Neuroscience & Medicine
Vol.2 No.2(2011), Article ID:5538,7 pages DOI:10.4236/nm.2011.22022
A Nucleotide-Based Drug Protects against Glutamate- and MPP+-Induced Neurotoxicity
![]()
1Basic Sciences Department, Faculty of Medicine and Health Sciences, Universitat Internacional de Catalunya, Barcelona, Spain; 2Neuroscience Institute, Universitat Autònoma de Barcelona, Barcelona, Spain; 3Centro de Investigación Biomédica en Red de la Fisiopatologia de la Obesidad y Nutrición (CIBER-OBN), Barcelona, Spain.
Email: agella@csc.uic.es
Received February 22nd, 2011; revised April 23rd, 2011; accepted May 10th, 2011.
Keywords: Cortical Cell Culture, Nucleotides, Excitotoxicity, Glutamate Neuroprotection, Parkinson’s Disease, SH-SY5Y Cells
ABSTRACT
Nucleo CMP Forte® is a nucleotide-based drug consisting of cytidinemonophosphate, uridinemonophosphate, uridinediphosphate and uridinetriphosphate. It has been prescribed for peripheral nervous system disorders, such as lumbosciatalgia, diabetic or alcoholic polyneuropathy, or trigeminal neuralgia. Its effects on brain pathologies has received little attention. We examined its neuroprotective effects on cell toxicity induced by glutamate excitotoxicity or by 1- methyl-4-phenyl-pyridinium (MPP+), an in vitro cell model of Parkinson’s disease. We used the human dopaminergic cell line SH-SY5Y and a primary culture of rat cortical cells pre-treated with the drug for 24 hours and then exposed to MPP+ or glutamate at a range of concentrations. Cell viability was measured at different times. Nucleo CMP Forte® pre-treatment significantly increased the rate of cell division in SH-SY5Y cells, as well as the synthesis of triglycerides and phospholipids. More interestingly, drug pre-treatment significantly reduced MPP+- and glutamate-induced cell death in SH-SY5Y cells and in rat cortical cells. These results indicate that the nucleotides included in Nucleo CMP Forte® are promising therapeutic molecules for the prevention of neuronal death in brain caused by focal ischemia, Parkinson’s disease or other neurodegenerative pathologies.
1. Introduction
Injury (trauma or ischemia) in the brain triggers an abnormal release of glutamate and other excitatory aminoacids that contribute significantly to neuronal death [1,2]. This phenomenon, named excitotoxicity, has also been implicated in epileptic seizures [3] and various chronic and progressive neurodegenerative diseases, such as Huntington’s chorea, Alzheimer’s disease, and Parkinson’s disease [4]. The neuronal death induced by glutamate is mediated by activation of the N-methyl-D-aspartate (NMDA)-subtype receptor of Glu and involves an increase in intracellular calcium concentrations that leads to an intracellular cascade of cytotoxic events [5]. Zhang and Bhavnani [6] report that changes that occur in apoptosis depend on the neuron cell type. On the other hand, treatment of neuronal cultured cells with 1-methyl 4- phenyl 1, 2, 3, 6-tetrahydropyridine (MPTP) is currently studied as a model of Parkinson’s disease [7]. MPP+ treated cells show signs of apoptosis and the toxin appears to act at oxidative-stress level, with the induction of p53, opening of the mitochondrial membrane transition pore and activation of the JNK pathway [8], although the specific mechanisms are not fully understood.
Nucleo CMP Forte® is a commercial nucleotide-based drug, consisting of uridinemonophosphate (UMP), uridinediphosphate (UDP), uridinetriphosphate (UTP) and cytidinemonophosphate (CMP). Nucleotides have shown to be effective in the treatment of diverse neurological peripheral syndromes and nucleotide containing drugs, such as Nucleo CMP Forte® have been prescribed to patients with lumbosciatalgia, alcoholic and diabetic polyneuropathies or trigeminal neuralgia, although their mechanism of action remains to be established [9-11]. Furthermore, nucleotides play a central role in many cellular processes, including those associated with nerve repair [12].
Uridine and cytidine are fundamental components of DNA and RNA, and are indispensable precursors in the biosynthesis of phospholipids and glycolipids, which include phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, gangliosides, cerebrosides, and sphigomyelin [13-15]. Sugar conjugates of uracilnucleotides also participate in protein and lipid glycosylation reactions, and in the synthesis of glycogen [16]. Uridine is the main source of cytidinetriphosphate (CTP) for the brain cells, since it can cross the blood-brain barrier and be phosphorylated to UTP, which in turn can be aminated to CTP [17].
Regarding peripheral nervous system, studies in vivo have shown that the addition of these nucleotides favors the regeneration of myelinatednerve fibers following crush injury to the sciatic nerve of the rat, thus accelerating the process of nerve repair [12], improving recovery after exhaustive exercise [18] and inhibiting spinal pain transmission [19].
Little is known about the beneficial effects of nucleotides on the central nervous system. Oral administration of UMP alone or in combination with unsaturated fatty acids increases spine density and the synthesis of synaptic proteins and ameliorates the impairment of hippocampal-dependent memory in impoverished rats, which provides a rationale for testing these compounds in the search for a way to treat neurological diseases characterized by synaptic loss [18,20-22]. Moreover, UTP administration to rats protects against cerebral ischemia reperfusion injury by reducing the infarct zone [26]. The present study demonstrates the neuroprotective effects of Nucleo CMP Forte® in two in vitro models of neuronal death: glutamate excitotoxicity and MPP+ toxicity.
