Journal of Environmental Protection
Vol.4 No.9(2013), Article ID:36802,5 pages DOI:10.4236/jep.2013.49114
Biosorption of Metal Ions by Exopolysaccharide Produced by Azotobacter chroococcum XU1
![]()
1Institute of Genetics and Plant Experimental Biology, Uzbekistan Academy of Sciences, Yukori Yuz, Uzbekistan; 2The Key Laboratory of Plant Resources and Chemistry of Arid Zone, China Xinjiang Technical Institute of Physics & Chemistry, CAS, Urumqi, China; 3Central Asian Drug Discoveries and Development Center of Chinese Academy of Sciences, Urumqi, China.
Email: rasulov.bakhtiyor@gmail.com, haji@ms.xjb.ac.cn
Copyright © 2013 Bakhtiyor A. Rasulov et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 5th, 2013; revised July 8th, 2013; accepted August 7th, 2013
Keywords: Azotobacter chroococcum XU1; Exopolysaccharide; Biosorption; Lead; Mercury
ABSTRACT
This paper deals with adsorption of Pb and Hg on the exopolysaccharide produced by Azotobacter chroococcum XU1. The adsorption of metal ions was significantly affected by the pH of metal solutions (Pb(NO3)2 and Hg(NO3)2), metal ions concentration and exopolysaccharide concentrations. At optimum pH the uptakes of the metals were 40.48% and 47.87% respectively. The metal ions biosorption was high at 4, 5-5.
1. Introduction
One of the major challenges in environmental biotechnology is the bioremediation of heavy metal pollution. Accumulation of heavy metals in ecosystems due to metal mining activities, electrical equipment production, application of artificial colours and pesticides, tannery industries, chemical fertilizers and municipal sewage disposal has concerned scientists and officials [1]. The search for novel and cost effective technologies has been directed to the application of biosorption, which constitutes an attractive and economical alternative remediation method. In this regard, extracellular polysaccharides or exopolysaccharides (EPSs) of bacterial, algal and fungal origin are recommended as surface active agents for heavy metal removal [2]. The adsorption of heavy metal by EPS is a metabolism-independent process, and it is attributed to interaction between metal cations and negative charges of acidic functional groups of EPS [3].
The purpose of this study was to study adsorption capacity of lead and mercury by EPS of Azotobacter chroococcum XU1.
2. Materials and Methods
2.1. Microorganism and Media
Azotobacter chroococcum XU1 used in this study was previously isolated from soil samples and shortly reported on its capability to produce EPS and indole- 3-acetic acid (IAA) [4]. It was cultivated at 30˚C, 150 rpm and pH 7.0, with supply of sterile air in the shaker for 3 days. The nitrogen-free Ashby broth consists of (g·l−1): mannose, 20; KH2PO4, 0.2; MgSO4·7H2O, 0.2; NaCl, 0.2; K2SO4, 0.1; CaCO3, 5.
2.2. EPS Purification
The culture broth of Azotobacter chroococcum XU1 centrifuged at 6000 rpm for 10 min to remove cells. Two volumes of cold ethanol (4˚C) were added to the supernatant and the crude EPS precipitate was dried in a dessicator overnight.
2.3. Metal Adsorption Experiments
The cell-free culture broth was added to metal solutions at optimized condition of pH at 25˚C. Two volumes of cold ethanol were added to the solutions. Then, the solution consisting of insoluble EPS was filtered through filter paper and the filtrate used for metal analysis. In order to account the effect of filter paper on adsorption of heavy metals, a separate set of control experiment was done with the same conditions for all experiments [5].
2.4. Metal Analysis
The total content of Pb in the digested solution were measured by inductively coupled plasma-mass spectrometry (ICP-MS), Hg were analyzed by atomic fluorescence spectrophotometer (AFS). All samples were analyzed in three replicates. Standard reference materials obtained from the China National Center for Standard Reference Materials were digested along with the samples and used for the quality assurance control program.
2.5. Calculation of Adsorption Isotherms Parameters
The isotherms data were characterized by the Langmuir (2) and Freundlich (3) equations:
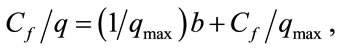 (2)
(2)
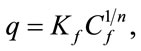 (3)
(3)
where q is the metal uptake by polysaccharide (mg·l−1), Cf is the equilibrium concentration of metal in solution (mg l−1), (b, qmax) and (Kf; n) are empirical constants of Langmuir and Freundlich isotherms, respectively.
