Food and Nutrition Sciences
Vol. 2 No. 10 (2011) , Article ID: 16366 , 6 pages DOI:10.4236/fns.2011.210151
Antioxidant Potential of Peel Extracts of Banana Varieties (Musa sapientum)
![]()
Department of Biotechnology, Kumaraguru College of Technology, Coimbatore, India.
Email: bhubaski@rediffmail.com
Received August 28th, 2011; revised October 28th, 2011; accepted November 6th, 2011.
Keywords: Antioxidants, Banana Peel, Lipid Peroxidation, Oxygen Radicals, Phytochemicals
ABSTRACT
The study has been aimed to evaluate and compare phytochemical content and the antioxidant activity in peel extracts of nine local varieties of banana, i.e. Musa sapientum species. Ethanolic extract of peels of these varieties were subjected to in vitro free radical scavenging assays like DPPH, ABTS and lipid peroxidation inhibition assay. Total antioxidant capacity assay to confirm the antioxidant potential and phytochemical content such as total phenols, flavonoids were also determined. The results obtained were analyzed statistically by ANOVA and DMRT analysis. The peel extracts of all the nine varieties of banana exhibited significant antioxidant and phytochemical activities with Musa spp. - Blueggoe (Monthan) - AAB and Musa spp. - Rasthali - AAB showing highest free radical scavenging activity and Musa spp. - Karpooravalli - ABB, Musa spp. - Rasthali - AAB, Musa spp. - Ney Poovan (Kadali) - AB and Musa spp. - Mysore (Poovan) - AAB having highest phytochemical content. The study suggests that peel extracts of these banana varieties could be useful to combat free radical mediated diseases.
1. Introduction
Free radicals are continuously produced in our body either naturally or on exposure to environmental stress as well as other factors and can be implicated in many diseases like cancer, atherosclerosis, arthritis, Parkinson’s disease, Alzheimer’s disease, aging and other age related problems [1]. Mammalian cells possess elaborate defense mechanisms for radical detoxification. Antioxidants are agents, which scavenge the free radicals and prevent the damage caused by them. Inspite of these in-built defense mechanisms, it seems more meaningful to utilize extra antioxidants available in diets, especially from fruits, vegetables and whole grains [2]. Due to their minimal side effects, there are growing interests in using natural products for preventive and therapeutic medicine [3].
Musa spp., comprising banana and plantain are among the world’s leading fruit crops and in terms of economical value, it is the number five agricultural crop in world trade. The edible fruit cultivars are a man-made complex based on two wild diploid species originating from South-East Asia: Musa acuminata Colla (AA), which is highly polymorphous, with spindly plants that grow in clumps, and Musa balbisiana Colla (BB), a homogeneous hardy plant with a massive pseudo-trunk. There are diploid, triploid or tetraploid genome groups. The main genome groups are AA, AB, AAA, AAB and ABB [4].
The peels of a variety of fruits have gained attention as a natural source of antioxidants and phytochemical content which are rich in compounds with free radical scavenging activity. Banana and Plantain peels are major agricultural wastes which have been used as medicine, animal feeds, blacking of leathers, soap making, fillers in rubber and so on [5]. Fruit wastes are highly perishable and seasonal and are a problem to the processing industries and pollution monitoring agencies. This problem can be recovered by utilizing its high value compounds, including the dietary fibre fraction that has a great potential in the preparation of functional foods [6]. Banana peel, an underutilized source of phenolic compounds is considered as a good source of antioxidants for foods and functional foods against cancer and heart disease [7]. The peel of the fruit contains various antioxidant compounds such as gallocatechin [7] and dopamine [6].
Recent trends focus on the isolation, characterization and utilization of natural antioxidants, especially growing interest in polyphenols as potential disease preventing agents. As these compounds are predominantly found in most of fruit tissues, it would be worthwhile investigating the nature of polyphenols that are present in banana peel, a potential source of antioxidant and antimicrobial activities. Hence, the present study has been aimed to evaluate and compare the phytochemical contents and their in vitro antioxidant activities in peel extracts of nine local varieties of banana to assess its protective role against free radical induced cell damage.