2. Methods
2.1. Materials and Reagents
First, Nucleo CMP Forte® was donated by GrupoFerrer (Barcelona, Spain). All cell culture media and supplements were obtained from Gibco® (Invitrogen™, Carlsbad, CA, USA), and cell culture plastic ware was from Nunc™ (Roskilde, Denmark). All other reagents were purchased from Sigma-Aldrich® (St. Louis, MO, USA).
2.2. SH-SY5Y Cell Culture
The human dopaminergicneuroblastoma SH-SY5Y cell line was obtained from the European Collection of Animal Cell Cultures (ECACC). The cells were grown in Dulbecco’s modified Eagle Medium/Ham’s F-12 medium, supplemented with 15% (v/v) heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 50 U/mL penicillin, 100 mg/L streptomycin and non-essential aminoacids, and maintained at 37˚C in a humidified 5% CO2 atmosphere. Medium was changed every 3 days and cells were passaged every 4 - 5 days. Cells were seeded at a 5.7 × 104 cells/cm2 density onto poly-D-lysine—precoated wells and starved at 1% (vol/vol) FCS for 24 h before treatments.
2.3. Primary Cultures of Rat Cortical Cell
Primary neuronal cultures of cerebral cortex were obtained from rat embryos (E16 - 18) according to Gallai [9] with certain modifications. Dissociated cells were plated at a density of 5.7 × 104 cells/cm2 in Basal Medium Eagle (BME) supplemented with 10% (v/v) FCS, 50 U/mL penicillin, 50 µg/mL streptomycin, 2 mmol/L L-glutamine and plated onto poly-D-lysine-precoatedwells. Cultures were kept for 7 days in vitro at 37˚C in a humidified 5% CO2 atmosphere. Immediately afterplating, the medium was replaced by BME, supplemented as above and with 10 µmol/Lcytosine arabinoside to prevent the proliferation of non-neuronal cells. Only mature cultures (11 to 14 days in vitro) were used for cell treatments. The procedures were followed in accordancewith guidelines of the Comissiód’ Èticaen l’ Experimentació Animal i Humana of the UniversitatAutònoma de Barcelona and animals were handled in accordance with the European legislation on the care of experimental animals (86/609/EEC).
2.4. Cell Treatment
To determine the highest non-toxic concentration of Nucleo CMP Forte®, itwas added to the medium 48 hours prior to cell viability assays, at concentrations ranging from 0 to 16 g/L. In experiments in which the protective effect of Nucleo CMP Forte® was tested, Nucleo CMP Forte® (3 g/L) was added 24 h before the addition of increasing MPP+ or glutamate concentrations and incubated for further 24 hours, and cell viability was then assayed.
2.5. Cell Viability Assay
Thiazolyl Blue Tetrazolium Bromide (MTT) assay performed as previously described by Hansen [24], with minor modifications. MTT was added 24 hours after cell treatment at a final concentration of 0.5 g/L for SHSY5Y cells or 0.2 g/L for cortical neurons and incubated at 37˚C in a CO2 incubator for 45 min. Then, MTT medium was removed and the resulting formazan dye was solubilised with dimethylsulfoxide. Absorbance was measured using a spectrophotometer (Synergy™ HT, BioTeK®, USA) at a test wavelength of 560 nm and reference wavelength of 620 nm. The results are expressed as a percentage with respect to untreated cells (100%).
2.6. Enzymatic Colorimetric Assay
Phospholipid, triglyceride and cholesterol concentrations of whole-cell protein extract were determined by an enzymatic colorimetric method from WakoChemicals (No. 990-54006, 419-10698 and 419-43998, respectively) according to the manufacturer’s instructions.
2.7. Statistical Analysis
All analysis was performed using SPSS® software (Chicago, USA). To establish differences between control and Nucleo CMP Forte® treated cells, one-way or twoway ANOVA tests were performed, followed by a MannWhitney post-test. Differences were considered significant when p-value was lower than 0.05 (p < 0.05).
3. Results
3.1. Cytotoxicity of the Nucleotide-Based Drug
In order to determine the highest non-toxic concentration of Nucleo CMP Forte® for the treatments, neuroblastoma cell line SH-S5Y5 (Figure 1(a)) and cortical neurons (Figure 1(b)) were incubated at increasing drug concentrations, and cell viability was measured using the MTT assay. Cell viability decreased with increasing concentrations of Nucleo CMP Forte® in both cell types. SH-S5Y5 cells showed a slight decrease in cell viability at 6.0 g/L, and a strong decrease (40%) at 8.0 g/L (Figure 1(a)). Similar results were obtained with cortical neurons, although the decrease appeared at lower concentrations. These cells showed only 60% viability when treated with 6.0 g/L (Figure 1(b)). In both cases higher drug concentrations steeply reduced cell survival: cell viability was 10% at the highest drug concentration tested (16 g/L). Based on these results, 3.0 g/L of Nucleo CMP Forte® (composed by 5.1 mM CMP, 1017 µM UMP, 837 µM UDP and 681 µM UTP) was considered a safe drug concentration (98.1 ± 2.7 % and 98.5 ± 0.7 % cell viability for cortical neurons and SH-S5Y5, respectively), so 3.0 g/L of Nucleo CMP Forte® was used for further cell treatments.
