Food and Nutrition Sciences
Vol. 2 No. 7 (2011) , Article ID: 7240 , 10 pages DOI:10.4236/fns.2011.27106
Stir-Fry Chicken with Green Curry Suppresses Inflammatory Gene Expression by Lipopolysaccharide-Induced Murine Macrophages
![]()
1Institute of Nutrition, Mahidol University, Nakhon Pathom, Thailand; 2Thalassemia Research Center, Institute of Molecular Biosciences, Mahidol University, Nakhon Pathom, Thailand; 3Department of Biochemistry, Faculty of Science, Mahidol University, Bangkok, Thailand.
Email: stuntipopipat@hotmail.com, nustt@mahidol.ac.th
Received June 6th, 2011; revised July 27th, 2011; accepted August 4th, 2011.
Keywords: Stir-Fry Chicken with Green Curry, Anti-Inflammation, Lipopolysaccharide, RAW264.7 Murine Macrophages
ABSTRACT
Inflammatory mediators produced during inflammatory response play an important role on pathological development of several chronic diseases. Although several dietary plants exhibited anti-inflammatory property, their impacts as a whole food has been rarely reported. The aim of the present study is to assess anti-inflammatory activity of an ethanol extract from a whole food namely “ready to eat stir-fry chicken with green curry” consisting of green curry paste, big egg plant, pea egg plant, red chili, kaffer lime and sweet basil as plant-based ingredients. The food extract at 55 - 220 μg/ml was incubated with RAW264.7 murine macrophage cells prior to stimulation with lipopolysaccharide (LPS) for 24 h. Inflammatory mediators [(inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor (TNF-a) and interleukine-6 (IL-6)] mRNA and protein were determined by RT-PCR, immunoblot and ELISA respectively. The modulation mechanism by the food extract was observed by measuring the phosphor-ylated form of mitogen activated protein kinases (MAPKs) and inhibitor kappa B alpha (IkB-a). The ready to eat stir-fry chicken with green curry extract significantly suppressed LPS-induced iNOS, COX-2, IL-6 and TNF-a gene expression in a dose-dependent manner without cytotoxicity. The suppressive effect was modulated partly by inhibiting phosphor-ylation of MAPKs and IkB-a. These results indicate that spices and vegetables in a complex diet still possess strong anti-inflammatory activities which warrant confirming such activities to ameliorate the pathogenesis of inflammatory-associated chronic diseases in vivo.
1. Introduction
More and more evidence supports the concept that inflammation plays a central role to develop pathology of various chronic diseases such as cardiovascular disease, diabetes, arthritis, neurode-generative, autoimmune diseases and cancer [1]. The macrophage is an inflammatory immune cell playing a predominant role during inflammation. It can be induced by various stimuli resulting in activating the inflammatory upstream pathway of inhibitor kappa B kinase-nuclear factor kappa B (IKK-NF-kB) and three mitogen-activated protein kinases (MAPKs): p- 38, c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinases (Erk1/2) [2]. Subsequent activation of downstream cascade signal transduction pathwaysNF-kB and activator protein-1 (AP-1) regulate expression of more than 400 genes associated with biological functions [2] such as pro-inflammatory cytokines, adhesion molecules and pro-inflammatory enzymes [1]. Proinflammatory status in turn enhances innate immune signaling resulting in activation of NF-kB to develop a vicious cycle leading to initiate and aggravate pathogenesis of many chronic inflammatory associated diseases [3].
A number of in vitro and in vivo studies supported the finding that nutraceuticals from dietary vegetables, spices and herbs suppressed inflammatory responses on multiple targets [4]. Both crude extracts and pure compounds isolated from spices and herbal vegetables have been demonstrated to suppress pro-inflammatory genes expression [5-6] by inhibiting phosphorylation of signaling proteins in signal transduction pathways which activate NF-κB mediated transcription of pro-inflammatory genes. Spices and herbs have long been used in ancient times in the tropical belt regions including Asia for culinary and medicinal purposes. Due to their daily consumption and relatively high intake compared with standard pharmaceutical agents, their impacts on human health are of increasing interest. In addition, various phytochemicals in a whole diet have been postulated to synergistically exert their health benefit functions via multiple pathways [5], rather than through a single specific one as for a pharmaceutical counterpart. A great challenge is to define potential mechanisms of spices and herbs in a whole dish to alleviate pathogenesis of such diseases. As with other Asian diets, habitual Thai foods usually consist of several spices and herbs in their recipes.