3. Results
The EPS produced by Azotobacter chroococcum XU1 was basically mannose containing (Figure 1) and it contained 3% of total protein. Analysis showed that protein of EPS-protein complex of the strain contains all ireplaceable aminoacids (Table 1; Figure 2).
The activity of functional groups of polysaccharide and the protein, associated with it (3%) and their competition for adsorption of metallic ions was affected by pH. It affects the solution chemistry of the metals, the activity of functional groups in biopolymer, and the competition of metallic ions for biosorption [5]. At neutral pH, the adsorption capacities of polysaccharide were obtained as 48.5% and 32.4% for Pb and Hg, respectively. Biosorption rates increase as pH of solution increases towards acidic. This may be due to interaction between cations and negative charges of acidic functional groups of polysaccharide. The decrease of biosorption at alkaline pHs might be attributed to start of insoluble hydroxide precipitating from solution at higher pH values, making true metal biosorption studies impossible [5].
The optimum pH values were obtained, less than 4.5 and 4 for Pb and Hg, respectively.
The adsorption capacity of EPS of Azotobacter chroococcum XU1 for lead and mercury at neutral pH showed that EPS had adsorbed 22.38 and 25.49 mg/g Pb and Hg respectively. The metal adsorption rates increase as initial pH of solution increases towards acidic. Therefore, at pH 4,5-5 the EPS adsorbed 33.5 mg/g of Pb, whereas at pH 5 absorbed 38.9 mg/g of Hg (Figure 3). At optimal pH values, the adsorption capacities of polysaccharide were obtained as 40.48% and 47.87% for Pb and Hg, respectively.
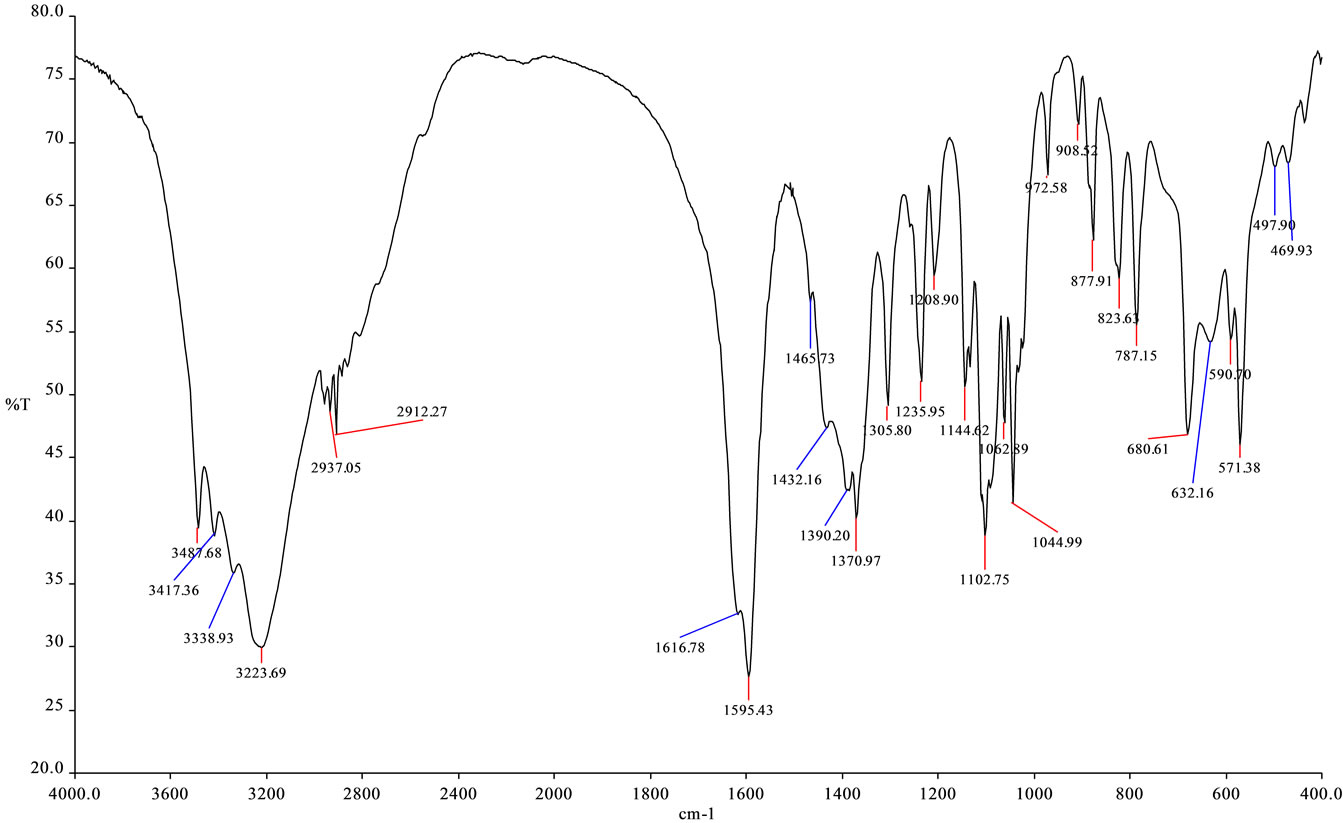
Figure 1. IR-spectra of EPS sample of Azotobacter chroococcum XU1.
