Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 206-212 doi:10.4236/jbise.2010.32027 Published Online February 2010 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online February 2010 in SciRes. http://www.scirp.org/journal/jbise Pressure shift mediated anoikis of endothelial cells in the flow field in vitro Jia Hu1, Er-Yong Zhang1, Jiang Wu2, Wei-Lin Xu3, Huai-Qinq Chen2, Ying-Kang Shi1, Ying-Qiang Guo1,3* 1Dept. Thoracic & Cardiovascular surgery, West China Hospital of Sichuan University, Chengdu, China; 2Institute of Biomedical Engineering, West China Medical Center, Sichuan University, Chengdu, China; 3The State Key Lab of Hydraulics on High Speed Flow, Sichuan University, Chengdu, China. Email: *drguoyq@hotmail.com Received 16 November 2008; revised 10 December 2009; accepted 13 December 2009. ABSTRACT Dramatic changes of pressure in the local circulation flow field would lead to alterations in biorheological characteristics of Endothelial cells(ECs), and futher resulted in the apoptosis induced by loss of anchorage, a form of cell death known as anoikis. In this study, we set levels of pressure(negative and positive pres- sure) loaded ECs groups and non-activated cultured ECs ,single shear stress loaded ECs as control group to demonstrate the effects of pressure shift on cell morphogenesis and adhesion. Furthermore, we in- vestigate the effects of pressure shift on ECs proli- feration and apoptosis to elucidate the influences of pressure shift on vitality of ECs. We present these data here to suggest that the negative pressure might be another important factor beyond velocity and shear stress in biomechanical impairment on ECs, then to trigger the apoptosis with the extracellular matrix (ECM) detachment (anoikis). As the negative pressure is thought to play a role in the anoikis process, these results have implications for both the path- ogenesis and therapeutics investigations of stenostic vessel dis- eases and the future vascular tissue engineering. Keywords: Endothelial Cells; Anoikis Cell Adhesion; Pressure; Flow Field 1. INTRODUCTION The role of the Extracellular matrix (ECM) goes beyond providing the physical scaffold on which the Endothelial cells (ECs) adhere, it also provides ECs with information for proliferation, migration, differentiation and survival through the structural and functional links. Loss of these links with ECM could induce apoptosis which has been termed as anoikis, a Greek ancient word meaning “homelessness”. Previous Hydrodynamics investigations on the mechanisms of the cardiovascular wall damage, in vitro assays and in vivo models, focused on the rela- tionship between the velocity, shear stress and the ECs, while investigations on the pressure shift (especially the negative pressure) mediated anchorage-related apoptosis (anoikis) of ECs in vitro were rarely described. Based on the engineering hydrodynamics advance, the dilated downstream of the stenosis could lead to a decrease of wall pressure and an increase of pressure pulsation, fur- ther resulted in a low pressure environment to generate the cavitation phenomenon which would damage the wall structure severely [1,2]. We therefore suggest that the distribution and variations of pressure located down- stream of the stenostic vessel may be another factor for biomechanical impairment on ECs, then