Thus, the present study aims to define the anti-inflammatory mechanism of a ready-to-eat recipe, namely “stir-fry chicken with green curry”, which consists of chicken meat, green curry paste (a mixture of 9 spices), big egg plant, pea egg plant, red chili, kaffer lime, and sweet basil in its recipe. A well defined LPS-induced murine macrophage RAW264.7 cell line was used to assess anti-inflammatory activities of this food extract. The results provide supportive scientific data to confirm the health benefit effects of the mixed spices and herbs present in the whole diet.
2. Materials and Methods
2.1. Chemicals and Reagents
Dulbecco’s modified Eagle’s medium (DMEM), lipopolysaccharide (LPS) (E. coli O11:B4), and anti-b-actinHRP were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was obtained from PAA Laboratories (Haidmannweg, Austria). Penicillin and streptomycin were obtained from Invitrogen (Grand Island, NY). Antibodies against inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), phosphop38 (p-p38), p38, phospho-JNK (p-JNK), JNK, phosphoErk1/2 (p-Erk1/2), Erk1/2, phospho-inhibitor kappa B alpha (p-IkB-α) and IkB-α were purchased from Cell Signaling Technology (Danvers, MA). All other chemicals and reagents were analytical or HPLC grade.
2.2. Cell Culture
Murine macrophage cell lines RAW 264.7 purchased from American Type Culture Collection (Manassas, VA, USA) were cultured in complete medium consisting of DMEM with 10% FBS, 15 mM [N-(2-Hydroxyethyl) piperazine-N’-(2-ethanesulfonic acid)] (HEPES), 100 units/ml penicillin and 100 mg/ml streptomycin at 37˚C in an atmosphere of 5% CO2. Cells at 7.5 × 105 cells/ml were cultured with serum and phenol red-free DMEM containing extract for 1 h prior to incubation with 5 ng/ml LPS for 24 h.
2.3. Preparation and Extraction Stir-Fry Chicken with Green Curry
The stir-fry chicken with green curry (198 g fresh weight per serving) consisted of 7.8 g chicken meat without skin, 13 g of green curry paste [a combination of fresh green chili pepper fruits (Capsicum annuum) 31%, fresh lemon grass stem (Cymbopogon citrates) 21%, garlic bulb (Allium vineale) 18.5%, fresh galangal root (Alpinia galangal) 8.5%, dried kaffer lime peel (Citrus hystrix) 2%, dried pepper seed (Piper nigrum) 0.5%, coriander seed (Coriandrum vulgare) 1%, cumin (Cuminum cyminum) 0.5%, tumeric root (Curcuma longa) 0.5%, and shrimp paste 4% with 12% less sodium salt], big egg plant fruits or brinjal (Solanum melongena) 39 g, pea egg plant fruits (Solanum torvum) 8 g, red chili fruit (Capsicum annuum) 4 g, kaffer lime leaves (Citrus hystrix) 1 g, sweet basil (Ocimum basilicum) 8 g, sorbitol 6 g, fishsauce 1 g, oil 10 g and water 31 g. The cooking method was prepared like the traditional fashion in Thai kitchen. The green curry paste was stir-fried in oil over medium heat for 5 min followed by addition of chicken and stir-fried until cooked. The big egg plant, pea egg plant, red chili fruit with water were subsequently stir-fried until cooked. Fishsauce and sorbitol were added as seasonings and topped up with kaffer lime leaves and sweet basil leaves. The ready-to-eat stir-fried chicken with green curry paste was freeze dried and packed in vacuum aluminum foil and stored at –20˚C until used. The freeze dried sample was extracted twice at ratio 0.1 g sample/3ml of 90% ethanol. After centrifugation at 4500 g for 10 min, the combined supernatant was evaporated under vacuum at 45˚C - 50˚C for 10 min. The yield of the dry extract was 22%. The dry extract was resolubilized with 0.2% final concentration of dimethyl sulfoxide (DMSO): ethanol (1:1) and further diluted to desired concentration with serum and phenol red-free DMEM prior to addition to cell cultures.