2. Materials and Methods
2.1. Materials, Chemicals and Reagents
The banana varieties belonging to Musa balbisiana viz., Monthan (Musa spp. - Blueggoe - AAB), Karpooravalli (Musa spp. - Karpooravalli - ABB) Nendran (Musa spp. - French Plantain - AAB), Kadali (Musa spp. - Ney Poovan - AB), and those belonging to Musa acuminata viz., Pachainadan (Musa spp. - Pachanadan - AABS), Poovan (Musa spp. - Mysore - AAB), Rasthali (Musa spp. - Rasthali - AAB), Robusta - Cavendish sub group (Musa spp. - Robusta - AAB) and Sevvazhai (Musa spp. - Red banana - AAA) were collected locally from different farms in the same locality in Coimbatore (Tamil Nadu, India) and authenticated by Dr. T. S. Balamohan, Professor and Head, Faculty of Horticulture, Tamil Nadu Agricultural University, Coimbatore. A voucher specimen of the samples has been deposited in the herbarium of the department.
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2-azobis-3- ethylbenzthiazoline-6-sulphonic acid (ABTS), sodium nitroprusside, sulphanilamide, naphthyl ethylenediamine dihydrochloride, 2-deoxyribose, thiobarbituric acid (TBA), trichloroacetic acid (TCA), sodium dodecyl sulphate (SDS) and ammonium molybdate were all of analytical grade.
2.2. Preparation of Peel Extracts
The peel of fresh naturally ripened yellow un-pigmented bananas were shade-dried for about a week and then crushed to make a coarse powder. The dried powder (10 g) was weighed and solvent extraction using ethanol was performed at a 10% concentration. Exhaustive extraction was carried out in triplicates for about 36 h in a shaker at 37˚C with a gentle shaking. The extracts were then evaporated at room temperature. The residues obtained were re-evaporated to remove impurities and stored at 4˚C to carry out radical scavenging assays. The remaining residue was stored in desiccators for further use.
2.3. Free Radical Scavenging Assays
2.3.1. Total Antioxidant Capacity Assay
Aliquots of suitable working solutions (1 - 10 mg/ml) of the samples were mixed with 1 ml of the reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) and incubated at 95˚C for 90 min [8]. The tubes were cooled to room temperature and the absorbance was measured at 695 nm against a blank. Ascorbic acid was used as a standard. Total antioxidant capacity was expressed as equivalents of ascorbic acid [9].
2.3.2. DPPH Radical-Scavenging Assay
DPPH scavenging activity was measured by the slightly modified spectrophotometric method of Brand-Williams et al. [10]. The absorbance of DPPH diluted in methanol was considered as control. The decrease in absorbance was measured at 517 nm. The antioxidant capacity to scavenge the DPPH radical was calculated by the following equation: Scavenging effect (%): [(1-absorbance of sample/absorbance of control) × 100]. Results were expressed as Mean ± SD of three experiments made by triplicate.
2.3.3. ABTS Radical Cation-Scavenging Assay
The assay was performed by a slightly modified protocol of Re et al. [11]. ABTS solution (7 mM) was reacted with ammonium persulphate (2.45 mM) solution to produce a dark coloured solution containing ABTS radical cations. The initial absorbance was measured at 745 nm. This stock solution was diluted with methanol to give a final absorbance value and equilibrated at 30˚C. The decrease in absorbance was measured exactly one minute after mixing the solution, up to six minutes. The final absorbance was noted. The percentage inhibition was calculated according to the formula:
% inhibition = [(Acontrol – Asample)/Acontrol] × 100%
2.3.4. Lipid Peroxidation Inhibition Assay
The lipid peroxidation assay was carried out by a modified procedure of Ohkawa et al. [12]. Different concentration of the sample (1 - 10 mg/ml) in water was added to 0.5 ml of the 10% goat liver homogenate. Lipid peroxidation was initiated by adding 0.05 ml of 0.07 mol/m3 ferrous sulphate to the reaction mixture. After 30 min, 1.5 ml of 20% acetic acid (pH 3.5), 1.5 ml of 0.8% TBA (in 1.1% SDS) and 0.05 ml of 20 × 10–2 TCA was added to the incubation solution, vortexed and boiled at 100˚C for 1 h, cooled to room temperature and read the absorbance at 532 nm. The percentage inhibition was then calculated.
