World Journal of Neuroscience
Vol.4 No.2(2014), Article ID:45935,8 pages DOI:10.4236/wjns.2014.42023
Evaluating “Cosmetic Therapy” by Using Near-Infrared Spectroscopy
Mayumi Ikeuchi1,2, Keishi Saruwatari3, Yumi Takada4, Mika Shimoda4, Ayako Nakashima4, Masao Inoue5, Takashige Oroguchi2,6, Naoaki Ishii2,6, Fumihito Yoshii7, Munetaka Haida7,8
1Department of Nursing, Tokai University School of Health Sciences, Kanagawa, Japan
2Life Care Center, Tokai University School of Medicine, Kanagawa, Japan
3Kanebo Cosmetics, Inc., Innovative Beauty Science Laboratory, Kanagawa, Japan
4Kanebo Cosmetics, Inc., Beauty Research Laboratory, Tokyo, Japan
5SHIMADZU CORPORATION, Market Development Group, Tokyo, Japan
6Department of Molecular Life science, Basic Medical Science and Molecular Medicine, Tokai University School of Medicine, Kanagawa, Japan
7Department of Neurology, Tokai University School of Medicine, Kanagawa, Japan
8Tokai University Junior College of Nursing and Medical Technology, Kanagawa, Japan
Email: ikeuchi@tokai-u.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 6 April 2014; revised 1 May 2014; accepted 7 May 2014
ABSTRACT
This study examined the effect of cosmetic therapy on frontal lobe activation as revealed by topographic near-infrared spectroscopy (NIRS). We evaluated emotional responses to a photograph of a face with/without makeup by 22 healthy female volunteers (mean age, 52 ± 10.5 years). The results of the first-round analysis showed a significant increase of oxy-Hb in the frontal lobe area when the subject looked at a photograph of herself made up as compared to not made up. In a later round of analysis, we divided the subjects into 2 groups having contrasting scores on the Profile of Mood States-Short Form Japanese version. One group was classed as “high vigor” (a common standard pattern) and the other as “low vigor” (depression-tendency pattern). The made-up/not made-up difference did not have any effect on the oxy-Hb level in the frontal lobe in the high vigor group. In contrast, makeup produced a significant increase in the oxy-Hb level over a wide frontal area in the low vigor group, which indicated widespread frontal lobe activation. This result indicates a beneficial effect of cosmetic therapy on the brain function of patients with depression and/ or dementia.
Keywords:NIRS, Frontal Lobe, Cosmetic Therapy, Depression, Emotion, POMS

1. Introduction
Japan’s ageing society faces the problem of an ever-increasing number of patients with dementia and/or depression. Many institutions such as nursing homes are experimenting with various approaches to preserve or recover brain function, e.g., cosmetic therapy, music therapy, recreation therapy, and exercise therapy. These therapies have been shown to improve the quality of life of older people [1] and are reported to be effective in reducing depression [2] . In addition, some reports show that they reduce agitation in Alzheimer’s disease [3] .
Cosmetic therapy tries to alleviate the symptoms of dementia and/or depression by introducing female patients with dementia to the use of makeup. Medical opinion concurs that changes of the patient’s orientation toward improvement in communication power, social life, and appearance underlie the acceptance of cosmetic therapy. However, the effects of cosmetic therapy on elderly women are not clear. Since cosmetic therapy has not yet established its usefulness as a medical therapy, it is necessary to show evidence that it can play complementary and alternative roles in medicine before it can be widely adopted.
Cerebral activation studies have been performed using functional magnetic resonance imaging (fMRI), xenon contrast computed tomography (Xe-CT) and positron emission tomography (PET) [4] -[8] . Recently, the development of near-infrared spectroscopy (NIRS) has enabled noninvasive and bedside measurements of regional cerebral blood volume changes in terms of the relative concentrations of oxy-hemoglobin (oxy-Hb), deoxy-hemoglobin (deoxy-Hb), and total hemoglobin (total-Hb), with high time resolution. NIRS has been applied to various cognitive studies, including sensorimotor, visual perception, language, development studies, and clinical studies [9] -[13] . We used a topographic NIRS system to investigate hemodynamic responses in the prefrontal area during performance of a task related to cosmetic therapy in an attempt to clarify the effects of this therapy.