may contribute to the pathogenesis of cardiovascular stenostic diseases. In the present study, we developed an efficient assay for the effects of pressure shift on the expression of cy- toskeleton(F-actin), Vascular adhesion molecule (VCAM) and one of the important transmembrane heterodimeric receptors(IntegrinαVβ3). Combined with our analysis of Ecs proliferation, apoptosis and the expression of apop- tosis-associated protein (Caspase-3, P53, Bcl-2 and Fas), we also presented correlative evidence that the negative pressure played a certain role in the genesis and progress of anoikis in the flow field in vitro. 2. MATERIALS AND METHODS 2.1. Cell Cultures and Maintenance Human umbilical vein ECs EA.Hy926 obtained from Jiangsu Institute of Hematology were cultured in a 5% CO2 atmosphere at 37³C, in RPMI medium (Gibco BRL, USA) supplemented with 10% fetal bovine serum(FBS). Cells were detached by D-Hank’s solution with 0.25% trypsin 1ml and then repelleted to suspend in the RPMI medium. The supernatants were collected and centri- fuged at 1000rpm for 5min in a MIKRO12–24 centri- *The study supported by NSF of China (Grant NO. 30700149, 30670515 and Youth Scientific Fund of Sichuan University (Grant NO.06062).  J. Hu et al. / J. Biomedical Science and Engineering 3 (2010) 206-212 207 Copyright © 2010 SciRes. JBiSE fuge at 4℃. The purified cells were collected, tested as previously described [3,4] and viability was determined by trypan blue exclusion. After been attached to the fi- bronectin plates (plastic plates coated for 30min at 37℃ in CO2 incubator with 50ug/ml of fibronectin, washed twice with D-Hanks solution before use), the cells were grown at 37℃ in 5% CO2 incubator (Heraeus, Germany) with RPMI medium to confluence. 2.2. Levels of Pressure Loading in the Flow Experiment The flow system was remanufactured from the system which was previously described by H.Q. Chen [5] (Fig- ure 1). The plastic slides containing the endothelial monolayer were inserted into the parallel-plate flow chamber that was installed between the upper and lower reservoir connected by tubing. Continuous flow in this system was maintained by circulating cell cul- ture medium (as arrow shown in Figure 1) by a peri- staltic pump installed between the upper and lower reservoir, while altitude difference provided constant flow through the chamber to expose the bottom of the inserts to laminar flow at a consistent levels of pressure. As the periodical fluctuation of the pressure that caused by the peristaltic pump and fluid flow could interfere the experiment results, we set two reservoirs as a feed- back regulator to maintain consistent and steady pres- sure. The numerical analysis of the chamber flow per- formed by Fluent 6.0 indicated the flow in chamber was 1) laminar flow; 2) two-dimensional flow; 3) suf- ficient developed steady flow; 4) the pressure distrib- uted in chamber averagely; 5) maintained steady flow in negative pressure environment. We set nonactivated cultured ECs(Con), single shear stress (1.85 dyn/cm2) loaded ECs (S-con)as control groups and levels of pressure loaded groups in the context of low shear stress (1.85dyn/cm2): –10cmH2O(N10), –20cmH2O(N20), –40cmH2O(N40), +20cmH2O(P20) and +40cmH2O(P40). Figure 1. The schematic diagram of the improved paral alized by using BODIPY FL phalloidin (Molecularlel plate flow chamber: 1) upper reservoir; 2) peristaltic pump; 3) lower reservoir and; 4) flow chamber. 