3.2. Effects of the Nucleotide-Based Drug on SH-SY5Y Cell Growth
The SH-S5Y5 cell line was grown and counted with Trypan Blue over five consecutive days (Figure 2). Cells treated with Nucleo CMP Forte®showed higher proliferation rates than untreated control cells, (EC50 1.50 vs 2.23 days, respectively). The experimental data show a typical sigmoid dose response curve, with r2 = 0.99. From the third day onwards, both cultures reached confluence, thus the number of cells was similar.
3.3. Effects of the Nucleotide-Based Drug on SH-SY5Y Lipid Concentration
Having seen the effects of the drug on cell growth, we
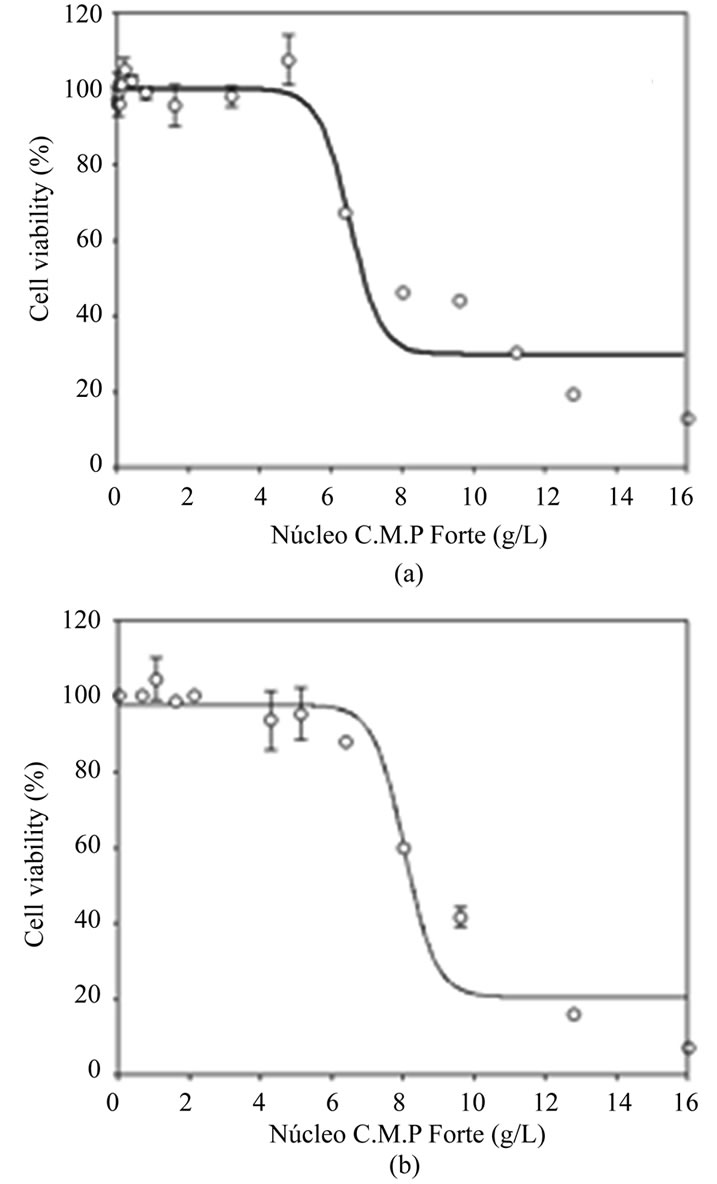
Figure 1. Cytotoxicity of Nucleo CMP Forte® in cell line SH-S5Y5 (a) and in primary cultures of rat cortical cells (b). Results are plotted as a sigmoid dose-response curve. The results are expressed as the mean percentage ± S.D.
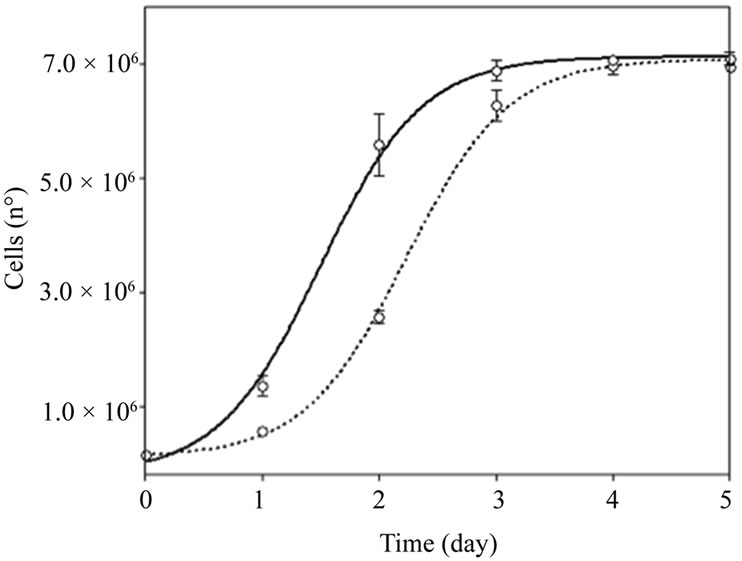
Figure 2. Cell growth in SH-S5Y5 cell line. Untreated control (dotted line) and in the presence of 3.0 g/L of Nucleo CMP Forte® for 5 days (solid line). Results are plotted as a sigmoid dose-response curve. The results are expressed as the mean ± S.D.
examined its effects on lipid concentration. Consistent with the cell proliferation observed, protein concentration was significantly higher in treated cell cultures than in untreated cultures (0.99 ± 0.04 vs 0.59 ± 0.04 g/L, respectively; p < 0.001). As shown in Table 1, the concentration of phospholipids and triglycerides was more than double (p < 0.001) in treated cells compared to untreated cells. Nevertheless, there were no significant changes in cholesterol concentration.