2. Methods
2.1. Subjects
The volunteers were recruited via posters placed in various locations around the study region of Kanagawa prefecture. Twenty-two healthy, right-handed, adult women, aged 30 - 60 years old (mean age, 52 ± 10.5 years) participated in this study. Informed consent was obtained prior to conducting the study, in compliance with the Declaration of Helsinki. The Ethical Review Board of Tokai University approved this study (No. 11R-114).
2.2. Makeup and Photography
Prior to the NIRS measurements, all subjects were photographed without makeup (real face) by a professional photographer. Then a professional makeup artist from the Kanebo Cosmetic Inc. Beauty Research Laboratory (Tokyo, Japan) made up the subjects, who were then photographed wearing makeup. The 2 photographs of each subject were not shown to her until the NIRS measurement. After the NIRS measurements, subjects were surveyed using an interview form to determine which photograph they preferred.
2.3. Profile of Mood
The participants’ subjective mental states were assessed using the Profile of Mood States-Short Form Japanese version (POMS-SFJ) just before NIRS measurement.
2.4. Near-Infrared Spectroscopy
NIRS measurements were performed using a multichannel instrument (FOIRE-3000, Shimadzu Co. Kyoto Japan). The system consisted of an array of optodes comprising 8 light source and 8 detector fibers arranged for 22-channel simultaneous recording. Three wavelengths (780, 805, and 830 nm) were used to determine hemoglobin concentrations. The system can detect changes in concentrations of oxygenated hemoglobin (oxy-Hb), deoxygenated hemoglobin (deoxy-Hb) and total hemoglobin in cortical regions of the brain. Inter-optode distances were set at 3.0 cm. The alignments of each fiber and channel are indicated in Figure 1. The optodes were brought firmly into contact with the head using a holder. The probes were placed on the subject’s frontal region and measured changes in the relative concentrations of hemoglobin states at 22 measurement points in a 14 × 11-cm area, with the lowest probe positioned along the Fp1-Fp2 line, according to the international 10/20 system of electro-encephalography (EEG), which was used for optode positioning. This optode array can create two-dimensional 14 × 11-cm Hb concentration images (i.e., topographies).
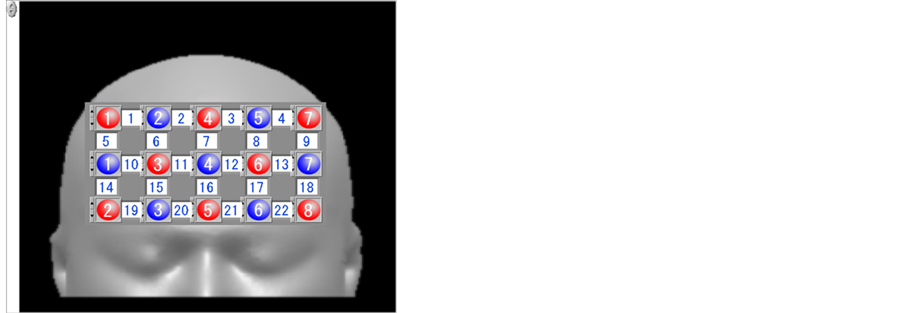
Figure 1. Layout of optical fiber connections and channels. Red circles show transmitters, blue circles show receivers, and white boxes show channels, with numbers indicated.
2.5. Study Task
Changes of oxy-Hb were measured with NIRS during the following 2 conditions.
Task A (15 s): Subject was shown her own real-face photograph.
Task B (15 s): Subject was shown her own made-up-face photograph.
The study design (Block design) is shown in Figure 2. Task A and task B were presented on a screen alternately (ABABA), for 15 s each. The subject was instructed to watch the photograph sequence.