2.4. HPLC Analysis
The dry residues of the ethanol extract were reconstituted with 1 ml of mobile phase solution prior to analysis of the carotenoid contents by reverse phase HPLC. The HPLC system consisted of a Waters 600 Controller module with photodiode array detector (PDA) (Agilent 1100 series, Santa Clara, CA, U.S.A). The column sytem was composed of guard cartridges C18 (Phenomenex KJO- 4282, Torrance, CA, USA) connected with a VYDAC 201TP54 (250 mm × 4.6 mm) C18 reversed-phase column, particle size 5-mm (GRACE, Hesperia, CA, U.S.A). Carotenoids were eluted by an isocratic mobile phase consisting of acetonitrile/methanol/ dichloromethane (80: 11:9 v/v/v) with 0.1% (v/v) triethylamine and 0.1% (w/v) ammonium acetate with 20 ml injection volume at flow rate of 0.7 ml/min. The chromatograms were monitored at 450 nm and quantified by comparison of retention time and peak area against a pure standard curve of carotenoids.
The phenolic contents in the dry extract were reconstituted in 2 ml of acid methanol (62.5% methanol: 6M HCl, 4:1 v/v) and incubated at 70˚C in a shaking water bath for 2 h prior to analysis by HPLC. The HPLC system (Agilent 1100 series; Agilent Technologies, Santa Clara, CA, U.S.A.) consisted of an auto-sampler injector at 4˚C. The column consisted of an Eclipse XDB-C18 guard column (4.6 mm × 12.5 mm, 5 µm, Agilent Technologies, U.S.A.) connected with a Zorbax Eclipse XDBC18 column (4.6 mm × 150 mm, 5 µm, Agilent Technologies, U.S.A.) and maintained at 30˚C. The mobile phase consisted of 100% water containing 0.5% (w/w) trifluoroacetic acid (TFA) (solvent A), 100% methanol containing 0.5% (w/w) TFA (solvent B), and 100% acetonitrile containing 0.5% (w/w) TFA (solvent C). Phenolic content was eluted by a gradient profile of mobile phase as previously described [7] with 5 ml injection volume at 0.6 mL/min. The chromatograms were monitored at 338 nm and evaluated by ChemStation program (Agilent Technologies, U.S.A.). The amount of each phenolic compound was quantified by comparing retention times, spectrum and peak area with pure standards.
2.5. Cytotoxic Assay
Cell viability was assessed by protein staining with Sulforhodamine B (SRB) [8]. The treated cells monolayer was fixed with 50% cold Trichloroacetic acid (TCA) at 4˚C for 2 h. Cells were stained with 0.1% w/v SRB in 1% acetic acid and dissolved with 10 mM Tris-hydroxylmethylaminomethane before measuring absorbance at 500 nm by microplate reader (TECAN Austria GmbH, Salzburg, Austria).
2.6. Measurement of Nitric Oxide (NO), Tumor Necrosis Factor-Alpha (TNF-(), Interlekin-6 (IL-6)
RAW 264.7 cells were incubated with food extract at 55 - 220 µg/ml for 1 h prior to incubate with LPS. The cell culture media were collected to determine nitrite, a stable product of nitric oxide (NO) by the Griess reaction [9]. Nitrite concentration was calculated by comparison with sodium nitrite. The sandwich ELISA was used to measure TNF-a and IL-6 by using paired antibodies purchased from eBioscience Inc. (San Diego, CA, USA). In brief, 96-well plates (NUNC, Roskilde, Denmark) were incubated with capture antibody against TNF-a and IL-6 overnight and incubated with 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS). Culture medium or recombinant mouse TNF-a and IL-6 proteins were incubated to the coated primary antibody at 4˚C overnight prior to addition with biotinylated antibodies. The complex was detected with streptavidin conjugated with peroxidase-tetramethylbenzidine detection system (Endogen, Rockford, IL, USA). The reaction was terminated by H2SO4 and absorbance at 450 nm was determined using a microtiter plate reader. Concentrations of TNF-a and IL-6 were calculated by comparing absorbance with the standard curve.