% inhibition = [(Acontrol – Asample)/Acontrol] × 100%
2.4. Determination of Phytochemicals
2.4.1. Determination of Total Polyphenol Content
Total phenolic contents were determined according to the spectrophotometric methods of Tanner and Brunner [13] and Kaur and Kapoor [14]. To 0.5 ml of a methanolic solution of the extracts, 7 ml of distilled water and 0.5 ml of Folin-Ciocalteau reagent were added and mixed well. After 3 min, 2 ml of 20% sodium carbonate was added and mixed well again. Absorbance of the resultant solution was read at 720 nm, after 1 h in a water bath at 25˚C [15]. The total polyphenol content was calculated from the standard calibration curve obtained from catechol.
2.4.2. Determination of Flavonoids
A slightly modified version of the spectrophotometric method was used to determine the flavonoid contents of samples [15]. A 0.5 ml aliquot of the sample in aqueous methanol was diluted with 3 ml of distilled water to which 0.3 ml of 5% sodium nitrite was added and mixed well. After 5 min at room temperature, 0.6 ml of 10% aluminium chloride was added. After 6 min, 2 ml of 1 M sodium hydroxide was added and the absorbance was read at 510 nm. Flavonoid contents were then calculated using a standard calibration curve, prepared from rutin.
2.5. Statistical Analysis
The experimental results were expressed as mean ± SD of three replicates. The data were subjected to two-way ANOVA (Analysis of Variance) and significance of difference between sample means were calculated by DMRT analysis using IRRISTAT software version 3.1. Difference in mean values were considered significant when P < 0.05.
3. Results and Discussion
3.1. Total Antioxidant Activity
The total antioxidant activities of various banana peel extracts are depicted in Table 1. The values represent the total antioxidant capacity of banana peel extracts expressed in terms of equivalents of ascorbic acid. This assay gives an estimate of the overall antioxidant potential of the banana peel [16]. In the presence of the extracts, Mo(VI) is reduced to Mo(V) and forms a greencoloured phosphomolybdenum V complex, which shows maximum absorbance at 700 nm. According to the results, the ethanolic extract of Pachainadan showed higher activity in the range of 5.85 mM·g–1 in comparison to other varieties of banana peel, whereas the ethanolic extract of Nendran showed least activity. Similar studies by Gonzalez-Montelongo et al. [17] showed the total antioxidant activity of banana peel extract under different solvent and incubation conditions.
3.2. DPPH Radical Scavenging Activity
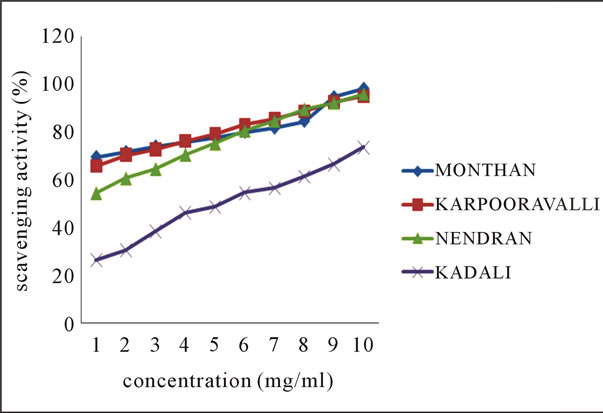
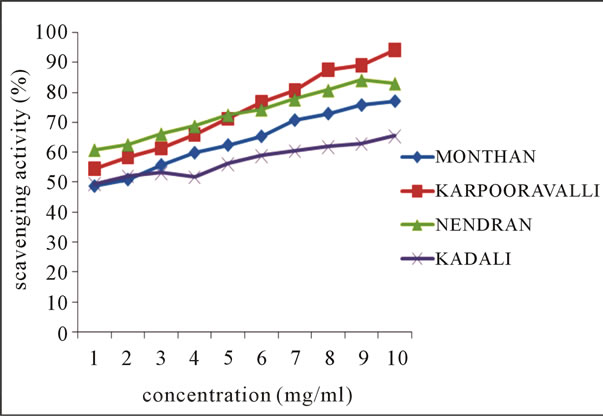
The line chart shown in Figures 1(a)-(b) for DPPH radical scavenging potential of the ethanolic extracts of
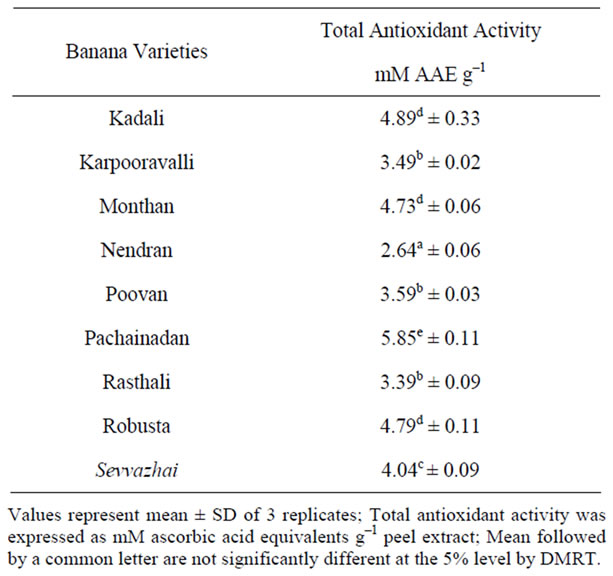
Table 1. Total Antioxidant activity in peels of banana varieties.