2.3. F-actin and Adhesion Molecule Analysis Loaded by levels of pressure for 2h and fixed by 4% paraformaldehyde at 4℃ for 15min, ECs were perme- abilized in 1% Triton X-100 and then F-actin was visu Probes, USA). The cell membranes were imaged at an excitation of 505nm and emission of 512nm by the laser confocal scanning microscope (Bio-Rad Mre-1024ES) and fluorescence density value analysis of F-actin were tested by IMAGEPRO plus 5.0(Media Cybernetics Inc.). The collected cells were incubated with specific mono- clonal antibody (PE-VCAM monoclonal antibody in 1:40 dilution, FITC-IntegrinαVβ3 monoclonal antibody LM609 in 1:100 dilution) respectively for 20 minutes. Cells were then resuspended in PBS to a density of 2~ 3×105cells/ml. The levels of cell surface fluorescently labeled protein were quantified by immunofluorescent flow cytometry (ELITE ESP, Coulter, USA): we set the fluorescent density value of non-activated cultured ECs(Con) as 100% and the fluorescent density value of other groups as Related fluorescent density value (RF%). 2.4. Proliferation and Apoptosis Assay To label chromosomal DNA with propidium iodide (PI), the pressure loaded cells were washed twice with PBS, 0.25% trypsin and the resulting cell pellet was resus- pended at 1×105 cells/ml in PBS containing 100ug/ml of PI and 500mg/ml RNase (Sigma; St. Louis, MO) for a 30min incubation at 4℃. The stained cells were then analyzed by flow cytometry and quantification was per- formed by using Cell Quest (Becton–Dickinson, Moun- tain View, CA). We use PI [Proliferation Index, PI=(S + G2M) / (G0/1+ S + G2M ) × 100%] and AI (Apoptosis Index, AI=number of cells displaying red fluorescence lower than the G0-G1 diploid peak / total number of cells × 100%) to assay ECs proliferation and apoptosis changes in cell cycle. RT-PCR technology as previously described [6,7,8] and Western blot analysis were applied to caspase–3, p53, Bcl–2 and Fas protein expression assay. 2.5. Statistics All experiments were repeated three times. Statistic in- formation was analyzed by one sample T-test with SPSS11.5 and differences at P<0.05 were considered statistically significant. 3. RESULTS 3.1. The Effects of Pressure Shift on Cells Morphological Changes Stained green-fluorescence, the F-actin filament of non-activated cultured ECs(Con) showed no oriented 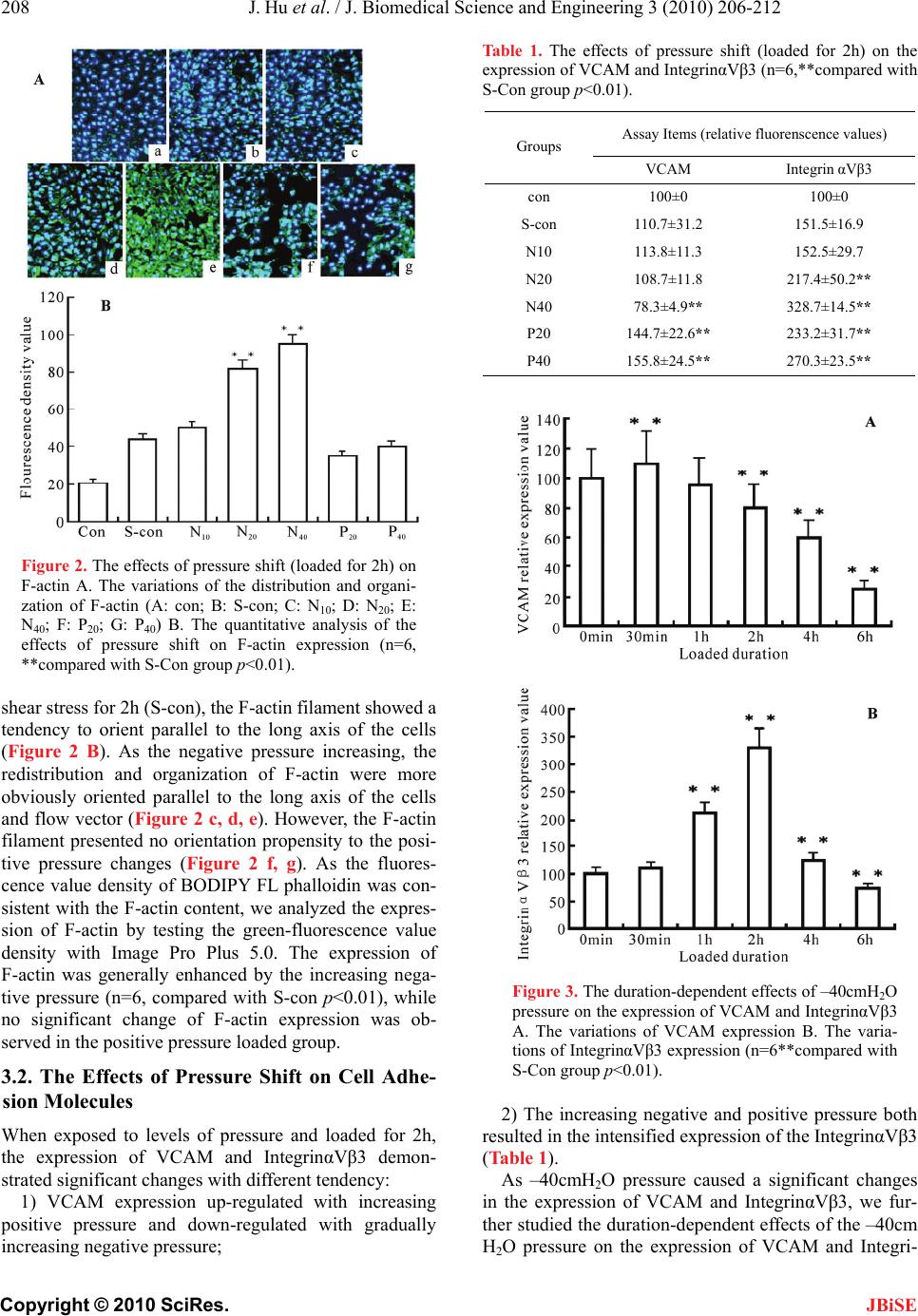 208 J. Hu et al. / J. Biomedical Science and Engineering 3 (2010) 206-212 Copyright © 2010 SciRes. JBiSE Figure 2. The effects of pressure shift (loaded for 2h) on F-actin A. The variations of the distribution and organi- zation of F-actin (A: con; B: S-con; C: N10; D: N20; E: N40; F: P20; G: P40) B. The quantitative analysis of the effects of pressure shift on F-actin expression (n=6, **compared with S-Con group p<0.01). shear stress for 2h (S-con), the F-actin filament showed a tendency to orient parallel to the long axis of the cells (Figure 2 B). As the negative pressure increasing, the redistribution and organization of F-actin were more obviously oriented parallel to the long axis of the cells and flow vector (Figure 2 c, d, e). However, the F-actin filament presented no orientation propensity to the posi- tive pressure changes (Figure 2 f, g). As the fluores- cence value density of BODIPY FL phalloidin was con- sistent with the F-actin content, we analyzed the expres- sion of F-actin by testing the green-fluorescence value density with Image Pro Plus 5.0. The expression of F-actin was generally enhanced by the increasing nega- tive pressure (n=6, compared with S-con p<0.01), while no significant change of F-actin expression was ob- served in the positive pressure loaded group. 