2.6. Data Analyses
Although 3 NIRS variables were obtained, here we mainly used the oxy-Hb results because we consider oxy-Hb to be the most sensitive variable of hemodynamic brain responses (Hoshi et al., 2001; Strangman et al., 2002b). We calculated an activation index (AI) as follows.
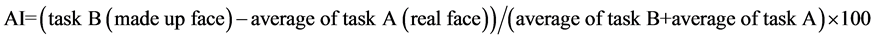
The AI becomes zero when there is no difference in task A and task B. The significance of hemodynamic changes was determined using the t-test, and we considered a change with p < 0.05 to be statistically significant.
3. Results
Figure 3 shows the results of our first round of analysis: AI calculated using the full oxy-Hb data of the block design. The AI was calculated by using 2 task B (makeup face) responses and 3 task A (real face) responses. In channel numbers 9, 15, 19, and 21, oxy-Hb was significantly increased when subjects looked at the photograph of the made-up face over when they looked at the unmade-up face. Figure 4 shows the AI changes calculated using only the first performances of task A and B. which were the first exposures to the photographs for the subjects. All channels except those in the right upper areas show significantly increased oxy-Hb. On the other hand, when the AI change was calculated using the second occurrences of tasks A and B (Figure 5), only channel number 9 showed a significant increase. Channel numbers 17, 18, and 22 showed a significant decrease.
Figure 6 shows the results of our second round of analysis: AI differences segregated by the subjects’ photograph preference. In this experiment, 15 subjects preferred their made-up face and 7 subjects did not. However, there was no statistically significant AI difference between the 2 groups.
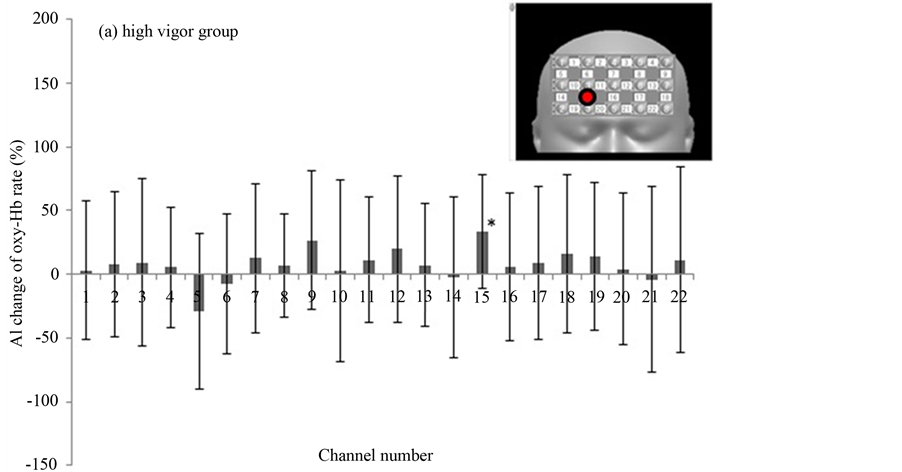
In the third round of analysis, we assessed the participants’ subjective psychological states by using the POMS, from which we calculated measures of “depression” and “vigor”. The subjects were divided into 2 groups based on POMS scores. The cutoff point was zero. Eleven subjects were in the high vigor (HV) group (a common standard pattern) and another group (11 subjects) were in the low vigor (LV) group (depression tendency pattern). In the HV group, few subjects showed a significant increase of AI to the photograph of made-up vs. real face. Only channel 15 was significantly increased in oxy-Hb (Figure 7(a)). In the LV group, however, the AIs were significantly increased in many channels (Figure 7(b)).
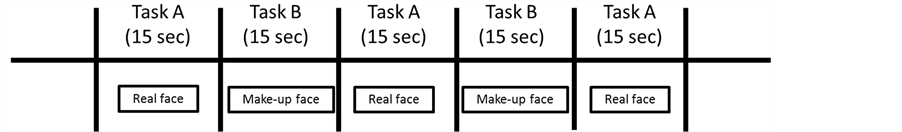
Figure 2. NIRS experimental paradigm (block design 15 s × 15 s).