3.4. Nucleotide-Based Drug Attenuates MPP+- and Glutamate-Induced Toxicity
To study the neuronal protection afforded by the drug, SH-S5Y5 and cortical neurons were exposed to various concentrations of MPP+, 24 hours after treatment with Nucleo CMP Forte®. Pretreatment, conferred significant protection (p < 0.05) in SH-S5Y5 cells against high concentrations of MPP+ (500 to 1000 µmol/L). Maximal protection was observedin SH-S5Y5 cells treated with 500 µmol/L of MPP+, where cell viability doubled (Figure 3(a)). Cortical neurons treated with Nucleo CMP Forte® show a similar response with significant protection (p < 0.05) at MPP+ concentrations above 100 µmol/L (Figure 3(b)).
Finally, we measured the protection conferred by Nucleo CMP Forte® against glutamate. Non-treated SHS5Y5 cells showed a 25% decrease in cell viability when the glutamate concentration was higher than 500 μmol/L (Figure 4(a), dotted line). Prior incubation with Nucleo CMP Forte®reduced cell death and significantly increased (p < 0.05) cell viability when the glutamate insult was 556 µmol/L (Figure 4(a), solid line). Cortical neurons were more sensitive than SH-S5Y5 cells to glutamate insult (Figure 4), showing more that 40% mortality at the highest concentrations. Drug pretreatment also conferred significant protection (p < 0.05) on cortical neurons, increasing cell viability to 86% (Figure 4(b)).
4. Discussion
In this study, we analyzed the neurotrophic and the neuroprotective effects of Nucleo CMP Forte®, a mixture of pyrimidine nucleotides, on human neuroblastoma SHSY5Y cell line and a primary culture of cortical neurons. Pyrimidine nucleotides have long been known as intermediates in cell metabolism [25]. They are key building blocks for nucleic acid synthesis, their amino derivatives are activators in phospholipid synthesis, and their sugar conjugates participate in glycogen biosynthesis, and in protein and lipid glycosylation. More recently, several Gprotein-coupled P2Y receptors that can be activated by pyrimidine nucleotides have been discovered. Therefore, new effects mediated by uracil derivatives have emerged, in particular in the nervous systems, and previously unexplored avenues for the pharmacological manipulation of this system are currently under investigation [26-28].
Uracil nucleotides control various physiological events such as cell survival, proliferation and differentiation. Neurotrophic assays, in the SH-SY5Y neuroblastoma cell
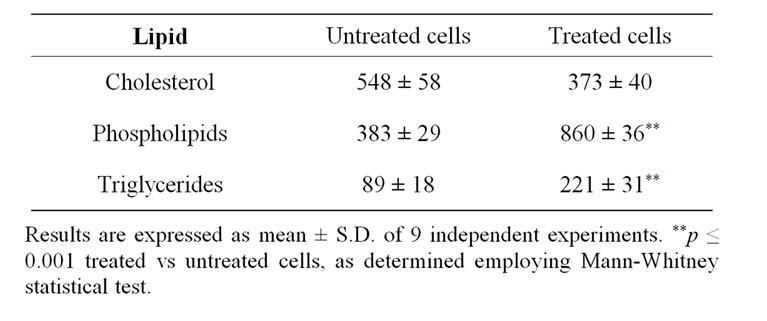
Table 1. Lipid concentrations (mmol/g) in untreated cells, and cells treated with Nucleo CMP Forte®.
