2, 4 - 6 mm, Lischrosorb) built in HPLC Merck model 2010 Shimadzu model, which consists of pumps LC-9A, Rheohydron injector (Syringe Loading Sample Injector-7125) and UV spectrophotometer detector(SPD-6A) adjusted to the 254 nm wavelength using Water: Methanol: Acetonitrile: Glacial acetic acid (50:20:30:1) as mobile phase with flow rate1.5 ml/min [6].
2.4. Biodistribution Study
Organ distribution studies were carried out in a group of three male Albino mice. Each animal was injected in the tail vein with 0.2 ml solution containing 3.7 MBq of 125ICephalexin. The mice were put in metabolic cages for the recommended time then sacrificed. Time dependent pharmacokinetic studies were carried out at (30 min, 1 h, 2 h, and 4 h) post injection. The organs as well as other body parts and fluids were dissected. Activity in each organ was counted and expressed as a percentage of the injected activity per organ. The weights of blood, bone and muscles were assumed to be 7%, 10% and 40% of the total body weight, respectively.
3. Results and Discussion
3.1. Factors Affecting the Labeling Yield
3.1.1. Effect of Substrate Amount
The radiochemical yield of 125I-Ceph as a function of Cephalexin amount was studied as shown in Figure 1. The results indicate, at low amounts of cephalexin, (25 μg - 100 μg the radiochemical yields of 125I-Ceph were 30% and 85%, respectively. The radiochemical yield was highly affected by increasing the amount of cephalexin to 200μg reaching 95%, but further increase in the amount of the substrate didn’t affect the radiochemical yield. This may be attributed to the fact that the yield reaches the saturation value (95%) because the entire generated iodonium ions in the reaction are captured at that concentration of Cephalexin [7].

Figure 1. Variation of the radiochemical yield of 125I-Ceph as a function of Ceph amount [200 μg iodogen and 10 μL of 3.7 MBq Na125I, x μg Ceph] at 40˚C for 20 min.
3.1.2. Effect of Chloramine-T (CAT) Amount
Sodium iodide is the simplest and versatile source of iodinating reagent for organic molecules [8]. Elemental iodine was formed by the oxidation of sodium iodide with oxidizing agents. Chloramine-T is the most famous oxidizing agent used to produce H2OI+ and HOI from sodium iodide [9]. The influence of CAT amount on the radioiodination of Cephalexin was studied as shown in Figure 2. The data clearly show that 25 μg and 50 μg of CAT was not sufficient to oxidize radioiodide ions in the reaction mixture [10]. By increasing the concentration of CAT to 100μg, the yield of 125I-Ceph was increased to 80%. Increasing the CAT concentration above 200 μg leads to a decrease in the yield due to the formation of undesirable oxidative side reactions like chlorinationand polymerization [11].
3.1.3. Effect of Iodogen Amount
The iodination of Ceph using iodogen as an oxidizing agent was achieved by the precipitation of iodogen on the wall of glass tube using chloroformic solution of iodogen. Figure 3 shows that the radiochemical yield of 125I-Ceph is low with small amount of iodogen. By increasing the concentration of iodogen to 200 μg, the radiochemical yield increased to 95%. Any further increase in the amount of iodogen to 300 μg didn’t cause any increase in the radiochemical yield of 125I-cephalexin.
3.1.4. Effect of N-Bromo Succinimide (NBS)
The effect of the NBS amount as an oxidizing agent was studied as shown in Figure 4. The data reveal that at low concentration the radiochemical yield is very low. But with further increase in the amount of NBS the radiochemical increased reaching 74% at 300 μg of NBS.