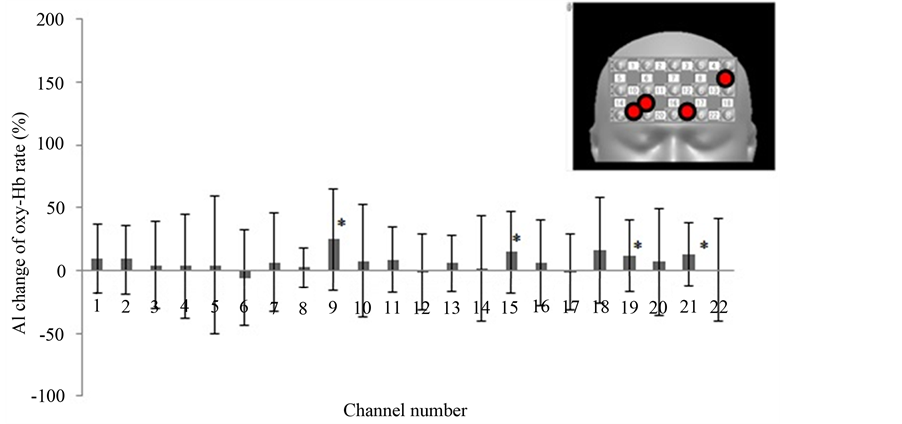
Figure 3. The AI change based on oxy-Hb concentration measured in each channel (ABABA block design). The data are expressed as an activation index (AI). AI = (time average of 2 performances of task B (makeup face) − time average of 3 performances of task A (real face))/(two-performance time average of task B + three-performance time average of task A) × 100. All figures: *, p < 0.05. Inset, upper right; red circles show channels of significantly increased oxy-Hb.
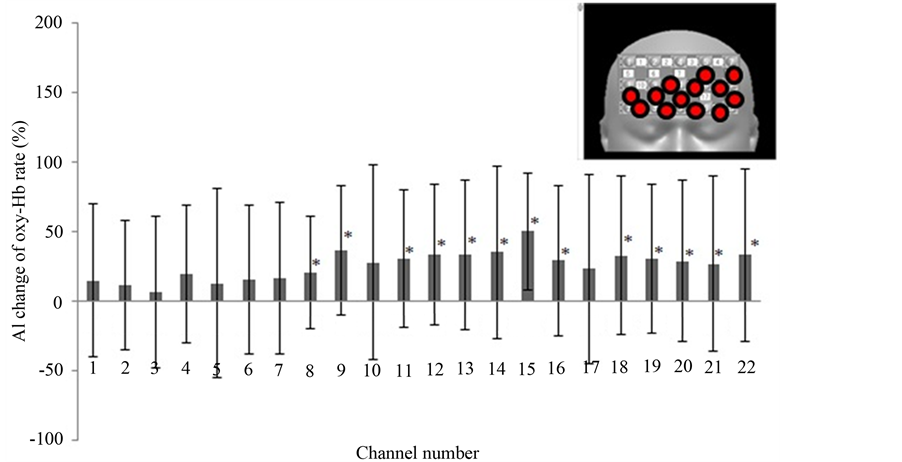
Figure 4. The AI changes in oxy-Hb in all channels (first presentations of pictures). The AIs were calculated from the time average of the first task B performance (makeup face) minus that of the first task A performance (real face). Inset, upper right; red circles show channels of significantly increased oxy-Hb.