 (a)
(a)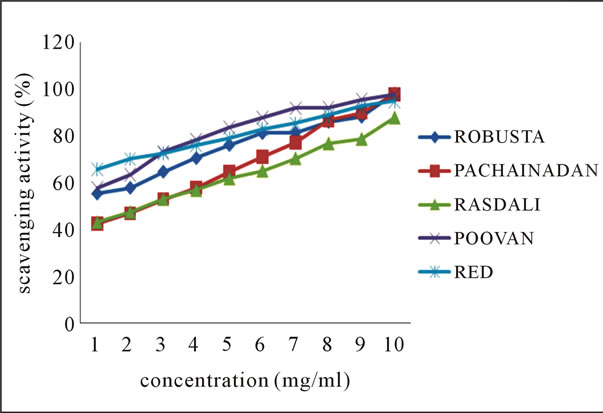 (b)
(b)
Figure 1. Scavenging activity (%) on DPPH radical by ethanolic extracts of (a) balbisiana (b) acuminate type of banana varieties.
balbisiana and acuminata type of local varieties of banana peel respectively, elucidating the mean values across the concentration range, clearly indicates the potential of Mondhan peel extract in scavenging free radicals as high percentage inhibition (98.19%) was noticed at 10 mg·ml–1 in comparison to the peels of other varieties from both groups. DPPH radicals react with suitable reducing agents, during which the electrons become paired off and the solution loses colour stoichiometrically depending on the number of electrons taken up [18]. In the experiment, the solution progressively reduced to a yellow coloured product, diphenylpicryl hydrazine, with the addition of the extracts in a concentration-dependent manner.
3.3. ABTS Radical Scavenging Activity
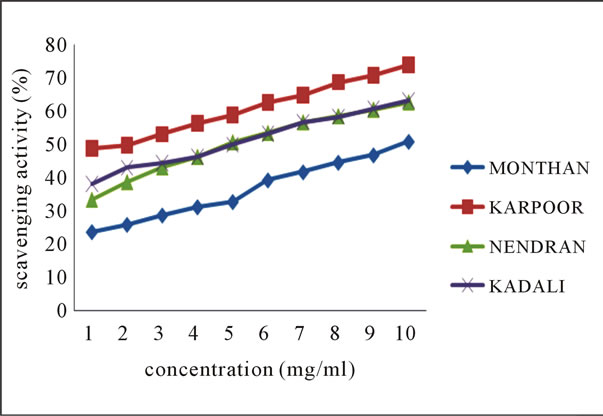
The line charts 2(a) and 2(b) of Figure 2 indicates the ABTS radical scavenging potential of the ethanolic extracts of the various peel extracts of both groups of bananas at various concentrations.
 (a)
(a)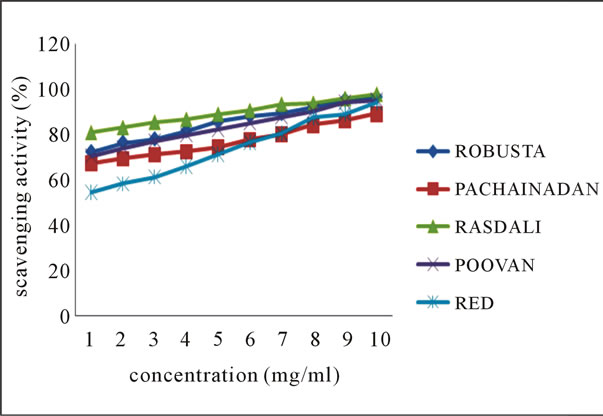 (b)
(b)
Figure 2. Scavenging activity (%) on ABTS radical by ethanolic extracts of (a) balbisiana (b) acuminate type of banana varieties.