3.1.5. Effect of the Reaction Temperature
The effect of the reaction temperature (25˚C - 80˚C) on the radioiodination of Cephalexin by 125I was carried out
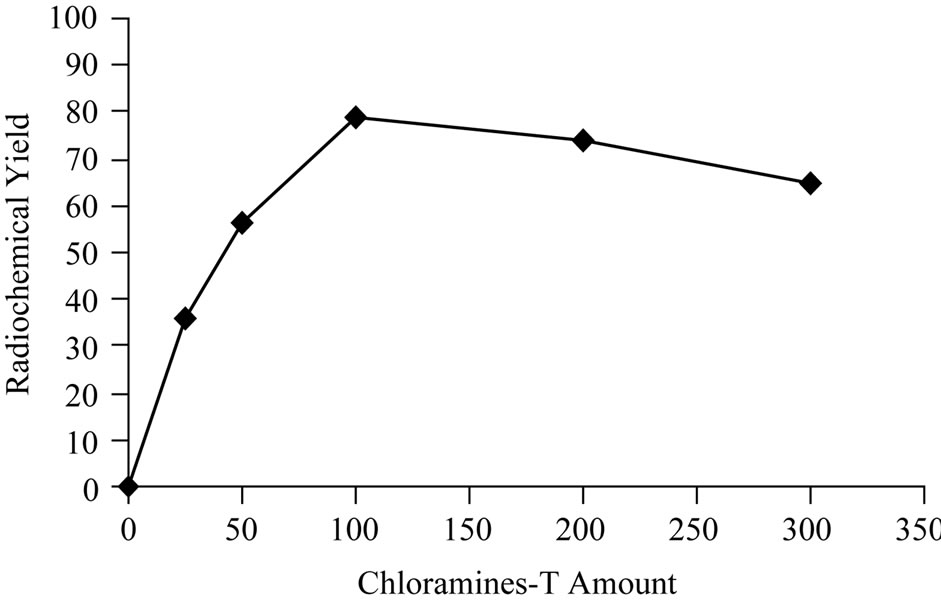
Figure 2. Variation of radiochemical yield 125I-Ceph as a function of Chloramine-T amount [200 μg Ceph., (x μg) CAT and 3.7 MBq Na125I] at 40˚C within 20 min.
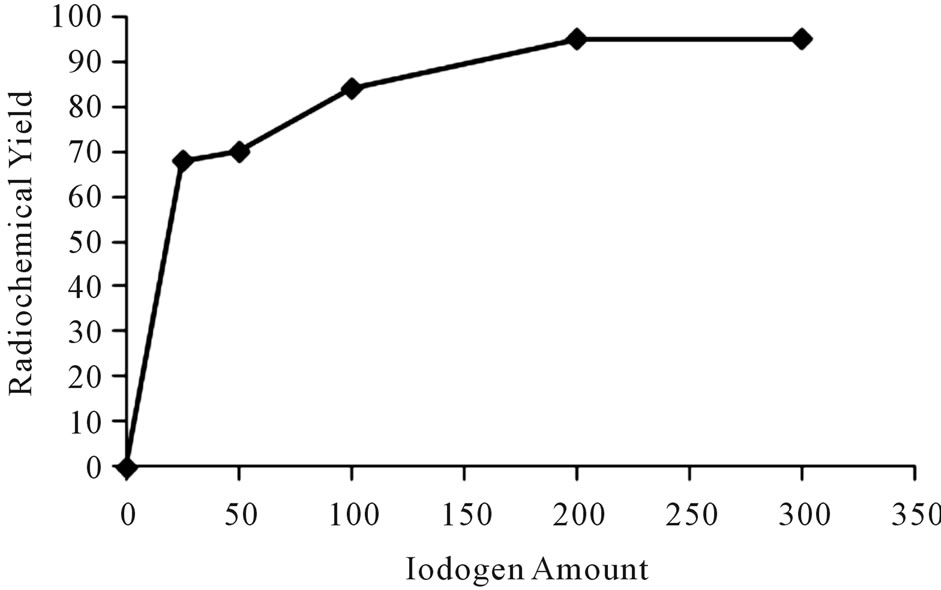
Figure 3. Variation of radiochemical yieldof 125I-Ceph as a function of Iodogen amount [200 μg Ceph, x μg I odogen and 3.7 MBq Na125I] at 40˚C within 20 min.
