Open Journal of Urology
Vol. 3 No. 2 (2013) , Article ID: 31407 , 4 pages DOI:10.4236/oju.2013.32021
The Effect of Switching Patients with Symptomatic Benign Prostatic Hyperplasia from Tamsulosin 0.2 mg to 0.4 mg
The Division of Urology, Department of Surgery, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Email: *sompolpermpong@gmail.com
Copyright © 2013 Supadach Teawongsuwon, Sompol Pempongkosol. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 29, 2013; revised March 1, 2013; accepted March 9, 2013
Keywords: Tamsulosin; Benign prostatic hypertrophy (BPH); Lower urinary tract symptoms (LUTS)
ABSTRACT
Objectives: In 2010, tamsulosin 0.2 mg (OD) was withdrawn from Thailand and replaced with tamsulosin 0.4 mg (OD). Therefore, we assessed the impact of this change on the patients, at a men’s health clinic, with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH). Material and Methods: Subjects were 100 men with BPH who had been taking tamsulosin 0.2 mg as needed for at least 3 months. The outcome measures were IPSS, AMS and IEFF5 scores and uroflowmetry. Tolerability was evaluated on by adverse events. Changes from baseline were assessed using the paired t-test. SPSS version 12.0 was used for statistical analysis, with p < 0.05 considered significant. Results: The mean follow up of tamsulosin 0.2 and 0.4 mg were 20.23 and 10.56 months respectively. On switching from tamsulosin 0.2 to 0.4 mg, mean IPSS score improved from 15.54 ± SD 1.25 to 14.13 ± SD 1.09 (p = 0.034), Q max 15.91 cm3/sec ± SD 1.36 to 16.69 cm3/sec ± SD 1.52 (p = 0.128), and nocturia 3.15 ± SD 0.32 to 2.68 ± SD 0.39 (p = 0.015), respectively. However IEFF-5 score and AMS score increased from14.78 ± SD 1.38 to 15.79 ± SD 1.03 (p = 0.0055) and 34.76 ± SD 2.76 to 33.21 ± SD 2.62 (p = 0.0853), respectively. Treatment-related adverse events of Tamsulosin 0.2 mg included dizziness (4%), postural hypotension (3%) and retrograde ejaculation (3%). Interestingly, no withdrawals resulted from adverse events during Tamsulosin 0.4 mg assessment. Conclusions: Switching to tamsulosin 0.4 mg improves LUTS. The change was well tolerated by the majority of patients. Increased symptoms scores of erectile dysfunction and aging male during the study may be due to increased age.
1. Introduction
Symptomatic benign prostatic hyperplasia (BPH) may affect up to 30% of men in their early 70s, causing urinary symptoms of bladder outlet obstruction [1]. Community and practice based studies suggest that men with lower urinary tract symptoms (LUTS) can expect slow progression of symptoms [2,3].
Medical therapies to treat LUTS/BPH include α1- adrenergic receptor antagonists (terazosin, doxazosin, tamsulosin, and alfuzosin), 5α-reductase inhibitors such as finasteride and dutasteride, and phytotherapy. Physicians choosing treatment to achieve symptom relief must take into account factors such as the clinical benefits, potential for morbidity, probable long-term efficacy, and costs [4]. Tamsulosin is a more selective α1A subtype antagonist, which maintains the α-antagonist effect on the prostatic capsule and bladder neck but has less of an effect on the vascular system and blood pressure. Tamsulosin has a favorable side effect profile in regard to problems related to hypotension and dizziness compared to those of terazosin and doxazosin [5].
In Thailand, tamsulosin 0.2 mg was the first line treatment in patients with BPH or LUTS but it was withdrawn and switched to tamsulosin 0.4 mg in 2010. Our study was designed to confirm previous research, which compared the efficacy of tamsulosin 0.4 mg and 0.2 mg in different groups, while our study was done in the same patients.
Our study observed patients who had BPH and LUTS symptoms and were first treated with tamsulosin 0.2 mg then, later, with tamsulosin 0.4 mg. The patients were evaluated for IPSS, side effects and uroflowmetry.