3.2. The Effects of Pressure Shift on Cell Adhe- sion Molecules When exposed to levels of pressure and loaded for 2h, the expression of VCAM and IntegrinαVβ3 demon- strated significant changes with different tendency: 1) VCAM expression up-regulated with increasing positive pressure and down-regulated with gradually increasing negative pressure; Table 1. The effects of pressure shift (loaded for 2h) on the expression of VCAM and IntegrinαVβ3 (n=6,**compared with S-Con group p<0.01). Assay Items (relative fluorenscence values) Groups VCAM Integrin αVβ3 con 100±0 100±0 S-con 110.7±31.2 151.5±16.9 N10 113.8±11.3 152.5±29.7 N20 108.7±11.8 217.4±50.2** N40 78.3±4.9** 328.7±14.5** P20 144.7±22.6** 233.2±31.7** P40 155.8±24.5** 270.3±23.5** Figure 3. The duration-dependent effects of –40cmH2O pressure on the expression of VCAM and IntegrinαVβ3 A. The variations of VCAM expression B. The varia- tions of IntegrinαVβ3 expression (n=6**compared with S-Con group p<0.01). 2) The increasing negative and positive pressure both resulted in the intensified expression of the IntegrinαVβ3 (Table 1). As –40cmH2O pressure caused a significant changes in the expression of VCAM and IntegrinαVβ3, we fur- ther studied the duration-dependent effects of the –40cm H2O pressure on the expression of VCAM and Integri- 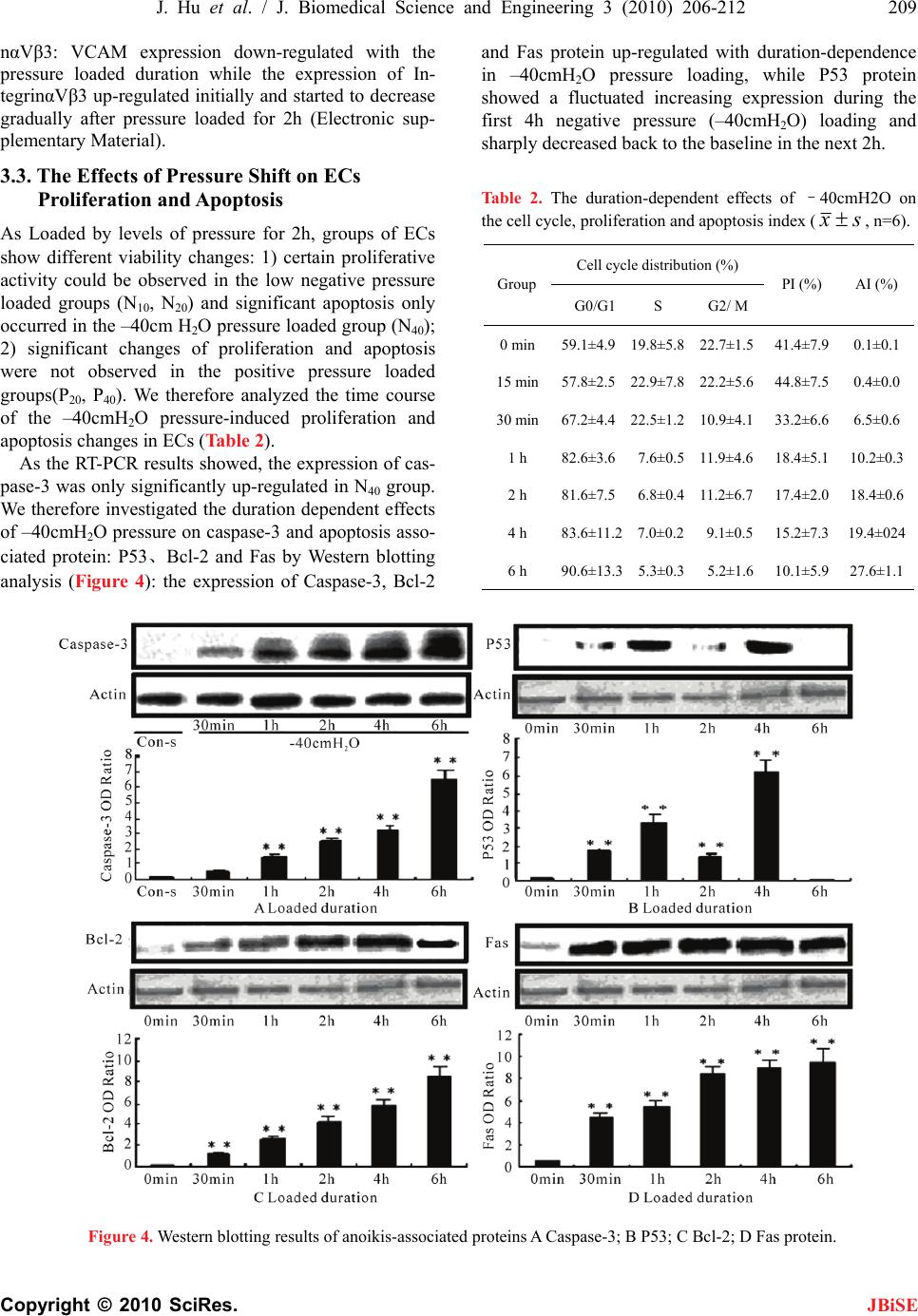 J. Hu et al. / J. Biomedical Science and Engineering 3 (2010) 206-212 209 Copyright © 2010 SciRes. nαVβ3: VCAM expression down-regulated with the pressure loaded duration while the expression of In- tegrinαVβ3 up-regulated initially and started to decrease gradually after pressure loaded for 2h (Electronic sup- plementary Material). JBiSE 3.3. The Effects of Pressure Shift on ECs Proliferation and Apoptosis As Loaded by levels of pressure for 2h, groups of ECs show different viability changes: 1) certain proliferative activity could be observed in the low negative pressure loaded groups (N10, N20) and significant apoptosis only occurred in the –40cm H2O pressure loaded group (N40); 2) significant changes of proliferation and apoptosis were not observed in the positive pressure loaded groups(P20, P40). We therefore analyzed the time course of the –40cmH2O pressure-induced proliferation and apoptosis changes in ECs (Table 2). As the RT-PCR results showed, the expression of cas- pase-3 was only significantly up-regulated in N40 group. We therefore investigated the duration dependent effects of –40cmH2O pressure on caspase-3 and apoptosis asso- ciated protein: P53、Bcl-2 and Fas by Western blotting analysis (Figure 4): the expression of Caspase-3, Bcl-2 and Fas protein up-regulated with duration-dependence in –40cmH2O pressure loading, while P53 protein showed a fluctuated increasing expression during the first 4h negative pressure (–40cmH2O) loading and sharply decreased back to the baseline in the next 2h. Table 2. The duration-dependent effects of –40cmH2O