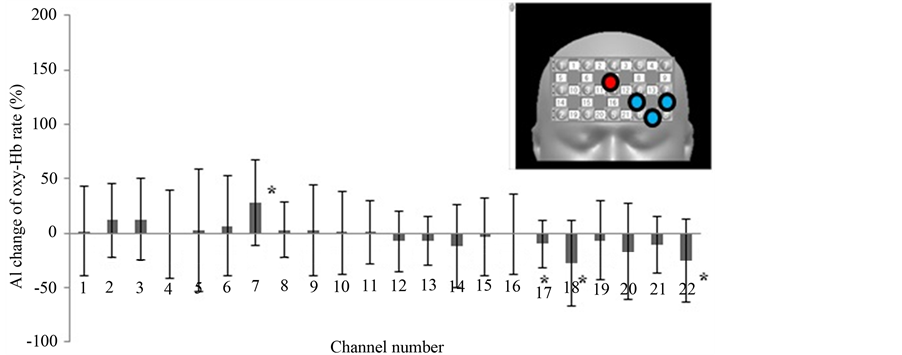
Figure 5. The AI changes in oxy-Hb in all channels (second presentations of pictures). The AIs were calculated from the time average of the second task B performance (makeup face) minus that of the second task A performance (real face). Inset, upper right; red circle shows a channel of significantly increased oxy-Hb. Blue circles show channels of significantly decreased oxy-Hb.
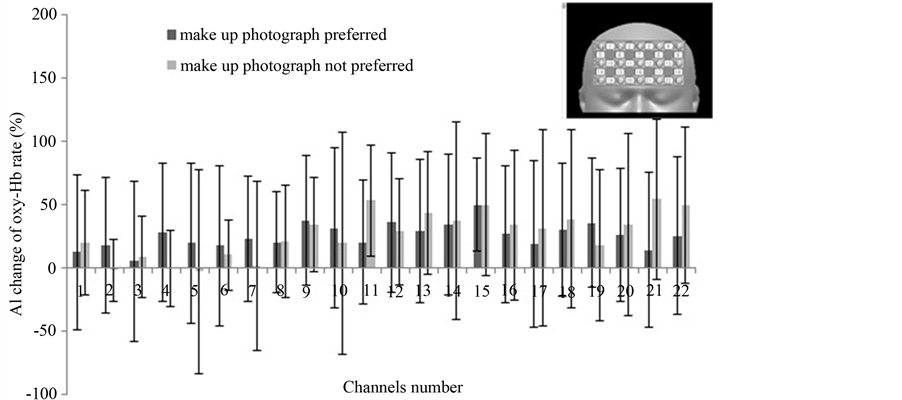
Figure 6. The AI changes in oxy-Hb broken down by preference for makeup vs. no makeup photograph. Values are mean ± standard deviation. No difference is significant.
4. Discussion
We used NIRS to investigate hemodynamic responses in prefrontal areas to cosmetic therapy. To our knowledge, this is the first report describing brain activation due to cosmetic therapy.
In this study, the subjects were made up by a professional makeup artist and were photographed by a professional photographer. Neither their made-up face nor the photographs were shown to them until the NIRS measurement. One reason why photographs were used as stimuli, rather than, say, mirrors, was to prevent the sight of the head-mounted apparatus from perturbing the experiment. In addition, it was important to measure brain activity when the subjects looked at their made-up face for the first time. In the AI change calculated using all instances of tasks A and B, when the subjects looked at the photographs, the oxy-Hb were increased only in 4 channels (Figure 3). However, when the subjects looked at the photographs for the first time (first B compared to first A), we found increased oxy-Hb on many channels (Figure 4). These results indicate that frontal lobe oxy-Hb had been increased over a large area, and that frontal lobe activation was improved. However, when the subjects looked at the photographs the second time (second B compared to second A), there was no sharp re-
 (a)
(a)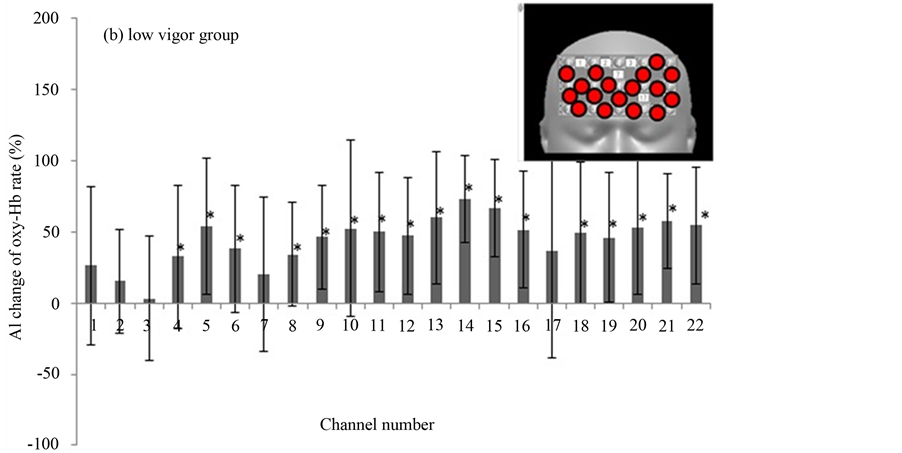 (b)
(b)
Figure 7. The AI changes broken down by high vigor group vs. low vigor group as defined by their Profile of Mood States scores. Values are mean ± standard deviation. Inset, upper right; red circles show channels of significantly increased oxy-Hb.
sponse such as occurred at the first time. This result indicates that the first impression is important for activating the frontal cortex. These results suggest that cosmetic therapy could be useful for activating brain functions.
In Japan, many elderly women use no makeup. Cosmetic therapy involving this first impression effect is therefore practical in nursing homes. Although there have been a few experiments with this cosmetic effect, we could not find reliable reports.
In our second round of analysis, we studied the effect of a preference for makeup on brain activation. Using an interview form, we found that 15 subjects preferred their made-up appearance and 7 subjects did not. However, there was no difference in change in oxy-Hb associated with this preference (Figure 6).