2. Materials and Methods
After obtaining the local ethics committee’s approval, we reviewed data between January 2007 and December 2010 from out patient cards for a retrospective study. A total of 130 patients were recruited in our study. Patients were diagnosed with LUTS due to BPH and aged above 50 years old at the first diagnosis and treated with tamsulosin (0.2 mg) in the outpatient department of a university hospital.
Thirty patients were excluded from our study due to having been treated with combined therapy, having other urological diseases (example: bladder cancer, prostate cancer, urinary tract infection (UTI)) or wanting to change to other drugs. A hundred patients remained in our study. The patients were treated with tamsulosin 0.2 mg in their first drug treatment and then switched to tamsulosin 0.4 mg in 2010 due to the withdrawal of tamsulosin 0.2 and its replacement with tamsulosin 0.4 mg. Age, time of follow up, IPSS scores, nocturia, erectile dysfunction using International Index Erectile Function (IIEF-5) scores, Uroflowmetry (maximum flow rate and post void residual urine), complications from tamsulosin were collected from records in outpatient cards. Patients were followed up every 3 months for IPSS scores, rectal examination and side effects, and uroflowmetry every 12 months. The mean follow up time for tamsulosin 0.2 mg was 20.23 ± 2.44 months and after switching to tamsulosin 0.4 mg the mean was 10.56 ± 1.09 months. For statistical analysis, we used the paired t-test and Stata version 12.0 with p-value < 0.05 for statistical significance.
3. Results
In result of our study, table 1 details the median ± standard deviation (SD) of each data. The average age was 68.13 ± 2.26 years when starting treatment with tamsulosin 0.2 mg and 70.46 ± 1.55 years after switching to tamsulosin 0.4 mg. Mean IPSS score before treatment was 20.23 ± 2.46, after treatment with tamsulosin 0.2 mg it was 15.54 ± 1.25 and after switching to tamsulosin 0.4 mg it was 14.13 ± 1.09. Regarding maximum flow rate and post residual urine: the maximum flow for tamsulosin 0.2 mg was 15.91 ± 1.36 cm3/sec and for tamsulosin 0.4 mg it was 16.69 ± 1.52 cm3/sec, average of post void residual urine for tamsulosin 0.2 mg was 73.57 ± 7.16 cm3 and for tamsulosin 0.4 mg it was 63.89 ± 5.54 cm3. The outcome of the International Index of Erectile Function questionnaire (IIEF-5) after treatment with tamsulosin 0.2 mg was 15.79 ± 6.60 and for tamsulosin 0.4 mg it was 14.78 ± 1.03. The