on the cell cycle, proliferation and apoptosis index ( s x , n=6). Cell cycle distribution (%) Group G0/G1 S G2/ M PI (%) AI (%) 0 min 59.1±4.9 19.8±5.8 22.7±1.5 41.4±7.90.1±0.1 15 min57.8±2.5 22.9±7.8 22.2±5.6 44.8±7.50.4±0.0 30 min67.2±4.4 22.5±1.2 10.9±4.1 33.2±6.66.5±0.6 1 h 82.6±3.6 7.6±0.5 11.9±4.6 18.4±5.110.2±0.3 2 h 81.6±7.5 6.8±0.4 11.2±6.7 17.4±2.018.4±0.6 4 h 83.6±11.2 7.0±0.2 9.1±0.5 15.2±7.319.4±024 6 h 90.6±13.3 5.3±0.3 5.2±1.6 10.1±5.927.6±1.1 Figure 4. Western blotting results of anoikis-associated proteins A Caspase-3; B P53; C Bcl-2; D Fas protein.  210 J. Hu et al. / J. Biomedical Science and Engineering 3 (2010) 206-212 Copyright © 2010 SciRes. JBiSE 4. DISCUSSION Anchorage-related apoptosis appeared in ECs which were experimentally detached from their extracellular matrix had suggested the role of ECM as a suppressor of apoptosis induced by biomechanical forces [9]. Since the discovery of adhesion-activated tyrosine kinase pp125 FAK [10,11], a web of signaling networks spread out and revealed the multiple pathways that could regulate the adhesion-related apoptosis(anoikis). Through these pathways, variety of biomechanical factors in flow field was capable of triggering the anoikis process. Many studies demonstrated [12,13] that observable changes in cytoskeleton and viability of ECs only occurred under the condition of loaded shear stress>8 dyn/cm2 and du- ration>24h. To differ the focus from previous reports in the literature, we therefore adopted extremely low shear stress(1.85dyn/cm2) and short pressure loading duration (≤6h) to evaluate the pressure shift effects specifically. The cytoskeleton, particularly the content and distri- bution of F-actin, is a strong determinant in the me- chanical properties of ECs, such as cell shape, stiffness and cytoplasm viscosity. We had identified that the sig- nificant changes in F-actin expression and cell shape were only observed after certain negative pressure ex- posure for 2h(N20 and N40 group). Interestingly, up- regulated expression of F-actin and streamlined cell shape resulted in down-regulated expression of VCAM、 up-regulated expression of apoptosis-associated pro- tein(especially Fas protein) and increased Apoptosis Index(AI%). However, these findings were different from previous studies that streched cells were found to be susceptible to rescuing from anoikis [14,15]. As our previous data demonstrated [5], when exposed to high shear stress, the adhesion ability of cells enhanced with the concentrated distribution of F-actin from the cortex to the perinuclear area of cells. We therefore considered that the increasing negative pressure may disassembly the F-actin filaments to monomers but inhibited its reor- ganizaton in the perinuclear area of cells, further resulted in the increased amount of F-actin presenting only in the cortex but sharply decreased the adhesion ability. Some evidence demonstrated that the cytoskeletal disruption regulated the FasL expression and were associated with anoikis [16], which was coincident with our findings in the consistent up-regulation of F-actin and Fas expres- sion with negative pressure(–40cmH2O) loaded for 2h. However, the detailed mechanism of this process re- mains to be determined by further investigations. In the experiment, we demonstrated the readily apparent dif- ferences