Studies of the effects of photograph viewing on brain function are rare. However, effects of fragrance (e.g., household fabric softener as agreeable stimulus and imitation sweat odor as disagreeable stimulus) on the electrocardiogram and brain blood flow of 5 subjects have been reported. An unpleasant smell was found to activate the prefrontal cortex more than did a pleasant smell [14] . The effect of palatability has also been tested. Although a preferred stimulus was found to activate the brain, individual differences were too large to permit statistical significance. In our data, brain activations in response to the no-makeup face were increased from baseline but there was likewise no statistically significant difference.
In third-round analysis, we studied the interaction of the mental state of the subjects with the effect of makeup on brain function. The subjects were divided into 2 groups based on POMS score. One group was the high vigor (HV) group (a common standard pattern) and the other group was the low vigor (LV) group (depression tendency pattern). The HV group did not show any significant changes in oxy-Hb signals when looking at the photograph of her made-up compared to no-makeup face, except in channel 15, which showed significantly increased oxy-Hb (Figure 7(a)). In contrast, in the LV group the oxy-Hb signal was significantly increased in many channels (Figure 7(b)). Suto et al. reported that oxy-Hb elevations in patients with major depressive disorder were smaller than in control subjects during a word fluency task [15] . Most previous NIRS studies on such patients used finger tapping [16] and word fluency tasks [9] . These authors reported that decreased oxy-Hb activation in depression is consistent with decreased cerebral blood flow and metabolism in the dorsolateral prefrontal cortex in the resting state. This has been observed in functional neuroimaging studies using other methodologies, such as PET, SPECT, and fMRI [17] -[20] . Moreover, the decreased cerebral blood flow activation during the cognitive task period in these studies indicates that the cerebral cortex of patients with depression cannot obtain a sufficient increase in blood supply to compensate increased oxygen consumption as in the case of healthy control subjects. The continued lack of adequate blood supply could result in a decrease of neuronal activity in the cerebral cortex, resulting in the diagnosis of depression [11] . However, we cannot distinguish whether decreased blood flow during the task period is a result of decreased frontal neuronal activity, or whether decreased neuronal excitability suppresses an increase of cerebral blood flow. In general, oxy-Hb activation in patients with depression seems to be smaller or suppressed relative to healthy people. In our study, although subjects were not patients with depression, but merely had a tendency toward depression as shown by the POMS score, they showed significant increases of oxy-Hb in many channels when watching the makeup photograph. Although we cannot clearly explain this result, we can speculate that a difference between the real and made-up face has greater impact on subjects in a depressive state than in those not in a depressive state, because it has emotional connotations. This might explain our results. Since it is difficult to evaluate individual emotional states, we could not get information about this point other than a preference for the made-up face. Our research results may enable us to evaluate the emotional change in the subject due to makeup.