average of frequency of nocturia after treatment with tamsulosin 0.2 mg was 3.15 ± 0.32/night and for tamsulosin 0.4 mg it was 2.68 ± 0.39/night. In aging male symptoms, we used the Aging Male Score (AMS) for evaluation, but in our analysis was limited to 60 patients due to some patients failed to complete AMS forms. The average score for AMS with tamsulosin 0.2 mg was 33.21 ± 2.76 and after switching to tamsulosin 0.4 mg it became 34.76 ± 2.62.
Table 2 Data analysis with statistical methods. IPSS scores data show a statistically significant increase after switching from tamsulosin 0.2 mg to treatment with tamsulosin 0.4 mg (p-value = 0.0034). In uroflowmetry, maximum flow rate in patients after treatment with tamsulosin 0.4 mg was better than with tamsulosin 0.2 mg but not statistically significant (p-value = 0.128). Post void residual urine after switching to tamsulosin 0.4 mg was less than that after treatment with tamsulosin 0.2 mg but, again, the difference was not statistically significant (p-value = 0.073). In erectile dysfunction it was shown that, after switching to treatment with tamsulosin 0.4 mg, IIEF-5 scores were less than when treated with tamsulosin 0.2 mg at a statistically significant level (p-value = 0.005). The higher dosage of tamsulosin also brought about a statistically significant decrease in the frequency of nocturia (p-value = 0.015). The aging male score after
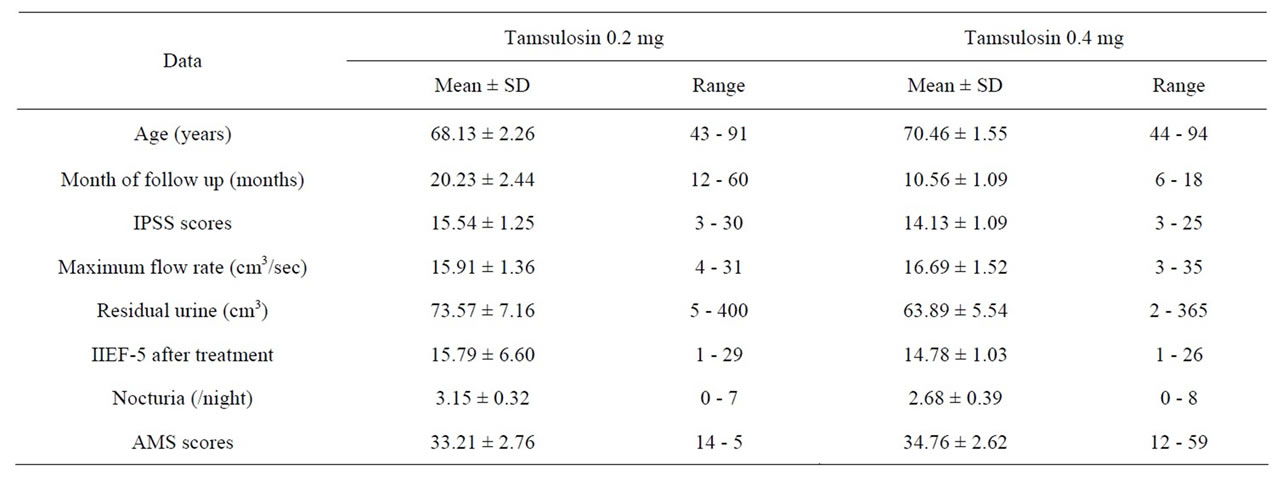
Table 1. Patients and data collection.
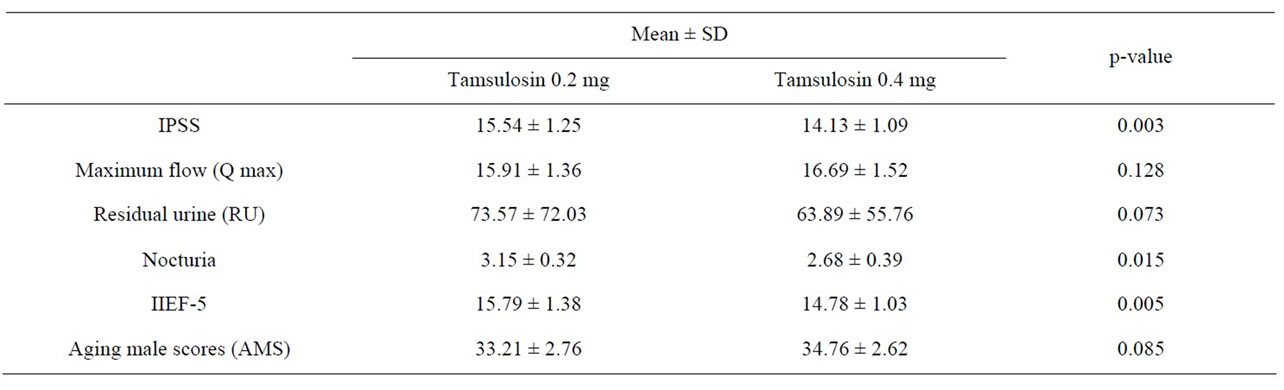
Table 2. Statistic analysis for data comparison in treatment with tamsulosin 0.2 mg and after to switched treatment with tamsulosin 0.4 mg.
the switch to treatment with tamsulosin 0.4 mg was higher than during treatment with tamsulosin 0.2 mg but not at a statistically significant level (p-value = 0.0853).
Complications of tamsulosin in our study included dizziness: 4 cases (4%) and postural hypertension: three cases (3%). Both of these problems were resolved within the first three months of treatment and before the change to the higher dosage of tamsulosin. We also found three cases (3%) of retrograde ejaculation in our study. The three patients concerned all opted to discontinue the use of tamsulosin 0.2 mg in favour of alternative medication after just two weeks. No further complications were found after the first three months of treatment with tamsulosin 0.2 mg and no complications arose within the sample group after the change to tamsulosin 0.4mg.