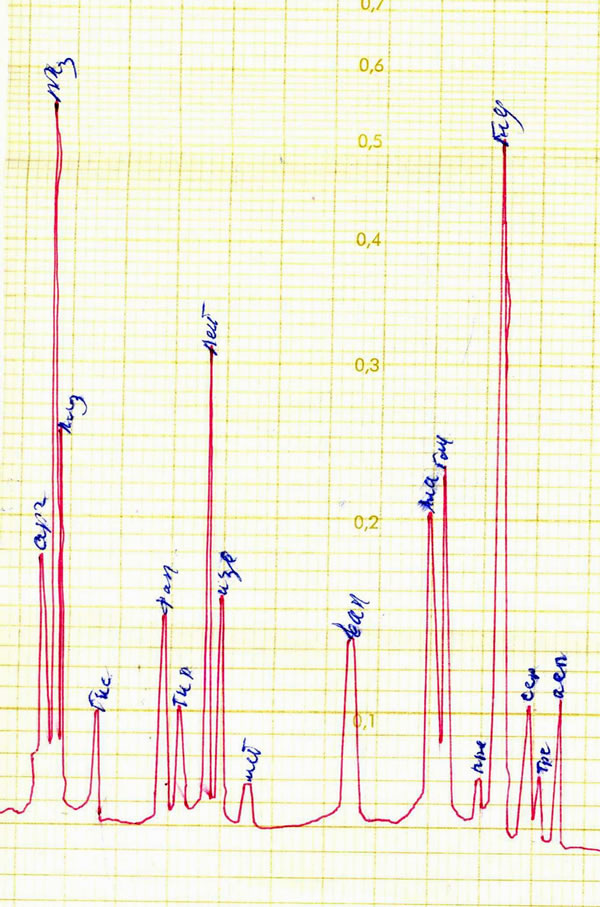
Figure 2. Chromatogram of aminoacids of protein, associated with EPS of Azotobacter chrooccoccum XU1.
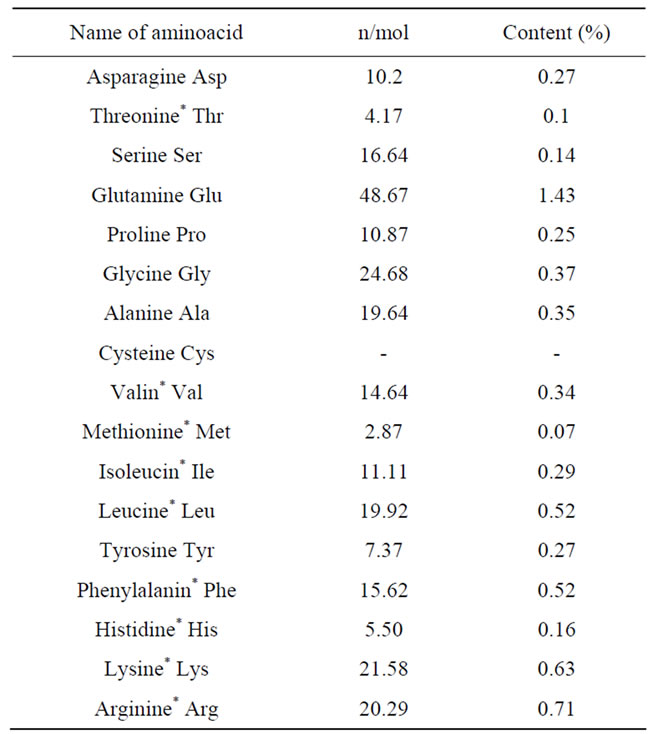
Table 1. Aminoacid content of EPS-protein complex of Azotobacter chroococcum XU1.