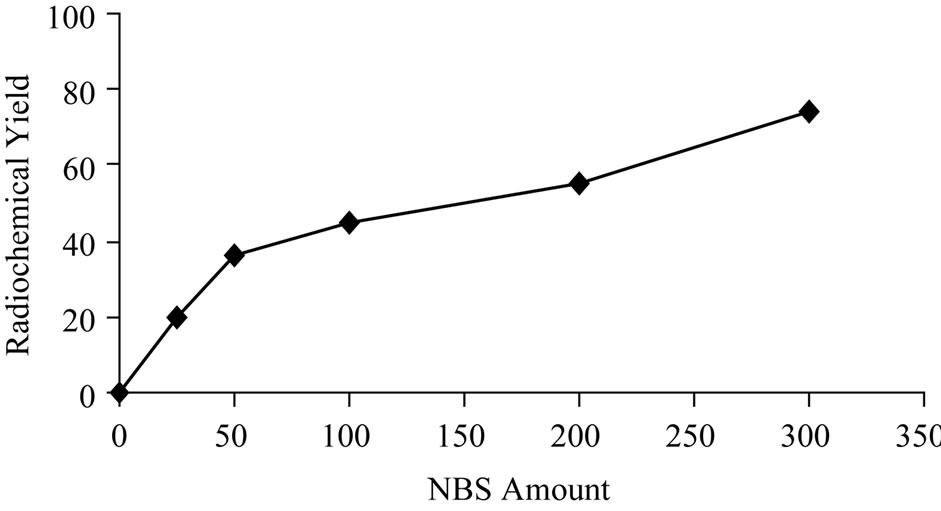
Figure 4. Variation of radiochemical yieldof 125I-Ceph as a function of NBS amount [200 μg Ceph, x μg NBS and 3.7 MBq Na125I] at 40˚C within 20 min.
at interval time using the optimum concentration of bothCeph and iodogen (200 μg Ceph and 200 μg iodogen).The data indicate that the reaction temperature was found to be significant factor affecting the radiochemical yield. As shown in Figure 5 the radiochemical yield of 125I-Ceph was low at room temperature all over the time. Increasing the reaction temperature to (40˚C) within 10 min the radiochemical yield of 125I-Ceph reached to (95%) and by increasing the reaction time the radiochemical yield wasn’t affected. On the other hand, increasing the reaction temperature to 60˚C up to 80˚C the radiochemical yield decreased. This may be attributed to the thermal decomposition of the labeled compound or distortion of the oxidizing agent [12].
3.1.6. Effect of pH
The effect of the hydrogen ion concentration of the reaction mixture on the radiochemical yield of 125I-Ceph in presence of iodogen as oxidizing agent was studied. Different buffers system at pH ranging from 2 to 11 were used. The results of this study were presented in Figure 6, which clearly showed that the optimum yield of 125ICeph (95%) was obtained at pH 2. This may be due to Cephalexin is an acid-stable cephalosporins [13]. When

Figure 5. Variation of radiochemical yield of 125I-Ceph as a function of reaction time [200 μg Ceph and 200 μg iodogen and 3.7 MBq Na125I] at different temperature.