4. Discussion
The mechanism of tamsulosin involves antagonism of both alpha1A and alpha1D-receptor subtypes. There is a low incidence of dizziness and hypotension with tamsulosin. In treatment with an alpha-blocker, comparison between tamsulosin 0.2 mg and 0.4 mg shows that tamsulosin 0.4 mg can improve symptoms in patients with lower urinary tract symptoms (LUTS); tamsulosin 0.4 mg generates significant differences in IPSS, quality of life, and Q max when compared with tamsulosin 0.2 mg [6].
LUTS symptoms are difficult to evaluate. In our study IPSS scores were used to evaluate and follow up the clinical progress of patients. Switching to treatment with tamsulosin 0.4 mg was shown to improve the clinical symptom of LUTS more than treatment with tamsulosin 0.2 mg. Therefore tamsulosin 0.4 mg has better efficacy in the treatment of BPH symptoms than tamsulosin 0.2 mg.
Uroflowmetry, maximum flow rate and post void residual urine are improved after treatment with tamsulosin 0.4 mg. Maximum flow rate increased by 1 cm3/sec and residual urine decreased by 10 cm3 after treatment with tamsulosin 0.4 mg. BPH causes bladder outlet obstruction in men, and it is often associated with detrusor overactivity [7]. BPH patients, particularly those > 65 years of age, commonly have neurogenic detrusor dysfunction [8]. Tamsulosin is selective for alpha1A and alpha1D-receptor subtypes, it can show effect on the prostate and the detrusor muscle to increase maximum flow rate and decrease post void residual urine. Tamsulosin 0.4 mg has higher efficacy than tamsulosin 0.2 mg as shown in uroflowmetry measurements.
Nocturia has been recognized as one of the most bothersome symptoms in men suffering from LUTS suggestive of BPH, due to its impact on the quality of life (QoL) [9]. Tamsulosin 0.4 mg is believed to result in a low risk of peak-associated adverse events and a good control of daytime and nighttime symptoms of BPH [10]. In our study for nocturia, tamsulosin 0.4 mg can decrease frequency compared to tamsulosin 0.2 mg by one time per night. Tamsulosin 0.4 mg can improve clinical symptoms and frequency of nocturia.
The prevalence of BPH and the loss of erectile function (ED) increase with advancing age, even though the pathogenetic relationship between LUTS and ED is not yet completely understood [11]. Alpha-blockers for LUTS have been associated with a decreased risk of sexual dysfunction. Improvement in sexual function correlated with the improvement in LUTS more strongly among those using alpha-blockers [12,13]. Erectile dysfunction is a problem in elderly male patients and we used IIEF-5 to evaluate it in this study. Treatment with tamsulosin 0.4 mg had minimal effecton IIEF-5 scores. Symptoms of ED progression may be due to the effect of increase in age, co-morbid diseases and cardiovascular disease on the clinical symptoms of ED. With either tamsulosin 0.4 mg or tamsulosin 0.2 mg, as age increases, clinical symptoms of ED will also increase.
Aging male symptoms are most problematic in elderly male LUTS and BPH patients. In our study it was found that after switching to treatment with tamsulosin 0.4 mg, aging male symptoms became worse. Increase of age, co-morbid disease and chronic illness can affect symptoms.
The most common side effects of tamsulosin include abnormal ejaculation occurring in up to 18.1 percent of patients, runny or stuffy nose up to 17.9 percent, dizziness up to 17.1 percent, infections, including the common cold or flu up to 10.8 percent, general body pain up to 8.5 percent, back pain up to 8.3 percent, diarrhea up to 6.2 percent and sore throat up to 5.8 percent. Less common tamsulosin side effects that occurred in 1 to 5 percent include increased coughing, sleepiness, chest pain, nausea, sinusitis, decreased libido, blurred vision, tooth problems, insomnia andvertigo [14]. In our study the side effects of tamsulosin were rare and no difference between the two dosage levels after the switch from 0.2 mg to 0.4 mg. Tamsulosin 0.4 mg is safe with regard to cardiovascular events and other side effects are equal to those of tamsulosin 0.2 mg.