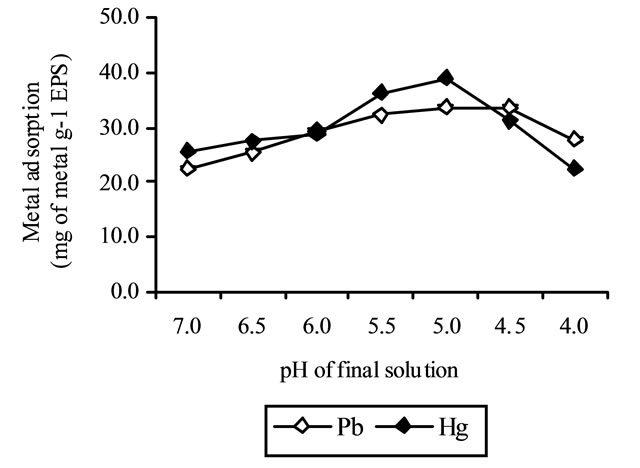
Figure 3. Effect of pH on metal adsorption of EPS by Azotobacter chroococcum XU1.
As shown in Figure 4, the kinetics of adsorption in tested samples was rapid during early minutes and leveled out after 20 min of metal solutions and supernatants mixing together at 25˚C. Similar results have been obtained by other authors working with Pb and Bacillus firmus [5].
Effects of initial concentration of metal ions on the biosorption capacity: Figure 5 shows the effect of initial concentration of the metals on their adsorption by EPS of Azotobacter chroococcum XU1. The results revealed that the amount of adsorbed metals increased by higher metal concentrations. Therefore, adsorption of Pb+2 and Hg+2 on EPS is controlled by their concentrations. As shown in Figure 3, lead and mercury adsorption patterns are affected by their initial concentrations, but it is obvious that the increasing trend would continue until the active adsorbing sites become saturated [1].
This increasing trend may depend on some properties of the metal sorbates (e.g. ionic size, reduction potential of the metal) and the properties of the biosorbent 15 (e.g. structure, functional groups and surface area).
4. Discussion
In recent years, increasing attention has been paid on exopolysaccharides produced by bacteria [6]. It is interesting that most bacterial polysaccharides are characterised by the presence of uronic acids, pentoses, a polypeptide moiety or other nonsaccharide components, such as organic (e.g., acetyl, pyruvyl, succinyl group) or inorganic (e.g., sulphate or phosphate group) substituents [7,8]. A wide variety of bacteria genera, in particular Bacillus [5,9], Pseudomonas [10], Paenibacillus [2], Azotobacter [11], Shewanella [12] and Sinorhizobium [1] have been documented for EPS synthesis, metal adsorption properties.
EPS from Azotobacter revealed high adsorption rate and played a major role in immobilization of heavy metals
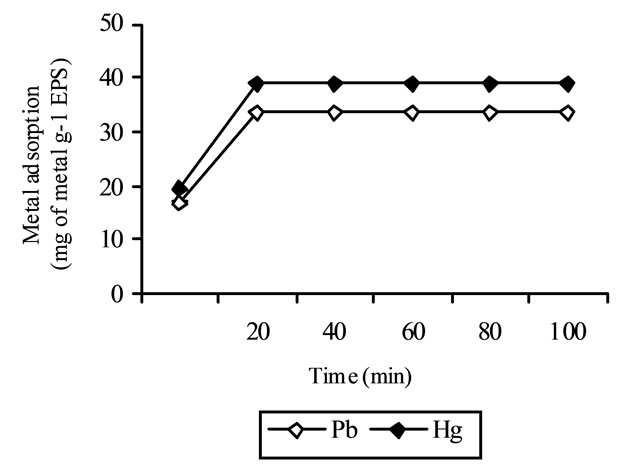
Figure 4. Time course of metal adsorption to EPS. Initial metal concentrations were 100 mg l−1.