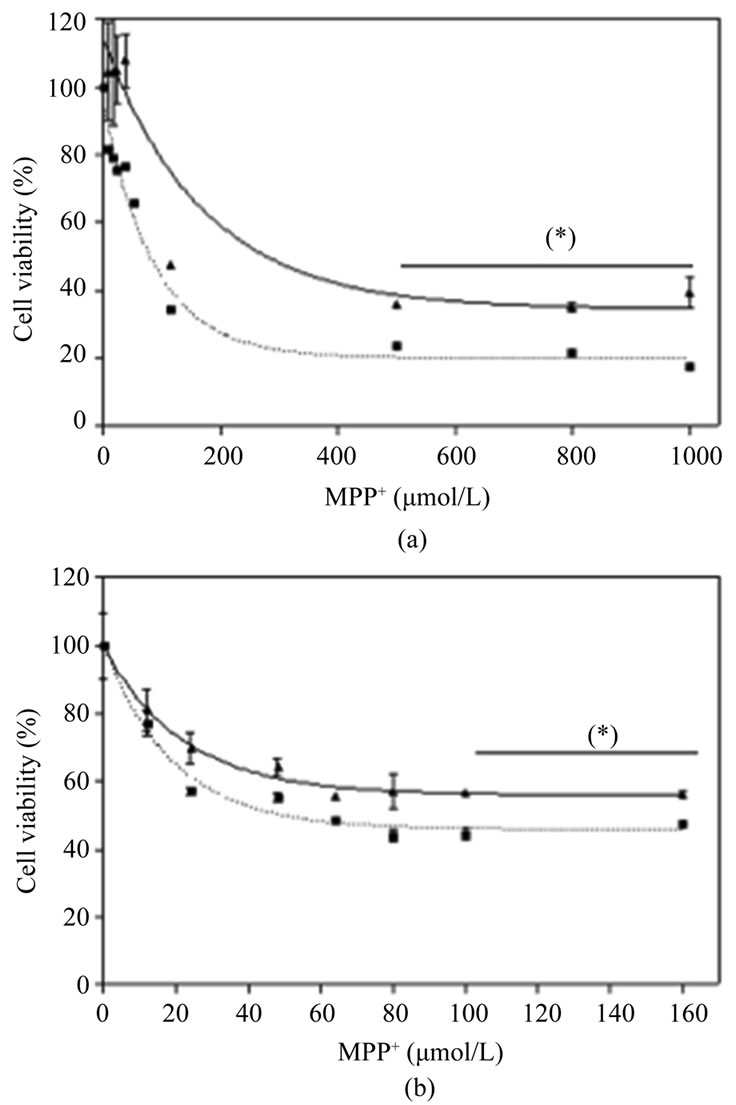
Figure 3. Effects of Nucleo CMP Forte® on toxicity induced by MPP+ in cell line SH-SY5Y (a) and in primary cultures of rat cortical cells (b). Cells were incubated without drug (dotted line) or with 3.0 g/L Nucleo CMP Forte® for 24 hours (solid line). Afterwards, increasing amounts of MPP+ were added and incubated for a further 24 hours, and cell viability was assessed by the MTT method. Values are expressed as mean ± S.D. of percentage respect to untreated cells. Statistical significance: (*) p < 0.05.
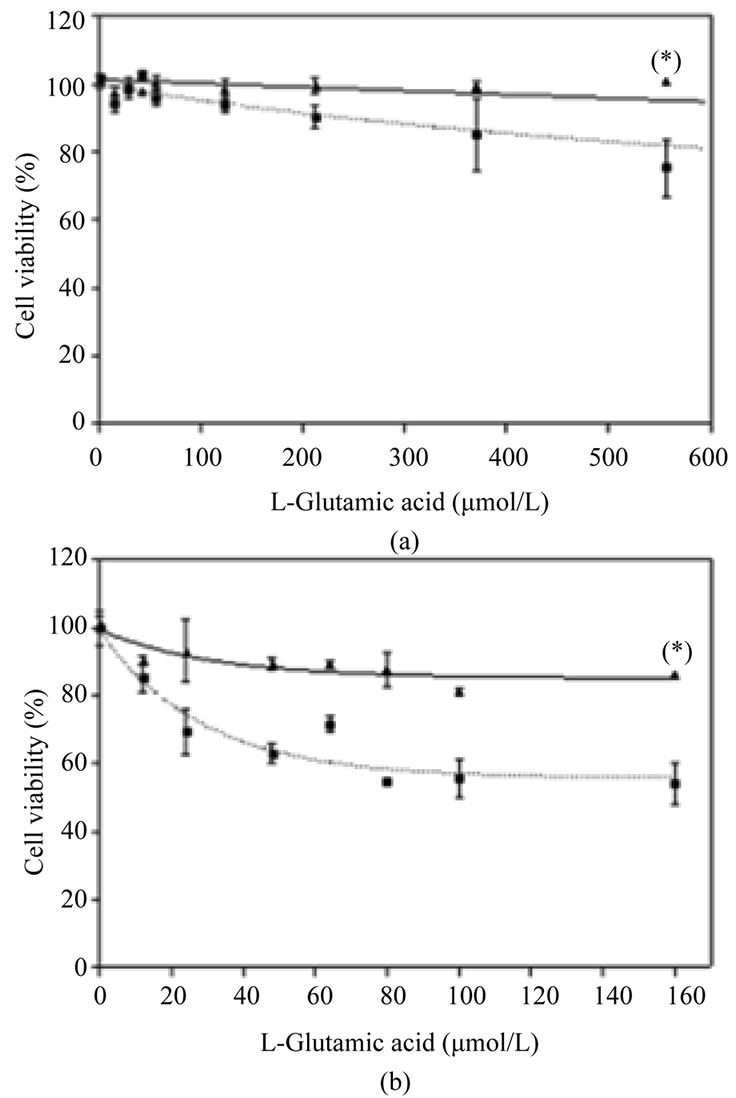
Figure 4. Effects of Nucleo CMP Forte® on toxicity induced by L-glutamate in cell line SH-SY5Y (A) and in primary cultures of rat cortical cells (B). Cells were incubated without drug (dotted line) or with 3.0 g/L Nucleo CMP Forte® for 24 hours (solid line). Afterwards, increasing amounts of glutamate were added and incubated for a further 24 hours, and cell viability was assessed by the MTT method. Values are expressed as mean ± S.D. of percentage respect to untreated cells. Statistical significance: (*) p < 0.05.