2.7. Western Blot Analysis
In brief, treated cells were washed twice with ice-cold PBS and resuspended in ice cold lysis buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 50 mM NaF, 10 mM sodium pyrophosphate and 0.2% protease inhibitor cocktail (Sigma) for 30 min at 4˚C. Cell lysate was obtained by centrifugation at 13,000 g at 4˚C for 10 min. Protein content was determined by BCA method (Pierce, Rockford, IL). Protein 40 mg (iNOS and COX-2) and 30 mg (MAPKs and IkB) was separated on 10% SDS-PAGE and transferred onto nitrocellulose membranes (Whatman GmbH, Dassel, Germany). Membranes were incubated with 5% nonfat dry milk in Tris-buffer containing 0.1% Tween (TBST) and incubated overnight at 4˚C with specific primary antibody in TBST containing 5% BSA. The membranes were reacted with peroxidase-conjugated with species specific secondary antibody (Sigma). The reactive bands on membranes were visualized by Super Signal solution (Endogen Inc, Rockford, IL). The membranes were stripped off the bound antibody and reprobed with anti-b actin or non-phosphorylated MAPKs antibodies as a control. The density of target bands was quantified by Image J program (download from http://rsb.info. nih.gov/ij/). Results are expressed as relative ratio of band density between target protein/b-actin proteins.
2.8. RNA Extraction and RT-PCR
Total RNA of treated cells was extracted with Trizol (Invitrogen, Grand Island, NY) following the manufacturer’s instruction. The cDNA was obtained from reverse transcription of one mg total RNA with oligo-dT18 primer by using reverse transcriptase (SuperScriptTM III Reverse Transcriptase, Invitrogen, USA) following the manufacturer’s instruction. The gene-specific primers sequences were previously described elsewhere [10]. Gene amplification was performed in a MyCycler thermal cycler (BioRad, Hercules, CA, USA) under the following conditions: denaturation at 95˚C for 15 min for the first cycle; 94˚C for 30 s annealing at 62˚C (COX-2 and IL-6) or 58.1˚C (iNOS) or 55˚C (TNF-a) for 30 s and extension at 72˚C (iNOS) and 68˚C (COX-2, TNF-a and IL-6) for 45 s for 25 repetitive cycles (COX-2, iNOS and IL-6) or 23 repetitive cycles (TNF-a). Final extension was done at 72˚C for 10 min. Each target gene was conducted by duplex amplification with b-actin primers as an internal control. The PCR products were separated on 1.5% agarose gel and stained with 1% ethidium bromide. The density of target bands was quantified by Image J program. Results were expressed as relative ratio of band intensity between target mRNA/b-actin mRNA.
2.9. Measurement of Intracellular Reactive Oxygen Species (ROS)
The intracellular ROS generated by LPS-challenged RAW264.7 cells was measured as previously described [11]. The treated cells were rinsed with PBS (37˚C) and incubated with 5 mM 2’,7’-dichlorofluorescein diacetate (DCF-DA) (Sigma) at 37˚C for 30 min. Cells diffused with DCF-DA were washed with PBS and lysed with 0.5% TritonX-100. The supernatant after centrifugation at 12,000 g for 5 min was collected to measure at excitation wavelength at 485 nm and emission wavelength at 530 nm by Luminescence Spectrometer LS55 (Perkin Elmer Instruments LCC, Shelton, CT).
2.10. Statistical Analysis
All statistical analysis was performed using SPSS (version 14.0, SPSS Inc., Chicago, Illinois). Data are presented as means ± SD. The mean values were calculated from at least three separate experiments conducted on separate days. Statistical differences were determined by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test for multiple comparison of group means. Significance was set at P < 0.05.
3. Results
3.1. Carotenoids and Phenolic Content
The food extract contained lutein at 4.4 ± 0.2 mg/g and bcarotene at 3.4 ± 0.1 mg/g dry weight. It also contained chlorogenic acid 17.9 ± 1.3, caffeic acid 23.2 ± 0.8, quercetin 28.9 ± 1.5, luteolin 6.5 ± 0.4 and apigenin 5.3 ± 0.4 mg/g dry weight.
3.2. Effect of Stir-Fry Chicken with Green Curry Extract on Cells Viability
The inhibitory effect of the food extract on LPS-induced proinflammatory mediator expression was investigated; therefore the extract concentration must not be cytotoxic. There was no cytotoxicity on RAW264.7 cells incubated with the food extract at 55 - 220 mg/ml prior to co-incubation with LPS for 24 h (data not shown). Therefore the observed effect on the next experiment was due to the potency of the extract itself.