between F-actin and ECs viability of negative versus positive pressure loaded ECs, which implied that the negative pressure could regulate both signaling molecules and apoptosis-related protein that were asso- ciated with the cytoskeleton, and as such may together regulate anoikis by serving as sensors of cytoskeletal integrity. Sufficient and appropriate adhesion to the ECM, rep- resented by the VCAM, is critical for proper ECs func- tion and signaling. With the organization of the cy- toskeleton and transduction of biochemical signals, the external (ECM) to internal (ECs) signaling transduction are mediated by the integrins [17]. As the member of the integrin family, IntegrinαVβ3 is capable of recognizing and bind many ECM proteins and induce intracellular biochemical responses to regulate anoikis process[18]. Therefore as a signaling transductant, the expression of IntegrinαVβ3 were observed significantly up-regulated in both positive and negative pressure groups with enough loaded duration and intensity (Table 1). Fur- thermore, we demonstrated the up-regulated expression of VCAM with low AI% in positive pressure loaded groups(P20, P40) and significant down-regulated expr- ession of VCAM accompanied by high AI% in N40 group with duration-dependence, which implied that the negative pressure (–40cmH2O) induced apoptosis could be adhesion-dependant. However, in our time course experiment on IntegrinαVβ3 and apoptosis-associated proteins (Figure 4), the up-regulated expression of In- tegrinαVβ3 in ECs were consistent with the intensified expression of Caspase-3、Bcl-2 and Fas protein when exposed to –40cmH2O pressure loaded for 2h, during which the expression of VCAM showed insignificantly changing. The findings give evidence of the Integri- nαVβ3 mediating some proapoptotic responses that could contribute to the integrated mechanism of negative pressure induced anoikis process with adhesion-inde- pendence. In support of our findings, previous studies [19] suggest the apoptotic effects observed with Integri- nαVβ3 antagonist (echistatin) in ECs were not due to detachment but rather due to activation of intracellular signals. Multiple pathways could initiate the caspase activa- tion to induce anoikis process converging at the level of effector caspases-3 [20]. As two main pathways in which caspases cascade are initially activated, the death recep- tor pathway and mitochondrial pathway are regulated by a host of key effector proteins, such as P53、Bcl-2 and Fas proteins which have been previously confirmed as important regulators in different pathways [21,22]. As we noted, the expression of P53 showed a fluctuated increasing within 4h negative pressure (–40cmH2O) loading (Figure 4), which might imply the multiple functions of P53 protein in regulating the early stage of anoikis process. During the first 2h, P53 expression up-regulated initially and then down-regulated with the up-regulation of Bcl-2 expression. Meanwhile, the de- creasing PI% that still exceeded the gradually increasing AI% (Table 2). Published studies [23] demonstrated that P53 could encode a transcription factor to activate genes  J. Hu et al. / J. Biomedical Science and Engineering 3 (2010) 206-212 211 Copyright © 2010 SciRes. JBiSE involved in growth arrest (p21, GADD45) and also could control the anti-apoptotic protein Bcl-2 in