From the results, it may be postulated that ethanolic extract of Rasthali, among all species, inhibit the radical or scavenge the radical in a concentration dependent manner with highest percentage inhibition (97.66%) noticed at 10 mg·ml–1. Studies by Okonogi et al. [19] determined the scavenging activity of banana peel extract on ABTS radical.
3.4. Lipid Peroxidation Inhibition Activity
Inhibition of lipid peroxidation by the ethanolic extracts of various banana peels is represented in Figures 3(a)-(b). Lipid peroxides are unstable and decompose to form reactive carbonyl compounds responsible for DNA damage, generation of cancer and age related diseases [20]. Most abundant among them is malondialdehyde (MDA), which reacts with thiobarbituric acid (TBA) to form a pink chromogen [21]. The decrease in the MDA levels in the presence of increased concentration of each extract indicates the role of extracts as antioxidants. TBARS assay was used to determine the anti-lipid per-
 (a)
(a)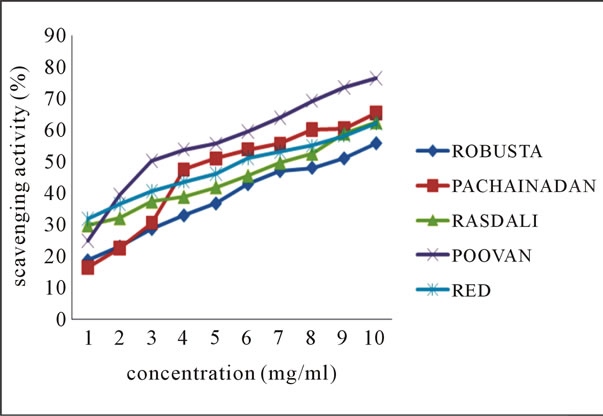 (b)
(b)
Figure 3. Scavenging activity (%) on lipid peroxidation by ethanolic extracts of (a) balbisiana (b) acuminate type of banana varieties.
oxidation properties of the banana peel extracts. The result values obtained indicate a moderate percentage inhibition with Poovan peel extract exhibiting highest inhibition among other varieties at 10 mg·ml–1.
3.5. Total Phenol and Flavonoid Content
The total phenol and flavonoid content of the different banana peel extracts is shown in Table 2.
The antioxidant activity of the plant products is associated to their bioactive compounds, mainly antioxidant phenolics, because of their ability to scavenge free radicals [22,23]. Phenols are secondary metabolites in plants and are known to possess a wide range of therapeutic uses, such as antioxidant, antimutagenic, anticarcinogenic, free radical-scavenging activities and also decrease cardiovascular complications [24]. From the results, it is inferred that Rasthali extract showed higher phenol content compared to other varieties, which could be related to its antioxidant potential.
Many flavonoids are found to be strong antioxidants capable of effectively scavenging the reactive oxygen species because of their phenolic hydroxyl groups [25]. In our study, from the table values, Poovan peel extract exhibited higher flavonoid content which might be correlated with its anti-lipid peroxidation activity.
Several mechanisms have been proposed to be involved in the antioxidant activity such as hydrogen donation, termination of free radical mediated chain reaction, prevention of hydrogen abstraction, chelation of catalytic ions, and elimination of peroxides [26].
Antioxidant activity is a dependent system and the characteristic of a particular system can influence the outcome of the analysis. Hence, a single assay would not be representative of the antioxidant potential of plant extracts. In the present study, we employed different models of antioxidant assays which could provide a more reliable approach to assess the antioxidant and radical scavenging potential of peel extracts of various banana varieties.