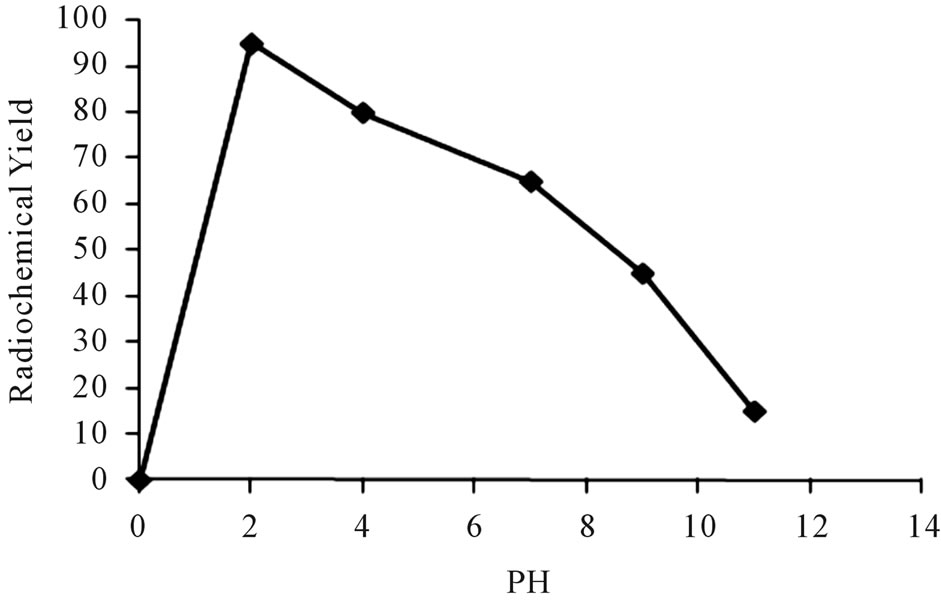
Figure 6. Variation of radiochemical yield of 125I-Ceph as a function of pH [200 μl Ceph, 200 μl iodogen and Na125I in 50 µL of buffer different pH] at 40˚C within 20 min.
the pH of the reaction medium was shifted toward alkaline pH, the radiochemical yield of 125I-Ceph decreased. This could be explained as increasing the pH value leads to a decrease in HOI which is responsible for electrophilic substitution reaction [14].
3.2. In Vitro Stability
The stability of 125I-Ceph was studied in order to determine the suitable time for injection to avoid the formation of the undesired products that result from the radiolysis of the labeled compound. These undesired radioactive products may be toxic or accumulated in undesired organ. Table 1 shows that 125I-Ceph is stable up to 24 h.
3.3. Radiochemical Purity
The free radioiodide was separated at retention time 4 min while 125I-Ceph is not completely separated from Ceph in Figure 7, which clarifies the overlapping between 125I-Ceph and Ceph, where the retention times are 10 and 8 min, respectively. The HPLC fractions were collected and evaporated under reduced pressure, dissolved in saline solution and sterilized by Millipore filter (0.22 mm) under aseptic conditions in a well type NaI (Tl)
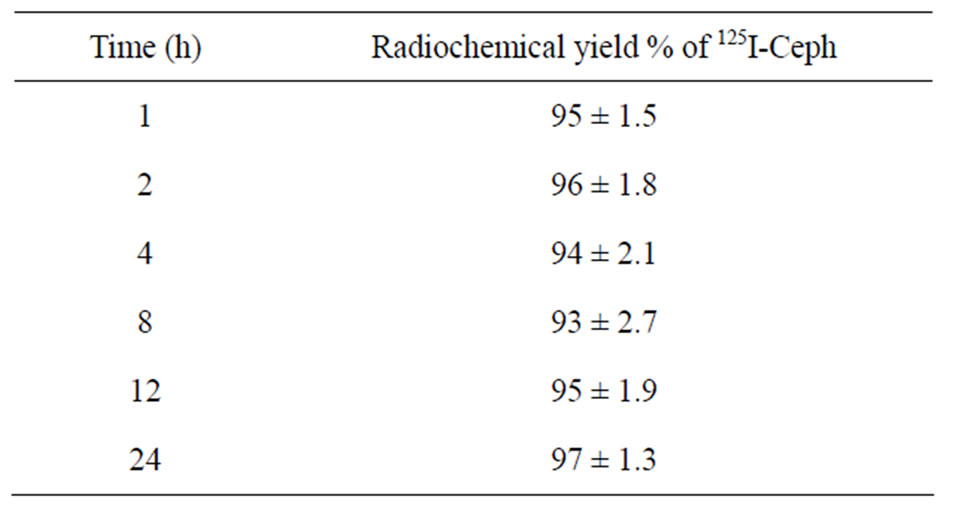
Table 1. In vitro stability of 125I-Ceph.

Figure 7. High-performance liquid chromatography elution profile of cephalexin, separated on reversed phase column (254*4.6 mm at flow rate 1.5 mL/min).
detector connected to a single-channel analyzer [15].