mito- chondrial pathway. Therefore, we considered the main function of P53 protein during the first 2h was inducing the growth arrest to reduce the sensitivity of ECs to apoptosis. While the slight down-regulated P53 expres- sion on the 2h checkpoint could be explained by some evidence that P53 and Bcl-2 may combined as p53-Bcl2 complexes in contributing to the direct mitochondrial p53 pathway of apoptosis [24]. Although much of p53- mediated apoptosis signals were through mitochondrial pathways, p53 inducible genes could alter the localiza- tion of death receptors normally found in the cytoplasm to the cell surface to enhance the sensitivity to death receptor-mediated apoptosis (Fas) [25]. Therefore, at the end of the first 2h, the homeostasis in the proliferation and apoptosis of ECs broke down (AI% started to ex- ceed PI %) with the significantly up-regulated Fas ex- pression and P53 expression. Meanwhile, the expression of VCAM showed a significant down-regulation, which further supported the notion that the negative pressure induced apoptosis could be adhesion-dependent (anoi- kis). As we noted, the P53 expression surprisingly de- creased back to the baseline with up-regulated Bcl-2 expression after being loaded by negative pressure (–40cmH2O) for 4h. During the same period, consistent with the up-regulated Fas expression, the expression of Caspase-3 still up-regulated with increasing AI%, which indicated the predominating function of Fas protein in the latter stage(after 4h) of negative pressure induced anoikis process. We have confirmed a certain role of negative pressure, particularly –40cmH2O pressure, in biomechanical im- pairment on ECs with adhesion-dependence. While our preliminary investigations on the mechanism of negative pressure induced anoikis demonstrated that P53 acts dual function in regulating the early stage of anoikis process and Fas protein (death receptor pathway) predominated the end stage of the negative pressure induced anoikis process. These data give us insights into integrated in- vestigations on mechanisms of downstream vascular impairment in stenostic vessel diseases (eg. atheroscle- rosis, post-stenostic aneurysm formation) and Integri- nαVβ3, P53, Fas represent attractive targets for protec- tive therapeutics aiming at downstream vessels for a better long-term results in the patients with stenostic vessel dieases. However, the key to anoikis regulation depends on the sum of intrinsic and extrinsic input, It will be of interest for us to sort out the precise manner by which the architectural state of the cytoskeleton, in- tegrin signal transduction events and posttranslational apoptotic factors are interrelated. REFERENCES [1] Xu, W.L., Liao, H.S., Yang, Y.Q. et al. (2002) Turbulent flow and energy dissipation in plunge pool of high arch dam. J Hydraulic Research, 40, 471–476. [2] Xu, W.L., Wang, W., Yang, Y.Q. et al. (1999) Numerical modeling of the water-air two-phase jet into a plunge pool. J Hydrodynamics, 11, 1–5. [3] Heurkens, A.H., Gorter, A., Vreede, T.M. et al. (1991) Methods for the detection of anti-endothelial antibodies by enzyme-linked immunosorbent assay. J Immunol Methods, 141, 33–39. [4] Edgell, C.J., McDonald, C.C. and Graham, J.B. (1983) Permanent cell line expressing human factor VIII related antigen established by hybridization. Proc Natl Acad