5. Conclusions
Our study suggests that facial makeup might activate the cerebral frontal lobe. These results may indicate beneficial effects of cosmetic therapy on brain function in female patients with depression and probably patients with dementia. However, few studies have also evaluated the emotional state of the subjects by means of NIRS. Although this study was limited to a small number of subjects, our result shows a beneficial effect of cosmetic therapy. NIRS could be a useful research tool for the examination of brain function, and for physiological and psychological research.
Furthermore detail examinations are required to depressed patient. In addition, comprehensive histopathological and immunohistochemical research are required to determine the mechanism by cosmetic therapy.
Acknowledgements
We would like to express deep gratitude to the Health Medical Industrial Promotion Conference and to many of the staff of the Tokai University for their support of this research.
References
- Hays, T. and Minichiello, V. (2005) The Contribution of Music to Quality of Life of Older People: An Australian Qualitative Study. Aging & Society, 25, 261-278. http://dx.doi.org/10.1017/S0144686X04002946
- Ashida, S. (2000) The Effect of Reminiscence Music Therapy Sessions on Changes in Depressive Symptoms in Elderly Persons with Dementia. Journal of Music Therapy, 37, 170-182. http://dx.doi.org/10.1093/jmt/37.3.170
- Jennings, B. and Vance, D. (2002) The Short-Term Effects of Music Therapy on Different Types of Agitation in Adults with Alzheimer’s. Act Adapt Aging, 26, 27-33. http://dx.doi.org/10.1300/J016v26n04_03
- Goldman, R., Stern, J., Engel Jr., J. and Cohen, M. (2002) Simultaneous EEG and fMRI of the Alpha Rhythm. Neuroreport, 13, 2487-2492. http://dx.doi.org/10.1097/00001756-200212200-00022
- Strangman, G., Boas, D. and Sutton, J. (2002a) Non-Invasive Neuroimaging Using Near-Infrared Light. Biological Psychiatry, 52, 679-693. http://dx.doi.org/10.1016/S0006-3223(02)01550-0
- Boas, D., Dale, A. and Franceschini, M. (2004) Diffuse Optical Imaging of Brain Activation: Approaches to Optimizing Image Sensitivity, Resolution, and Accuracy. Neuroimage, 23, S275-S288. http://dx.doi.org/10.1016/j.neuroimage.2004.07.011
- Feige, B., Scheffler, K., Esposito, F., Di Salle, F. and Herrnann, M.J. (2005) Cortical and Subcortical Correlates of Electroencephalographic Alpha Rhythm Modulation. Journal of Neurophysiology, 93, 2846-2872. http://dx.doi.org/10.1152/jn.00721.2004
- Strangman, G., Gulver, J., Thompson, J. and Boas, D. (2002b) A Quantitative Comparison of Simultaneous BOLD fMRI and NIRS Recordings during Functional Brain Activation. Neuroimage, 17, 719-731. http://dx.doi.org/10.1006/nimg.2002.1227
- Hock, C., Villringer, K., Muller-Spahn, F., Wenzel, R., Heekeren, H., Schuh-Hofer, S., Hofmann, M., Minoshima, S., Schwaiger, M., Dirnagl, U. and Villringer, A. (1997) Decrease in Parietal Cerebral Hemoglobin Oxygenation during Performance of a Verbal Fluency Task in Patients with Alzheimer’s Disease Monitored by Means of Near-Infrared Spectroscopy (NIRS)—Correlation with Simultaneous rCBF-PET Measurements. Brain Research, 755, 293-303. http://dx.doi.org/10.1016/S0006-8993(97)00122-4
- Hoshi, Y., Oda, I., Wada, Y., Ito, Y., Yamashita, Y., Oda, M., Ohta, K., Yamada, Y. and Tamura, M. (2000) Visuospatial Imagery Is a Fruitful Strategy for the Digit Span Backward Task: A Study with Near-Infrared Optical Tomography. Cognitive Brain Research, 9, 339-342. http://dx.doi.org/10.1016/S0926-6410(00)00006-9
- Hoshi, Y., Kobayashi, N. and Tamura, M. (2001) Interpretation of Near-Infrared Spectroscopy Signals: A Study with a Newly Developed Perfused Rat Brain Model. Journal of Applied Physiology, 90, 1657-1662.