This study is a retrospective study because tamsulosin 0.2 mg was withdrawn in Thailand. This was an advantage because collecting data for a prospective study may have been difficult to justify to an ethics committee. With regard to cost of treatment, tamsulosin 0.4 mg is more expensive than tamsulosin 0.2 mg but when compared for efficacy and side effects tamsulosin 0.4 mg is a worthwhile and safe treatment. Limitations of this study included: 1) Time for follow up after switching to tamsulosin 0.4 mg was shorter than that for tamsulosin 0.2 mg. 2) This study collected data from out patient department cards, data may not include all complications after treatment with tamsulosin.
5. Conclusion
Switching to tamsulosin 0.4 mg improves clinical symptoms, improves quality of life in patients with LUTS due to BOO associated with BPH. The most common side effects of tamsulosin are dizziness, postural hypotension and retrograde ejaculation found in first three months of treatment.
REFERENCES
- R. Webber, “Benign Prostatic Hyperplasia,” Clinical Evidence, Vol. 15, 2006, pp. 1213-1226.
- S. J. Jacobsen, C. J. Girman, H. A. Guess, et al., “Natural History of Prostatism: Longitudinal Changes in Voiding Symptoms in Community Dwelling Men,” Journal of Urology, Vol. 155, No. 2, 1996, pp. 595-600.
- M. J. Barry, F. J. Fowler, L. Bin, et al., “The Natural History of Patients with Benign,” 1997.
- P. Narayan and H. S. G. R. Tunuguntla, “Long-Term Efficacy and Safety of Tamsulosin for Benign Prostatic Hyperplasia,” Review in Urology, Vol. 7, 2005, pp. 42-45.
- F. C. Lowe, “Summary of Clinical Experiences with Tamsulosin for the Treatment of Benign Prostatic Hyperplasia,” Review of Urology, Vol. 7, No. 3, 2005, pp. 13-21.
- J.-W. Chung, S. H. Choi, B. S. Kim, T.-H. Kim, E. S. Yoo, C. Il Kim, K. S. Lee and T. G. Kwon, “Efficacy and Tolerability of Tamsulosin 0.4 mg in Patients with Symptomatic Benign Prostatic Hyperplasia,” Korean Journal of Urology, Vol. 52, No. 7, 2011, pp. 479-484.
- A. K. Erik, “Storage and Voiding Symptoms: Pathophysiologic Aspects,” Journal of Urology, Vol. 62, No. 3, 2003, pp. 3-10.
- R. Sakakibara, S. Hamano, T. Uchiyama, Z. Liu, T. Yamanishi and T. Hattori, “Do BPH Patients Have Neurogenic Detrusor Dysfunction? A Uro-Neurological Assessment,” Journal of Urology, Vol. 74, No. 1, 2005, pp. 44-50.
- R. Christopher, J. E. Batista, R. Berges, E. Chartier-Kastler, A. Tubaro, P. Van Kerrebroeck and H. Stoevelaar, “The Impact of Nocturia in Patients with LUTS/BPH: Need for New Recommendations,” Journal of European Urology, Vol. 5, Suppl. 5, 2006, pp. 12-18.
- P. Van Kerrebroeck, “Nocturia and Tamsulosin OCAS,” Journal of European Urology, Vol. 6, 2007, pp. 695-760.
- M. H. Braun, F. Sommer, G. Haupt, M. J. Mathers, B. Reifenrath and U. H. Engelmann, “Lower Urinary Tract Symptoms and Erectile Dysfunction: Co-Morbidity or Typical ‘Aging Male’ Symptoms? Results of the ‘Cologne Male Survey’,” European Urology, Vol. 44, No. 5, 2003, pp. 588-594.
- R. Kumar, A. Nehra, D. J. Jacobson, M. E. McGree, N. M. Gades, M. M. Lieber, S. J. Jacobsen and J. L. St. Sauver, “Alpha-Blocker Use Is Associated with Decreased Risk of Sexual Dysfunction,” Journal of Urology, Vol. 74, No. 1, 2009, pp. 82-87.
- S. Permpongkosol, S. Krilad-O-Larn and K. Ratana-OLarn, “Treatment with a Uroselective α1-Blocker Improves Voiding and Sexual Function: A Study in Thai Men with lower Urinary Tract Symptoms,” Journal of Sex Medicine, Vol. 13, No. 4, 2011, pp. 534-536.
- Kristi Monson, 2007. http://www.emedtv.com/tamsulosin-side-effect.htm
NOTES
*Corresponding author.