Figure 5. Effect of initial concentrations of lead and mercury on their adsorption by EPS of Azotobacter chroococcum XU1 after a 20 min incubation (pH 7.0).
[11]. EPS of Azotobacter directly bind and uptake heavy metals like Cd and Cr in the contaminated soils [13]. The metal absorption (like Cd2+, Cu2+, Pb2+, Zn2+) behavior of alginate from Azotobacter in soil and water environment helps by removing toxic metals or by creating microenvironment of essential metal ions to maintain soil ecology and accelerate the normal growth of plant. The productions of biosurfactant like polysaccharide by A. vinelandii AV01 were evidenced for improve hydrocarbon remediation with intrinsic soil nitrogen fixer [14].
By some researchers, there have obtained different data on Pb adsorption by microbial EPS. The maximum uptake capacities was 54.92 mg/g (37.52% removal) by Abbas H. Sulaymon et al., 2013, who researched some anaerobic bacteria, yeast and protozoa, whereas by H. Salehizadeh and S.A. Shojaosadati [5] obtained the maximum lead uptake—1103 mg/g (98.3% removal) by B. firmus MS-102, and it was an excellent adsorbent compared with previously documented M. organophilum [3], and Rhizopus nigricans [15]. In comparing with earlier reported works, Azotobacter chroococcum XU1 adsorbed 33.5 mg/g of lead at pH 4.5 - 5 with 40.48% of removal. It is necessary to point out that in our researches experiments are carried out in low concentrations of salts, which are crucial in final adsorption capacities. Analogical results are observed with Hg in comparing to earlier studies.
Figure 3 indicates the metal uptake isotherms for Pb and Hg ions plotted against final metal concentration Cf in aqueous solutions. The finding from this figure, particularly with regard to our maximum value of uptake of 33.5 mg/g of Pb and 38.9 mg/g of Hg, lead us to believe EPS produced by A. chroococcum XU1 is a promising adsorbent. This can attribute to charge density, attractive interaction and types of conformation of polymer with adsorbed ions. As is known, charge densities of bivalent ions increase with decrease in ionic radius (Charge density: Pb, Hg).
But, the role of protein, synthesized together with EPS by Azotobacter chroococcum XU1, and other functional groups, such as hydroxyl (-OH) and carboxylic groups (basically C=O) is not clarified yet. It is known that the main biosorption mechanisms are complexation and physical adsorption onto natural active functional groups [16].
Based on the results presented in this study, the EPS by Azotobacter chroococcum XU1 indicates the possibility of application of this biosorbent for removal of lead and mercury from waste waters, which affect environment.
5. Acknowledgements
This work was financially supported by grant of Drug R & D Center of Central Asian, Chinese Academy of Sciences as well as Talents introduction and exchange from neighboring countries Program of Chinese Academy of Sciences.
REFERENCES
- A. Lakzian, A. R. Berenji, E. Karimi and S. Razavi, “Adsorption Capability of Lead, Nickel and Zinc by Exopolysaccharide and Dried Cell of Ensifer meliloti,” Asian Journal of Chemistry, Vol. 20, No. 8, 2008, pp. 6075- 6080
- J. A. Morillo Perez, R. Garcia-Ribera, T. Quesada, M. Aguilera, A. Ramos-Cormenzana and M. MonteolivoSanchez, “Biosorption of Heavy Metals by the Exopolysaccharide Produces by Paenibacillus jamilae,” World Journal of Microbiology and Biotechnology, Vol. 24, No. 11, 2008, pp. 2699-2704. http://dx.doi.org/10.1007/s11274-008-9800-9
- S. Y. Kim, J. H. Kim, C. I. Kim and O. K. Oh, “Metal Adsorption of the Polysaccharide Produced from Methylobacterium organophilum,” Biotechnology Letters, Vol. 18, No. 10, 1996, pp. 1161-1164. http://dx.doi.org/10.1007/BF00128585
- B. A. Rasulov, A. Yili and H. A. Aisa, “A New Azotobacter chroococcum XU1 Strain, Producer of Indolic Compounds and Exopolysaccharides,” Proceedings of 5th Congress of Microbiologists of Uzbekistan, Tashkent, 12-13 October 2012, pp. 3-4.