3.3. Stir-Fry Chicken with Green Curry Extract Suppresses LPS-Induced iNOS Expression
Nitric oxide is a well known reactive nitrogen species playing pathological role in several inflammatory associated diseases. Exposure of macrophages with LPS activates NO generation from L-arginine through iNOS activity in macrophage. In the present study, LPS-induced RAW- 264.7 cells significantly enhanced production of nitrite (Figure 1(a)). The LPS-induced NO production was significantly reduced by pretreatment with the extract at 55 - 220 mg/ml by 26% - 88% (Figure 1(a)) with the IC50 at 97 mg/ml. To confirm whether the reduction of NO levels by the extract was due to reduction of iNOS expression, iNOS mRNA and protein level were measured. The results indicated that reduction of NO level correlated with decrease iNOS protein and mRNA levels. The extracts inhibited iNOS protein expression at 40% - 78% with IC50 at 103 mg/ml and mRNA level at 11% - 25% (Figures 1(b) and (c)). Thus, this food extract attenuated LPS-induced NO concentration by suppressing iNOS mRNA level.
3.4. Stir-Fry Chicken with Green Curry Extract Attenuates LPS-Induced COX-2, TNF-α and IL-6 Expression
Moreover, COX-2 protein, a pro-inflammatory enzyme, is also known to be induced by LPS. Upon challenging with LPS, RAW264.7 cells highly expressed COX-2 protein and mRNA (Figures 2(a) and (b)). Pretreated cells with the extract suppressed COX-2 protein by 30% - 87% with IC50 at 90 mg/ml (Figure 2(a)) and mRNA expression by 14% - 61% with IC50 at 180 mg/ml (Figure 2(b)). To be expected, LPS also up regulated TNF-a and IL-6 expression by RAW 264.7 cells in the present study (Figures 3,4). The extract reduced TNF-a level by 50% - 76% with IC50 at 55 mg/ml (Figure 3(a)) and mRNA content by 11% - 47% (Figure 3(b)). Similar to TNF-a, the extract diminished IL-6 secretion by 83% - 99% with IC50 at 33 mg/ml (Figure 4(a)) and mRNA by 29% - 95% with IC50 at 68 mg/mL (Figure 4(b)). These
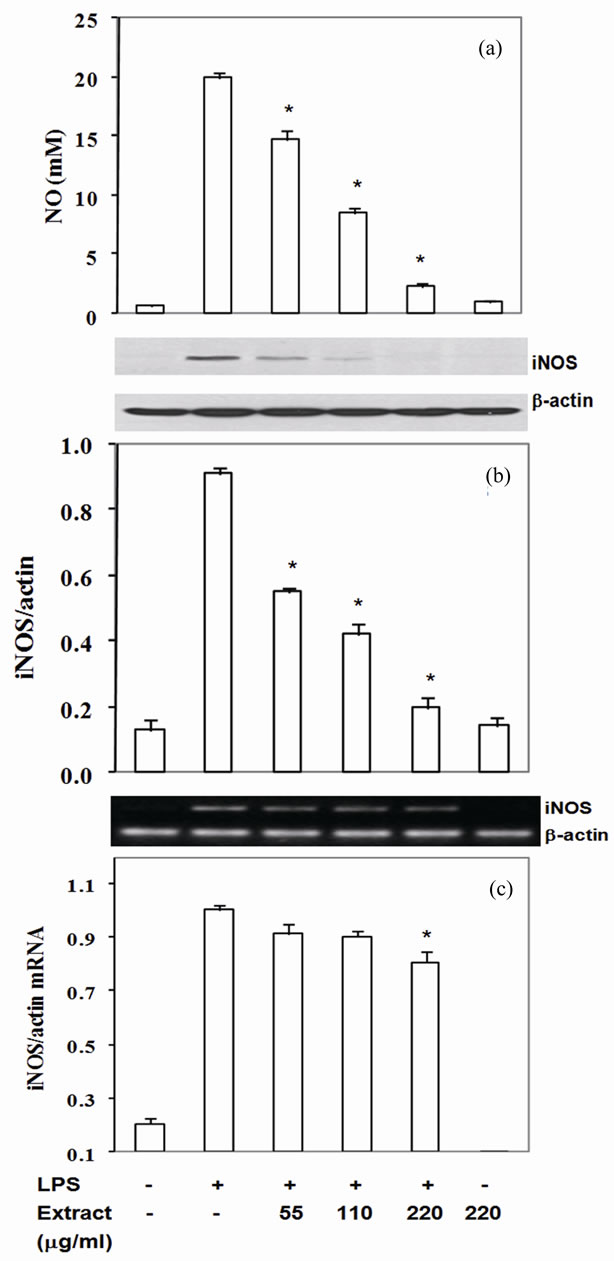
Figure 1. Stir-Fry chicken with green curry extract suppresses LPS-induced iNOS expression. Cells were treated as described in materials & methods. The culture medium was assayed for NO (a) iNOS protein was measured in cell lysate using β-actin as a control (b) iNOS mRNA was quantitated by RT-PCR using β-actin as an internal control (c) Values are expressed as mean ± SD in three independent experiments. *P < 0.05 compared with LPS treatment alone.
results indicated that the active compounds in this food extract decreased COX-2, TNF-a and IL-6 production by suppression of their cognate mRNA levels.