The electrophoresisanalysis of samples from the reaction mixture resulted in two peaks as shown in Figure 8, one corresponding to the free iodide which moved towards the anode with 11 cm distance while 125I-Cephalexin remained at the point of spotting.
3.4. Biodistribution
The 125I-Cephalexin was injected into normal mice via intravenous route to estimate its biological distribution. The data of this study is presented in Table 2. Data were collected and coded prior to analysis; all data were expressed as mean ± SD. It is noticed that the blood uptake is high at 1/2 h equal to 24% post injection. This may be due to the binding of the drug with plasma protein. The uptake of the tracer by the liver is about 6.5% at 1/2 h post injection. This uptake slowly decreased to 5% at 4 h post injection. This low percentage uptake might be due to that the drug is not excreted from the liver. At 4 h post injection, the activity held by the collected urine increased to 20% due to the normal excretion of Cephalexin in urine, as 80% of cephalexin is excreted in the urine [16]. Also the data show that 125I-Cephalexin is held in the intestine by 8% and increased to 17% after 4 hr post injection. It was also absorbed by the stomach reaching 10% at 30 min post injection. This might be due to the
Figure 8. Electrophoretic pattern of 125Cephalexin; condition: Solvent: 0.02 M phosphate buffer pH 7 paper type: cellulose acetate; 300 V; running time: 1.5 hours.
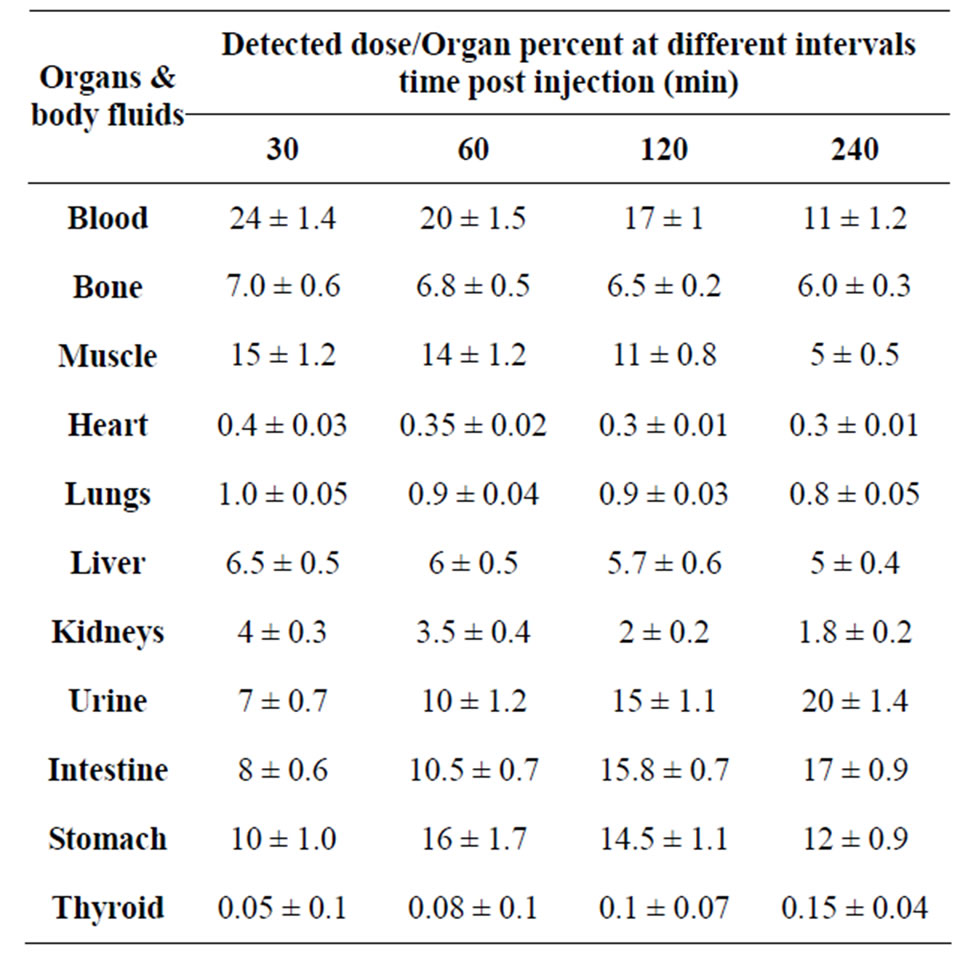
Table 2. Biodistribution of 125I-Ceph in normal mice.