line as an in vitro model of dopaminergic neurons for Parkinson’s disease [29], show that the addition of pyridine nucleotides stimulates cell division as well as the synthesis of lipids (phospholipids and triglycerides). The increase in phospholipid levels observed in our study agrees with other studies involving nucleotides. For example, Gallai et al. 1992 [9] shows that the administration of uridine to patients with diabetic polyneuropathy led to a significant improvement in nerve transmission and these results could be explained by an increase in lipid synthesis, above all polyphosphoinositides, which are important components of neuron membranes [30]. In relation to cell proliferation, it was previously described that UTP increases cell proliferation in C6 glioma cell line and astrocytes, and it was associated with Ras-RAfMEK-MAPK pathways [31,32]. Indeed, the mitogenic effects of Nucleo CMP Forte® in the SH-SY5Y neuroblastoma cell line could be related to the activation of the Gq protein coupled P2Y purinoreceptors that stimulate MAPK pathways leading to DNA synthesis and cell proliferation. This ligand-activated upregulation of P2Y receptors by UTP is correlated with cell differentiation, promoting neurite outgrowth and elongation [33]. Interestingly, other studies using human neural stem/precursor cells demonstrate that UTP augments proliferation in these neural cells [34], which is consistent with our results. In addition, they also demonstrate that UTP and UDP promote dopaminergic differentiation of neural stem cells. Importantly, due to the selective loss of dopaminergic neurons in Parkinson’s disease, the finding that UTP and UDP can induce both neural stem cell proliferation and dopaminergic differentiation could represent a starting point for the development of new therapeutic approaches for this pathology.
In the present study, we also performed neuroprotection experiments on human neuroblastoma SH-SY5Y cell line and on a primary culture of cortical neurons injured with two chemical products, MPP+ and glutamate. Our purpose was to examine the neuroprotective effects of Nucleo CMP Forte® in neuron cell death caused by excitotoxicity and in a model of Parkinson disease. Our results clearly show a protective effect of cytidine and uridine against the two kinds of cytotoxicity. In neuronal cells, MPP+ is taken up by catecholaminergic neurons via the dopaminergic reuptake system (dopamine transported, DAT). It is concentrated in the mitochondria, where it inhibits complex I of the respiratory chain, leading to ATP depletion and, eventually, to cell death of dopaminergic neurons [14]. In cardiomyocytes, UTP preserves mitochondrial function by inducing transient membrane depolarization, which protects against subsequent chemical or hypoxic stress [35]. On the other hand, glutamate (Glu)-induced excitotoxicity during brain ischemia leads to an intracellular cascade of cytotoxic events [36]. It has been described in an astrocytic cell line that UTP positively regulates the anti-apoptotic genes bcl-2 and bcl-x and down-regulates the pro-apoptotic gene bax, but little is known about neuroprotective effects of pyrimidine nucleotides on neuronal cell death. Our results show that Nucleo CMP Forte®protects against glutamate-induced neuronal death. The role of uracil nucleotides in brain ischemia remains to be established. However, several demonstrations of their protective role against cell death are now emerging [29]. Cell lysis such as necrotic death causes the extracellular release of the whole cytoplasmic and organelle content including nucleotides, whose concentration increases dramatically in the close proximity of the damaged tissue. The increased release of uracil nucleotides accompanied by receptor up-regulation might be the first response to damage, which triggers signals to the surrounding tissue. It is conceivable that pharmacological manipulation of this system may thus increase the beneficial effects of uracil nucleotides and reduces their noxious effects.
5. Conclusions
Nucleo CMP Forte® has shown significant neuroprotective effects against glutamateand MPP+-induced toxicity. We conclude that the data reported in this study may lead to the investigation of new pharmacological applications of Nucleo CMP Forte®. However, it will be necessary to extend these studies to more complex models in order to evaluate possible therapeutic applications in detail, and to elucidate the molecular mechanism of the neuroprotective effects.
6. Acknowledgements
The authors acknowledge and thank the following organizations and persons for their comments, scientific contribution and logistic support for this project: J. L. Lorenzo, Medical Department Ferrer, S. A. (Barcelona). Ferrer, S. A. provided an unrestrictive research grant. This work was partially supported by a public research grant from the Ministerio de Ciencia y Tecnología, Spain, (BIO2002-00128 and BIO2005-01591) and a grant from the Generalitat de Catalunya (2005SGR00270).
REFERENCES
- D. W. Choi and S. M. Rothman, “The Role of Glutamate Neurotoxicity in Hypoxic-Ischemic Neuronal Death,” Neuroscience, Vol. 13, 1990, pp. 171-182.
- R. L. Hayes, L. W. Jenkins and B. G. Lyeth, “Neurotransmitter-Mediated Mechanisms of Traumatic Brain Injury: Acetylcholine and Excitatory Amino Acids,” Journal of Neurotrauma, Vol. 1, 1992, pp. S173-S187.