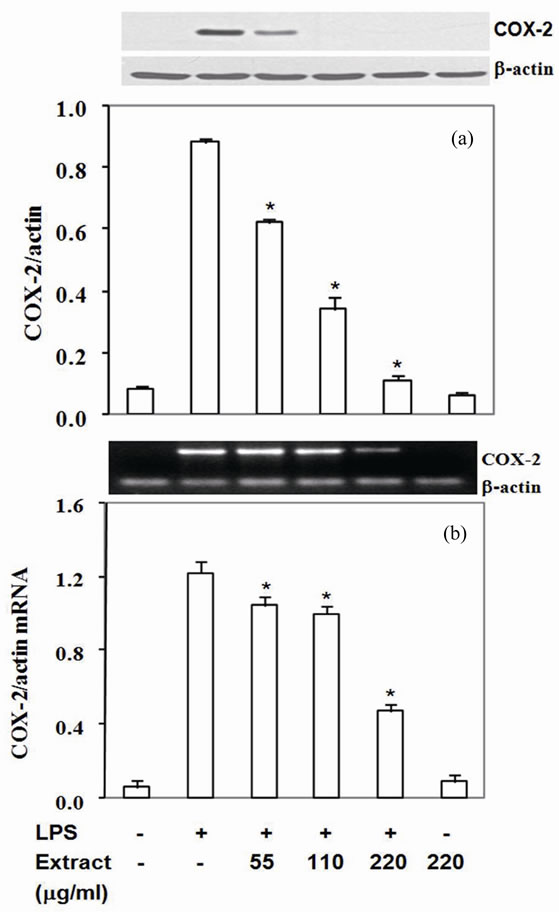
Figure 2. Stir-Fry chicken with green curry extract inhibits COX-2 expression. COX-2 protein was measured in cell lysate using β-actin as a control (a) COX-2 mRNA was quantitated by RT-PCR using β-actin as an internal control (b) Results are expressed as relative ratio of COX-2 to bactin protein and mRNA. Values are presented as mean ± SD in three independent experiments. *P < 0.05 relative to LPS treatment alone.
3.5. Inhibition by Stir-Fry Chicken with Green Curry Extract of LPS-Induced MAPK Signaling Pathways
Mitogen activated protein kinases (MAPKs) including Erk1/2, p38 and JNK are upstream signal transduction kinases which involve in expression of iNOS, COX-2, TNF-α and IL-6 induced by LPS. Therefore, to define whether the extract inhibited pro-inflammatory mediator expression via modulation of MAPKs phosphorylation, phosphorylated form of MAPKs in experimental condition was monitored as described in the methods. RAW- 264.7 cells exposed with LPS significantly activated Erk1/2, p38 and JNK phosphorylation (Figures 5(a)-(c)). Pretreatment the cells with the extract prior to co-incu-
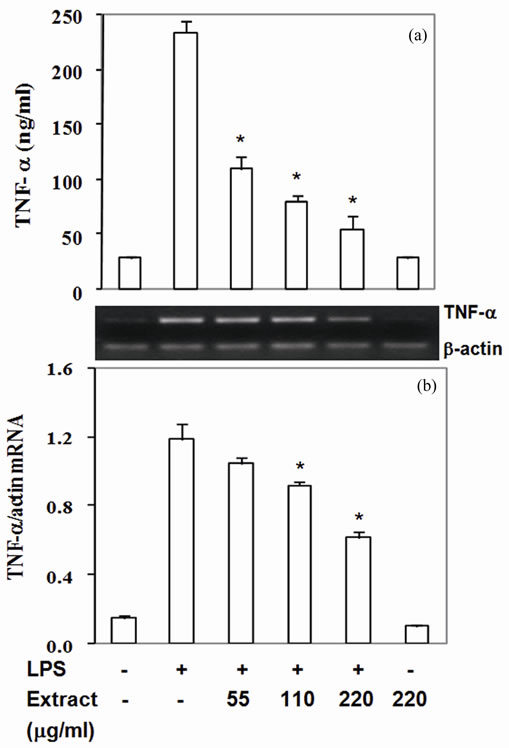
Figure 3. Stir-fry chicken with green curry extract attenuates LPS-enhanced TNF-a expression. Results are presented as mean ± SD in three independent experiments. *P < 0.05 relative to LPS treatment alone.