Sci., 80, 37342–37371. [5] Chen, H.Q., Wei, T., Chen, Y.S. et al. (2004) Effect of steady and oscillatory shear stress on F-actin content and distribution in neutrophils. Biorheology, 41, 655–664. [6] Klein, D. (2002) Quantification using real-time PCR technology: Applications and limitations. Trends Mol Med, 8, 257–260. [7] Cosa, G., Focsaneanu, K.S., McLean, J.R.N. et al. (2001) Photophysical properties of fluorescent DNA-dyes bound to single- and double-stranded DNA in aqueous buffered solution. Photochem Photobiol, 73, 585–599. [8] Ginzinger, D.G. (2002) Gene quantification using real- time quantitative PCR: an emerging technology hits the main stream. Exp Hematol, 30, 503–511. [9] Meredith, J.E., Fazeli, B. and Schwartz, M.A. (1993) The extracellular matrix as a cell survival factor. Mol Biol Cell, 4, 953–961. [10] Schaller, M.D., Borgman, C.A., Cobb, B.S. et al. (1992) pp125 FAK, a structurally distinctive proteintyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci., USA, 89, 5192–5196. [11] Schaller, M.D. and Parson, J.T. (1993) Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol., 3, 258–262. [12] Dewey, C.F., Bussolari, S.R., Gimbrone, M.A. et al. (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech Engineering, 103, 177–188. [13] Davies, P.F. (1995) Flow-mediated endothelial mecha- notransduction, Physiol Rev., 75, 519–560. [14] Chen, C.S., Mrkisch, M., Huang, S. et al. (1997) Geo- metric control of cell life and death. Science, 276, 1425– 1428. [15] Flusberg, D.A., Numaguchi, Y. and Ingber, D.E. (2001) Cooperative control of Akt phosphorylation, bcl-2 ex- pression, and apoptosis by cytoskeletal microfilaments and microtubules in capillary endothelial cells. Mol. Biol. Cell, 12, 3087–3094. [16] Ishida, K., Nagahara, H. and Kogiso, T. (2003) Cell ad- hesion aside from integrin system can abrogate anoikis in rat liver cells by down-regulation of FasL expression, not by activation of PI-3K/Akt and ERK signaling pathway. Biochem. Biophys. Res. Commun, 300, 201–208. [17] Frisch, S.M., Screaton, R.A. (2001) Anoikis mechanisms. Curr Opin Cell Biol., 13, 555–562. [18] Stupack, D.G. and Cheresh, D.A. (2002) Get a ligand, get a life: integrins, signalling and cell survival. J. Cell Sci., 115, 3729–3738. [19] Brassard, D.L., Maxwell, E., Malkowski, M. et al. (1999) IntegrinαVβ3-Mediated Activation of Apoptosis. Exp  212 J. Hu et al. / J. Biomedical Science and Engineering 3 (2010) 206-212 Copyright © 2010 SciRes. JBiSE Cell Res., 251, 33–45. [20] Simpson, C.D. et al. (2008) Anoikis resistance and tumor metastasis. Cancer Lett., doi:10.1016/j.canlet.2008.05. 029. [21] Kitsis, R.N. and Mann, D.L. (2005) Apoptosis and the heart: a decade of progress. J. Mol Cell Cardiol, 38, 1–2. [22] Stoneman, V.E.A., Bennett, M.R. (2004) Role of apop- tosis in atherosclerosis and its therapeutic implications. Clin Sci., 107, 343–354. [23] Mercer, J., Mahmoudi, M. and Bennett, M. (2007) DNA damage, p53, apoptosis and vascular disease. Mutat Res., 621, 75–86. [24] Tomita, Y., Marchenko, N., Erster, et al. (2006) WT p53, but not tumor-derived mutants, bind to Bcl-2 via the DNA binding domain and induce mitochondrial perme- abilization. J. Biol Chem., 281, 8600. [25] Fais, S., Milito, A.D. and Lozupone, F. (2005) The role of FAS to ezrin association in FAS-mediated apoptosis. Apoptosis, 10, 941–947. |