rapid absorption of the drug from the gastrointestinal tract [12]. The uptake of the muscle was 15% at 30 min post injection and decreased to reach only 5 % at 4 h post injection.
The biodistribution of 125I-Cephalexin tracer in E. coliinflamed mice as cleared from the data presented in Table 3. Inflammation was induced by the injection of a suspension of Escherichia coli (E. coli) into the right thigh muscle and the left thigh muscle was taken as control. The uptake by the septic (inflamed) muscle was 30% at 30 min post injection and reached 15% at 4 h post injection, while the uptake of the non inflamed muscle (control) was 10% at 30 min post injection and decreased to 4% after 4 h post injection. Using the unpaired Student's test, statistical differences were assumed to be reproducible when p < 0.05, the difference between the uptake of the inflamed muscle and the normal muscle was significant. This means that 125I-Ceph is able to localize in inflammatory foci induced by E. coli. The thyroid uptake was mostly ranged from 0.05 to 0.15% within 4h indicating that 125I-Cephalexin free form radioiodide and it is stable in vivo.
Furthermore, Figure 9 summarizes the elimination of 125I-Ceph tracer from the blood and its washout from the normal muscle and the inflamed one (induced by E. coli). It was observed that at 30 min and 2 h post injection the uptake of the inflamed muscle was higher than the background, which is blood in our case, which gives us an indication that it is the best times for the imaging of the inflamed muscle to obtain a clear picture. While at 4 h post injection the ratio between the uptake of the blood and the inflamed muscle decrease which may affect the

Table 3. Biodistribution of 125I-Ceph in inflamed mice with E. coli.
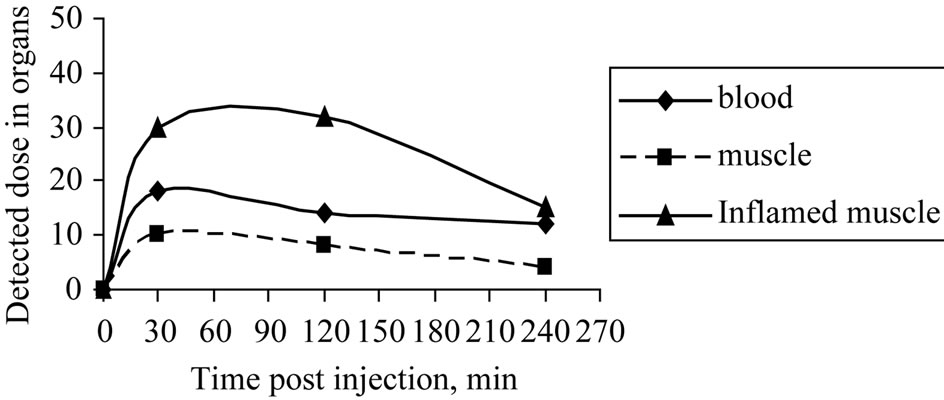
Figure 9. Time course of blood elimination, normal muscle and inflamed muscle washout 30 - 240 minutes after the injection of 125I-Ceph into inflamed mice (induced with E. coli).
clarity of the imaging. The results also show that the uptake of the normal mice was lower than that of the blood along the different times. This will help us to differentiate easily between the normal and the inflamed muscle.
4. Conclusion
The labeling of Cephalexine with radioactive iodine-125 was done. The optimum conditions of the labeling of Cephalexine to give a radiochemical yield of 95% were 200 mg Cephalexine, 200 ml of iodogen at pH 2 when the reaction mixture was heated at 40˚C for 20 min. The ligand studied provides efficient labeling and good in vitro stability. All the obtained data demonstrate that the tracer was distributed rapidly throughout the body after intravenous injection and cleared through the urinary system. In addition, the biodistribution of the tracer in inflamed mice demonstrated the possibility of the use of this tracer as imaging agent for inflammatory foci.