4. Conclusions
The investigation of the antioxidant potential and phytochemical content of banana peel of nine different local varieties showed that the content of total phenols were higher in Rasthali compared to other species which might be correlated with its high ABTS scavenging activity. In contrast, the content of flavonoids was higher in Poovan banana and is highly correlated with its lipid peroxidation inhibition activity. Hence the relationship between phytochemical content and free radical scavenging activity of banana peel indicates that peel extracts from these varieties may be useful to combat free radical mediated diseases. These observations may be used to substantiate
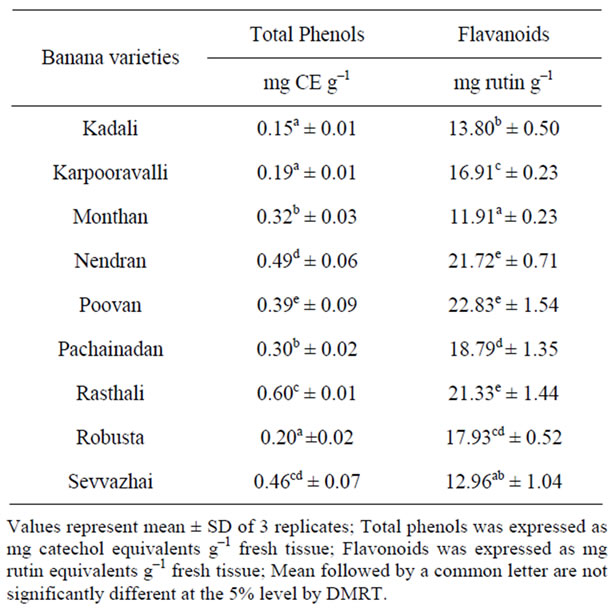
Table 2. Phytochemical contents in peels of banana varieties.
the scientific reasoning that free radical-scavenging is indeed the mode of operation of these peel varieties in the treatment or prevention of the onset of diseases that evidence free radical activity.
5. Acknowledgements
The authors gratefully acknowledge the Management of Kumaraguru College of Technology, Coimbatore for financial assistance to carry out this work.
REFERENCES
- B. Halliwell and J. M. C. Gutteridge, “Free Radicals in Biology and Medicine,” 2nd Edition, Oxford Science Publications, Clarendon, 1989.
- B. A. Silva, F. Ferreres, J. O. Malva and A. C. P. Dias, “Phytochemical and Antioxidant Characterization of Hypericum perforatum Alcoholic Extracts,” Food Chemistry, Vol. 90, No. 1-2, 2005, pp. 157-167. doi:10.1016/j.foodchem.2004.03.049
- K. J. Jo, M. R. Cha, M. R. Lee, M. Y. Yoon and H. R. Park, “Methanolic Extracts of Uncaria rhynchophylla Induce Cytotoxicity and Apoptosis in HT-29 Human Colon Carcinoma Cells,” Plant Foods for Human Nutrition, Vol. 63, No. 2, 2008, pp. 77-82. doi:10.1007/s11130-008-0074-z
- A. Guyle`ne, P. Berthe and F. Louis, “Bananas, Raw Materials for Making Processed Food Products,” Trends in Food Science and Technology, Vol. 20, No. 2, 2008, pp. 1-3.
- J. O. Arawande and E. A. Komolafe, “Antioxidative Potentials of Banana and Plantain Peel Extracts on Crude Palm Oil,” Ethnobotanical Leaflets, Vol. 14, 2010, pp. 559-569.
- K. Kanazawa and H. Sakakibara, “High Content of Dopamine, a Strong Antioxidant in Cavendish Banana,” Journal of Agricultural Food Chemistry, Vol. 48, No. 3, 2000, pp. 844-848. doi:10.1021/jf9909860
- S. Someya, Y. Yoshiki and K. Okubo, “Antioxidant Compounds from Bananas (Musa cavendish),” Food Chemistry, Vol. 79, No. 3, 2002, pp. 351-354. doi:10.1016/S0308-8146(02)00186-3
- M. Umamaheswari and T. K. Chatterjee, “In Vitro Antioxidant Activities of the Fractions of Coccinia grandis L. Leaf Extract,” African Journal of Traditional, Complementary and Alterrnative Medicine, Vol. 5, No. 1, 2008, pp. 61-73. doi:10.4314/ajtcam.v5i1.31258
- G. Raghavan, M. Vijayakumar and S. Mehrotra, “Free Radical Scavenging Potential of Chlorophytum tuberosum Baker,” Journal of Ethnopharmacology, Vol. 104, No. 3, 2003, pp. 423-425.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, “Use of Free Radical Method to Evaluate Antioxidant Activity,” Lebensmittel Wissenschaft und Technologie, Vol. 28, No. 1, 1995, pp. 25-30. doi:10.1016/S0023-6438(95)80008-5
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, “Antioxidant Activity Applying Improved ABTS Radical Cation Decolorization Assay,” Free Radical Biology and Medicine, Vol. 26, No. 9-10, 1999, pp. 1231-1237. doi:10.1016/S0891-5849(98)00315-3
- H. Ohkawa, N. Ohishi and K. Yagi, “Assay for Lipid Peroxidation in Animal Tissues by Thiobarbituric Acid Reaction,” Annals in Biochemistry, Vol. 95, No. 2, 1979, pp. 351-358. doi:10.1016/0003-2697(79)90738-3
- H. Tanner and H. R. Brunner, “Gentranke-Analytik,” Verlag Heller Chemie und Verwaltunsgesellschaft mbH, Germany, 1979.