- Wenzel, R., Wobst, P., Heekeren, H., Kwnong, K., Brandt, S., Kohl, M., Obrig, H., Dirnagl, U. and Villringer, A. (2000) Saccadic Suppression Induces Focal Hypooxygenation in the Occipital Cortex. Journal of Cerebral Blood Flow & Metabolism, 20, 1103-1110. http://dx.doi.org/10.1097/00004647-200007000-00010
- Baird, A., Kagan, J., Gaudette, T., Walz, K., Hershlag, N. and Boas, D. (2002) Frontal Lobe Activation during Object Permanence: Data from Near-Infrared Spectroscopy. Neuroimage, 16, 1120-1125. http://dx.doi.org/10.1006/nimg.2002.1170
- Kanai, H., Tsuji, H., Asanomi, M., Iahizawa, H., Nishimatsu, T. and Miyasaka, H. (2008) Influence on Heart Rate Variability and Neuronal Activity by Inhalation of Fragrance with Different Preference. Kansei Engineering International Journal, 7, 469-476. (In Japanese with English abstract).
- Suto, T., Fukuda, M., Ito, M., Uehara, T. and Mikuni, M. (2004) Multichannel Near-Infrared Spectroscopy in Depression and Schizophrenia: Cognitive Brain Activation Study. Biological Psychiatry, 55, 501-511. http://dx.doi.org/10.1016/j.biopsych.2003.09.008
- Colier, W., Quaresima, V., Oeseburg, B., Ferrari, M. (1999) Human Motor-Cortex Oxygenation Changes Induced by cyclic Coupled Movements of Hand and Foot. Experimental Brain Research, 129, 457-461. http://dx.doi.org/10.1007/s002210050913
- Mayberg, H., Brannan, S., Tekell, J., Silva, J., Mahurin, R., McGinnis, S. and Jerabek, P. (2000) Regional Metabolic Effects of Fluoxetine in Major Depression: Serial Changes and Relationship to Clinical Response. Biological Psychiatry, 48, 830-843. http://dx.doi.org/10.1016/S0006-3223(00)01036-2
- Kennedy, S., Evans, K., Krüger, S., Mayberg, H., Meyer, J., McCann, S., Arifuzzman, A., Houle, S. and Vaccarino, F. (2001) Changes in Regional Brain Glucose Metabolism Measured with Positron Emission Tomography after Paroxetine Treatment of Major Depression. American Journal of Psychiatry, 158, 899-905. http://dx.doi.org/10.1176/appi.ajp.158.6.899
- Strakowski, S., Adler, C. and DelBello, M. (2002) Volumetric MRI Studies of Mood Disorders: Do They Distinguish Unipolar and Bipolar Disorder? Bipolar Disorders, 4, 80-88. http://dx.doi.org/10.1034/j.1399-5618.2002.01160.x
- Moore, G. and Galloway., M. (2002) Magnetic Resonance Spectroscopy: Neurochemistry and Treatment Effects in Affective Disorders. Psychopharmacology Bulletin, 36, 5-23.