REFERENCES
- W. Sneader, “Cephalosporin Analogues,” Wiley, New York, 2005, p. 324. doi:10.1002/0470015535
- The American Society of Health-System Pharmacists, “Cephalexin,” Retrieved 3 April 2011. http://www.drugs.com/monograph/cephalexin.html
- M. E. Pichichero, “Use of Selected Cephalosporins in Penicillin-Allergic Patients: A Paradigm Shift,” Diagnostic Microbiology and Infectious Disease, Vol. 57, No. 3, 2007, pp. 13S-18S. doi:10.1016/j.diagmicrobio.2006.12.004
- F. Rojo, J. A. Ayala, E. J. de la Rosa , M. A. de Pedro, V. Arán, J. Berenguer and D. Vázquez, “Binding of 125ILabeled Beta-Lactam Antibiotics to the Penicillin Binding Proteins of Escherichia Coli,” Journal of Antibiotics (Tokyo), Vol. 37, No. 4, 1984, pp. 389-393.
- D. A. Spyker, B. L. Thomas, M. A. Sande and W. K. Bolton, “Pharmacokinetics of Cefaclor and Cephalexin: Dosage Nomograms for Impaired Renal Function,” Antimicrob Agents Chemother, Vol. 14, No. 2, 1978, pp. 172-177.
- V. M. Shinde, B. S. Desai and N. M. Tendolkar, Indian Journal of Pharmaceutical Sciences, Vol. 56, No. 2, 1994, pp. 58-60.
- K. M. El-Azony, A. A. El-Mohty, H. M. Killa., U. Seddik, and S. I. Khater, “An Investigation of the 125I-Radioiodination of Colchicine for Medical Purposes,” Journal of Labelled, Compounds and Radiopharmaceuticals, Vol. 52, No. 1, 2008, pp. 1-5.
- E. A. EL-Ghany, R. El-Sheikh, A. S. El-Wetery, A. Z. Saleh and H. Hussien, Arab Journal of Nuclear Sciences and Applications, Vol. 42, 2007, pp. 80.
- W. M. Hunter, Proceedings of the Society for Experimental Biology and Medicine, Vol. 133, 1970, pp. 989.
- V. Nara, V. Howell, R. Harapanhali, K. Sastry and D. Rao, Journal of Nuclear Medicine, Vol. 33, 1992, pp. 2196.
- W. G. Wood, C. Wachter and P. C. Scriba, “Comparison of 125I-Labelling of Polypeptides Using Chloramine-T and 1,3,4,6-Tetrachloro-3α,6α-diphenyl-glycoluril (Iodogen®) with Subsequent Separation of Iodide and Labelled Protein on Cation-Exchange Dextran,” Fresenius’ Journal of Analytical Chemistry, Vol. 301, No. 2, 1980, p. 119. doi:10.1007/BF00467765
- K. M. El-Azony, Arab Journal of Nuclear Sciences and Applications, Vol. 37, 2004, p. 81.
- H. R. Adams, “Veterinary Pharmacology and Therapeutic,” 8th Edition, Wiley-Blackwell, New York, 2001, p. 21.
- J. C. Saccavini, C. Bruneau, IAEA-CN 4519, 1984, p. 153.
- R. F. Verbruggen, “Kit Preparation and Rapid Quality Control of I-Labelled Hippuran,” International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes, Vol. 37, No. 12, 1986, pp. 1249-1250. doi:10.1016/0883-2889(86)90017-1
- J. A. Davies and J. M. Holt, “Clinical Pharmacology of Cephalexin Administered by Intravenous Injection,” Journal of Clinical Pathology, Vol. 25, No. 6, 1972, pp. 518-520. doi:10.1136/jcp.25.6.518
NOTES
*Corresponding author.