- S. M. Rothman and J. W. Olney, “Excitotoxicity and the NMDA Receptor—Still Lethal after Eight Years,” Trends in Neuroscience, Vol. 10, No. 7, 1995, pp. 299-302. doi:10.1016/0166-2236(87)90177-9
- B. B. Meldrum and J. Garthwaita, “Excitatory Amino Acid Neurotoxicity and Neurodegenerative Disease,” Trends in Pharmacological Sciences, Vol. 11, No. 9, 1990, pp. 379-387. doi:10.1016/0165-6147(90)90184-A
- D. W. Choi, “Enhancement of Outward Potassium Current May Participate in Beta-Amyloid Peptide-Induced Cortical Neuronal Death,” Neuron, Vol. 1, No. 8, 1998, pp. 623-634. doi:10.1016/0896-6273(88)90162-6
- Y. Zhang and B. R. Bhavnani, “Glutamate-Induced Apoptosis in Neuronal Cells is Mediated via Caspase-Dependent and Independent Mechanisms Involving Calpain and Caspase-3 Proteases as well as Apoptosis Inducing Factor (AIF) and This Process is Inhibited by Equine Estrogens,” BMC Neuroscience, Vol. 7, 2006, p. 49. doi:10.1186/1471-2202-7-49
- M. J. Zigmond and E. M. Stricker, “Animal Models of Parkinsonism Using Selective Neurotoxins: Clinical and Basic Implications,” International Review of Neurobiology, Vol. 31, 1989, pp. 1-79. doi:10.1016/S0074-7742(08)60277-9
- O. Eberhardt and J. B. Schulz, “Apoptotic Mechanisms and Antiapoptotic Therapy in the MPTP Model of Parkinson’s Disease,” Toxicology Letters, Vol. 139, No. 2-3, 2003, pp. 135-151. doi:10.1016/S0378-4274(02)00428-9
- V. Gallai, G. Mazzotta, S. Montesi, P. Sarchelli and F. Del Gatto, “Effects of Uridine in the Treatment of Diabetic Neuropathy: An Electrophysiological Study,” Acta Neurologica Scandinavica, Vol. 86, No. 1, 1992, pp. 3-7. doi:10.1111/j.1600-0404.1992.tb08045.x
- L. Cartier, J. L. Castillo and R. Verdugo, “Effect of the Nucleus CMP Forte in 46 Patients with Progressive Spastic Paraparesis. Randomized and Blind Study,” Revista Medica de Chile, Vol. 124, No. 5, 1996, pp. 583-587.
- C. Kretschmar, S. Kaumeier and W. Hasse, “Medicamentous Therapy of Alcoholic Polyneuropathy. Randomized Double-Blind Study Comparing 2 Vitamin B Preparations and a Nucleotide Preparation,” Fortschritte der Medizin, Vol. 114, No. 32, 1996, pp. 439-443.
- B. Watting, G. Schalow, F. Heydenreich, R. Warzok and J. Cervós-Navarro, “Enhancement of Nerve Fibre Regeneration by Nucleotides after Peripheral Nerve Crush Damage. Electrophysiologic and Morphometric Investigations,” Arzeinmittelforshung, Vol. 42, No. 9, 1992, pp. 1075-1078.
- W. Araki and R. J. Wurtman, “How is Membrane Phospholipid Biosynthesis Controlled in Neural Tissues?” Journal of Neuroscience Research, Vol. 51, No. 6, 1998, pp. 667-674. doi:10.1002/(SICI)1097-4547(19980315)51:6<667::AID-JNR1>3.0.CO;2-9
- J. R. Marszalek and H. F. Lodish, “Docosahexaenoic Acid, Fatty Acid-Interacting Proteins, and Neuronal Function: Breastmilk and Fish Are Good for You,” Annual Reviews of Cell and Developmental Biology, Vol. 21, No. 21, 2005, pp. 633-657. doi:10.1146/annurev.cellbio.21.122303.120624
- S. I. Rapoport, “In Vivo Fatty Acid Incorporation into Brain Phosholipids in Relation to Plasma Availability, Signal Transduction and Membrane Remodeling,” Journal of Molecular Neuroscience, Vol. 16, No. 2-3, 2001, pp. 234-261. doi:10.1385/JMN:16:2-3:243
- E. S. Haugaard, K. B. Frantz and N. Haugaard, “Effect of Uridine on Cellular UTP and Glycogen Synthesis in Skeletal Muscle: Stimulation of UTP Formation by Insulin,” Proceedings of the National Academy of Sciences of USA, Vol. 74, No. 6, 1977, pp. 2339-2342. doi:10.1073/pnas.74.6.2339
- N. N. Suzuki, K. Koizumi, M. Fukushima, A. Matasuda and F. Inagaki, “Structural Basis for the Specificity, Catalysis, and Regulation of Human Uridine-Cytidinekinase,” Structure, Vol. 12, No. 5, 2004, pp. 751-764. doi:10.1016/j.str.2004.02.038
- A. Gella, J. Ponce, R. Cussóand N. Durany, “Effect of the Nucleotides Cmp and Ump on Exhaustion in Exercise Rats,” Journal of Physiology Biochemistry, Vol. 64, No. 1, 2008, pp. 9-17. doi:10.1007/BF03168230
- M. Okada, T. Nakagawa, M. Minami and M. Satoh, “Analgesic Effects of Intrathecal Administration of P2Y Nucleotide Receptor Agonists UTP and UDP in Normal and Neuropathic Pain Model Rats,” Journal of Pharmacology and Experimental Therapeutics, Vol. 303, No. 1, 2002, pp. 66-73. doi:10.1124/jpet.102.036079
- L. A. Teather and R. J. Wurtman, “Chronic Administration of UMP Ameliorates the Impairment of Hippocampal-Dependent Memory in Impoverished Rats,” Journal of Nutrition, Vol. 136, No. 11, 2006, pp. 2834-2837.