- H. Salehizadeh and S. A. Shojaosadati, “Removal of Metal Ions from Aqueous Solution by Polysaccharide Produced from Bacillus firmus,” Water Research, Vol. 37, No. 7, 2003, pp. 4231-4235. http://dx.doi.org/10.1016/S0043-1354(03)00418-4
- Z. Chi and Y. Fang, “Exopolysaccharides from Marine Bacteria,” Journal of Ocean University of China, Vol. 14, No. 1, 2005, pp. 67-74. http://dx.doi.org/10.1007/s11802-005-0026-2
- R. De Philippis and M. Vincenzini, “Exocellular Polysacharides from Cyanobacteria and Their Possible Applications,” FEMS Microbiology Reviews, No. 22, 1998, pp. 151-171.
- R. De Philippis, C. Sili, R. Paperi and M. Vincenzini, “Exopolysaccharide-Producing Cyanobacteria and Their Possible Exploitation,” Journal of Applied Phycology, Vol. 13, No. 4, 2001, pp. 293-299. http://dx.doi.org/10.1023/A:1017590425924
- R. E. Martinez, D. S. Smith, E. Kulczycki and F. G. Ferris, “Determination of Intrinsic Bacterial Surface Acidity Constants Using a Donnan Shell Model and a Continuous pKa Distribution Method,” Journal of Colloid and Interface Science, Vol. 253, No. 1, 2002, pp. 130-139. http://dx.doi.org/10.1006/jcis.2002.8541
- D. M. Borrok and J. B. Fein, “The Impact of Ionic Strength on the Adsorption of Protons, Pb, Cd, and Sr onto the Surfaces of Gram Negative Bacteria: Testing Non-Electrostatic, Diffuse, and Triplelayer Models,” Journal of Colloid and Interface Science, Vol. 286, No. 1, 2005, pp. 110-126. http://dx.doi.org/10.1016/j.jcis.2005.01.015
- S. S. Gauri, S. Archanaa, K. C. Mondal, B. R. Pati, S. M. Mandal and S. Dey, “Removal of Arsenic from Aqueous Solution Using Pottery Granules Coated with Cyst of Azotobacter and Portland Cement: Characterization, Kinetics and Modelling,” Bioresource Technology, Vol. 102, No. 10, 2011, pp. 6308-6312. http://dx.doi.org/10.1016/j.biortech.2011.02.037
- J. Claessens and P. van Cappellen, “Competitive Binding of Cu2+ and Zn2+ to Live Cells of Schewanella Putrefaciens,” Environmental Science & Technology, Vol. 41, No. 3, 2007, pp. 909-914. http://dx.doi.org/10.1021/es0620944
- P. Joshi and A. Juwarkar, “In Vivo Studies to Elucidate the Role of Extracellular Polymeric Substances from Azotobacter in Immobilization of Heavy Metals,” Environmental Science & Technology, Vol. 43, No. 15, 2009, pp. 5884-5889. http://dx.doi.org/10.1021/es900063b
- Q. Helmy, E. Kardena, Z. Nurachman and Wisjnuprapto, “Application of Biosurfactant Produced by Azotobacter vinelandii AV01 for Enhanced Oil Recovery and Biodegradation of Oil Sludge,” International Journal of Civil & Environmental Engineering, Vol. 10, No. 1, 2008, pp. 334-338.
- Z. Li, Z. Li, Y. Yu and C. Chen, “Removal of Lead from Aqueous Solution by Non-Living Rhizopus nigricans,” Water Research, Vol. 32, No. 5, 1998, pp. 1437-1444. http://dx.doi.org/10.1016/S0043-1354(97)00348-5
- H. S. Abbas, E. E. Shahla and M. J. Mohammed-Ridha, “Equilibrium, Kinetic, and Thermodynamic Biosorption of Pb (II), Cr (III), and Cd (II) Ions by Dead Anaerobic Biomass from Synthetic Wastewater,” Environmental Science and Pollution Research, Vol. 20, No. 1, 2013, pp. 175-187. http://dx.doi.org/10.1007/s11356-012-0854-8