- C. Kaur and H. C. Kapoor, “Antioxidant Activity and Total Phenolic Content of Some Asian Vegetables,” International Journal of Food Science and Technology, Vol. 37, No. 2, 2002, pp. 153-161.
- F. Karadeniz, H. S. Burdurlu, N. Koca and Y. Soyer, “Antioxidant Activity of Selected Fruits and Vegetables Grown in Turkey,” Turkish Journal of Agricultural Forum, Vol. 29, 2005, pp. 297-303.
- P. Preito, M. Pineda and M. Aguliar, “Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E,” Annals in Biochemistry, Vol. 269, No. 2, 1999, pp. 337-341. doi:10.1006/abio.1999.4019
- M. Gonzalez-Montelongo, L. Gloria and G. Monica, “Antioxidant Activity in Banana Peel Extracts: Testing Extraction Conditions and Related Bioactive Compounds,” Food Chemistry, Vol. 119, No. 3, 2010, pp. 1030-1039. doi:10.1016/j.foodchem.2009.08.012
- B. Subhasree, R. Baskar, R. Laxmi Keerthana, R. L. Susan and P. Rajasekaran, “Evaluation of Antioxidant Potential in Selected Green Leafy Vegetables,” Food Chemistry, Vol. 115, No. 4, 2009, pp. 1213-1220. doi:10.1016/j.foodchem.2009.01.029
- C. Okonogi, A. Songyot, T. Suganya and C. Sombat, “Comparison of Antioxidant Capacities and Cytotoxicities of Certain Fruit Peels,” Food Chemistry, Vol. 103, No. 3, 2007, pp. 839-846. doi:10.1016/j.foodchem.2006.09.034
- T. Kulisic, V. Ddagovic-Uzelac and V. Milos, “Antioxidant Activity of Herbal Tea Infusions,” Food Technology and Biotechnology, Vol. 44, No. 4, 2006, pp. 485-492.
- R. Baskar, V. Rajeswari and T. S. Kumar, “In Vitro Antioxidant Studies in Leaves of Annona Species,” Indian Journal of Experimental Biology, Vol. 45, No. 5, 2007, pp. 480-485.
- E. Casanova, J. M. García-Mina and M. I. Calvo, “Antioxidant and Antifungal Activity of Verbena officinalis L. Leaves,” Plant Foods for Human Nutrition, Vol. 63, No. 3, 2008, pp. 93-145. doi:10.1007/s11130-008-0073-0
- K. Saito, M. Kohno, F. Yoshizaki and Y. Niwano, “Extensive Screening for Edible Herbal Extracts with Potent Scavenging Activity against Superoxide Anions,” Plant Foods for Human Nutrition, Vol. 63, No. 2, 2008, pp. 65- 70. doi:10.1007/s11130-008-0071-2
- G. C. Yen, P. D. Duh and C. L. Tsai, “Relationship between Antioxidant Activity and Maturity of Peanut Hulls,” Journal of Agricultural Food Chemistry, Vol. 41, No. 1, 1993, pp. 67-70. doi:10.1021/jf00025a015
- G. Cao, E. Sofic and R. L. Prior, “Antioxidant and ProOxidant Behaviour of Flavonoids: Structure Activity Relationships,” Free Radical Biology and Medicine, Vol. 22, No. 5, 1997, pp. 749-60. doi:10.1016/S0891-5849(96)00351-6
- M. F. Gordon, “The Mechanism of Antioxidant Action in Vitro,” In: B. J. F. Hudson, Ed., Food Antioxidants, Elsevier Applied Science, London, 1990, pp. 1-18.