- T. Sakamoto, M. Cansev and R. J. Wurtman, “Oral Supplementation with Docosahexaenoic Acid and Uridine- 5’-monophosphate Increases Dendritic Spine Density in Adult Gerbil Hippocampus,” Brain Research, Vol. 28, 2007, pp. 50-59. doi:10.1016/j.brainres.2007.08.089
- L. Wang, M. A. Albrecht and R. J. Wurtman, “Dietary Supplementation with Uridine-5’-monophosphate (UMP), a Membrane Phosphatide Precursor, Increases Acetylcholine Level and Release in Striatum of Aged Rat,” Brain Research, Vol. 1133, No. 1, 2007, pp. 42-48. doi:10.1016/j.brainres.2006.11.048
- M. L. Tian, Z. Zou, H. B. Yuan, C. C. Wang, Q. F. Zhu, H. T. Xu, X. Gao and X. Y. Shi, “Uridine 5’-triphosphate (UTP) Protects against Cerebral Ischemia Reperfusion Injury in Rats,” Neuroscience Letters, Vol. 465, No. 1, 2009, pp. 55-60. doi:10.1016/j.neulet.2009.08.076
- M. B. Hansen, S. E. Nielsen and K. Berg, “Re-Examination and Further Development of a Precise and Rapid Dye Method for Measuring Cell Growth/Cell Kill,” Journal of Immunological Methods, Vol. 119, No. 2, 1989, pp. 203-210. doi:10.1016/0022-1759(89)90397-9
- S. M. Weissman, “Human Pyrimidine Metabolism,” The Journal of the American Medical Association, Vol. 195, No. 1, 1966, pp. 27-30. doi:10.1001/jama.195.1.27
- D. Lecca and S. Ceruti, “Uracil Nucleotides: From Metabolic Intermediates to Neuroprotection and Neuroinflammation” Biochemical Pharmacology, Vol. 75, No. 10, 2008, pp. 1869-1881. doi:10.1016/j.bcp.2007.12.009
- W. Fischer and U. Krügel, “P2Y Receptors: Focus on Structural, Pharmacological and Functional Aspects in the Brain,” Current Medicinal Chemistry, Vol. 14, No. 23, 2007, pp. 2429-1455. doi:10.2174/092986707782023695
- A. Brunschweiger and C. E. Müller, “P2 Receptors Activated by Uracil Nucleotides—An Update,” Current Medicinal Chemistry, Vol. 13, No. 3, 2006, pp. 289-312. doi:10.2174/092986706775476052
- H. R. Xie, L. S. Hu and G. Y. Li, “SH-SY5Y Human Neuroblastoma Cell Line: In Vitro Cell Model of Dopaminergic Neurons in Parkinson’s Disease,” Chinese Medical Journal, Vol. 123, No. 8, 2010, pp. 1086-1092.
- A. Cuppelo, A. Dierich, P. Mandel and M. Wintzerith, “In Vitro Protein Synthesis Activity of Poly(A) RNA from Neuroblastoma Cells,” Neurochemical Research, Vol. 5, No. 3, 1980, pp. 271-279. doi:10.1007/BF00964615
- M.-T. Tu, S.-F. Luo, C.-C. Wang, C.-S. Chien, C.-T. Chiu, C.-C. Lin and C.-M. Yang, “P2Y2 Receptor Mediated Proliferation of C6 Glioma Cells via Activation of Ras/Raf/MEK/MAPK Pathway,” British Journal of Pharmacology, Vol. 129, No. 7, 2000, pp. 1481-1489. doi:10.1038/sj.bjp.0703182
- J. T. Nearg, Y. Kang, Y. Bu, E. Yu, K. Akong and C. M. Peters, “Mitogenic Signaling by ATP/P2Y Purinergic Receptors in Astrocytes: Involvement of a CalciumIndependent Protein Kinase C, Extracellular SignalRegulated Protein Kinase Pathway Distinct from the Phosphatidylinositol-Specific Phospholipase C/Calcium Pathway,” The Journal of Neuroscience, Vol. 19, No. 11, 1999, pp. 4211-4220.
- F. Cavaliere, V. Nestola, S. Amadio, N. D’Ambrosi, D. F. Angelini, G.Sancesario, G. Bernardi and C. Volonté, “The Metabotropic P2Y4 Receptor Participates in the Commitment to Differentiation and Cell Death of Human Neuroblastoma SH-SY5Y Cells,” Neurobiology of Disease, Vol. 18, No. 1, 2005, pp. 100-109. doi:10.1016/j.nbd.2004.09.001
- J. Milosevic, A. Brandt, U. Roemuss, A. Arnold, F. Wegner, S. C. Schwarz, A. Storch, E. Zimmermann and J. Schwarz, “Uracil Nucleotides Stimulate Human Neural Precursor Cell Proliferation and Dopaminergic Differentiation: Involvement of MEK/ERK Signaling,” Journal of Neurochemistry, Vol. 99, No. 3, 2006, pp. 913-923. doi:10.1111/j.1471-4159.2006.04132.x
- S. Yitzhaki, E. Hochhauser, E. Porat and A. Shainberg, “Uridine-5’-triphosphate (UTP) Maintains Cardiac Mitochondrial Function Following Chemical and Hypoxic Stress,” Journal of Molecular and Cellular Cardiology, Vol. 43, No. 5, 2007, pp. 653-662. doi:10.1016/j.yjmcc.2007.07.060
- H. Y. Cheng, M. T. Hsieh, C. R. Wu, F. H. Tsai, T. C. Lu, C. C. Hsieh, W. C. Li, Y. T. Lin and W. H. Peng, “Schizandrin Protects Primary Cultures of Rat Cortical Cells from Glutamate-Induced Excitotoxicity,” Journal of Pharmacological Science, Vol. 107, No. 1, 2008, pp. 21-31. doi:10.1254/jphs.FP0072394
NOTES
#To our deep regret Ms NúriaDurany died during this study.