bate with LPS reduced phosphorylation of p38 (p-p38) by 11% - 77% with IC50 at 122 mg/ml (Figure 5(a)), pJNK by 22% - 73% with IC50 at 134 mg/ml (Figure 5(b)) and p-Erk1/2 22% - 79% with IC50 at 115 mg/ml (Figure 5(c)). Thus, the phytochemicals in this food extract suppressed LPS-induced pro-inflammatory mediators production partly through inactivated upstream signal transduction pathway MAPKs proteins.
NF-kB, a master transcription factor, is known to up regulate proinflammatory mediator expression after functional cells including macrophage expose to LPS. In resting state, NF-kB is sequestered by IkB-α in the cytoplasm. When macrophages expose to LPS, NF-kB signaling cascade is activated and results in IkB-α phosphorylation and degradation. NF-kB is released and translocates into the nucleus where it up regulates expression of pro-inflammatory mediators. Thus, we investigated the effect of food extract on phosphorylated IkBa. The food extract significantly decreased p-IkB-a level by 20% - 75% with IC50 at 128 mg/ml (Figure 6) without affecting the degradation of IkB-a (data not shown). These activities of the extract lasted up to 24 h. These results indicated that the stir-fried chicken with green
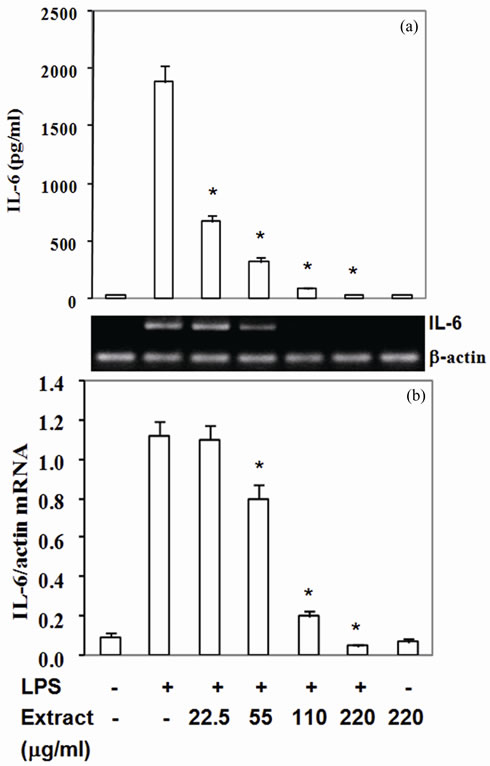
Figure 4. Stir-fry chicken with green curry extract suppresses LPS-enhanced IL-6 expression. Results are presented as mean ± SD in three independent experiments. *P < 0.05 relative to LPS treatment alone.
curry extract inhibited LPS-induced iNOS, COX-2, TNFa and IL-6 by RAW264.7 cells via partly blocking phosphorylation of IkB-a.
3.6. Stir-Fry Chicken with Green Curry Extract Decreases Intracellular ROS Level
Reactive oxygen species are produced via the activation of a membrane-bound NADPH oxidase after macrophage stimulation with LPS which leads to activation of MAPKs and NF-κB nuclear translocation and results in expression of pro-inflammatory mediator. High level of intracellular ROS is one of contributing factors of artherosclerosis development. Inactivation of MAPKs and IkB by this food extract may be due to its reactive scavenging capacity, therefore the LPS-induced ROS level cultured with extract was monitored. The intracellular ROS significantly enhanced in LPS-induced RAW264.7 cells (Figure 7) but decreased by 26% - 86% with IC50 at 103 mg/ml when treatment with the food extracts (Figure 7). Thus, inactivated phosphorylation of MAPKs and IkB by this food extract may be due to reactive scavenging capacity of the food extract to reduce ROS accumulation.
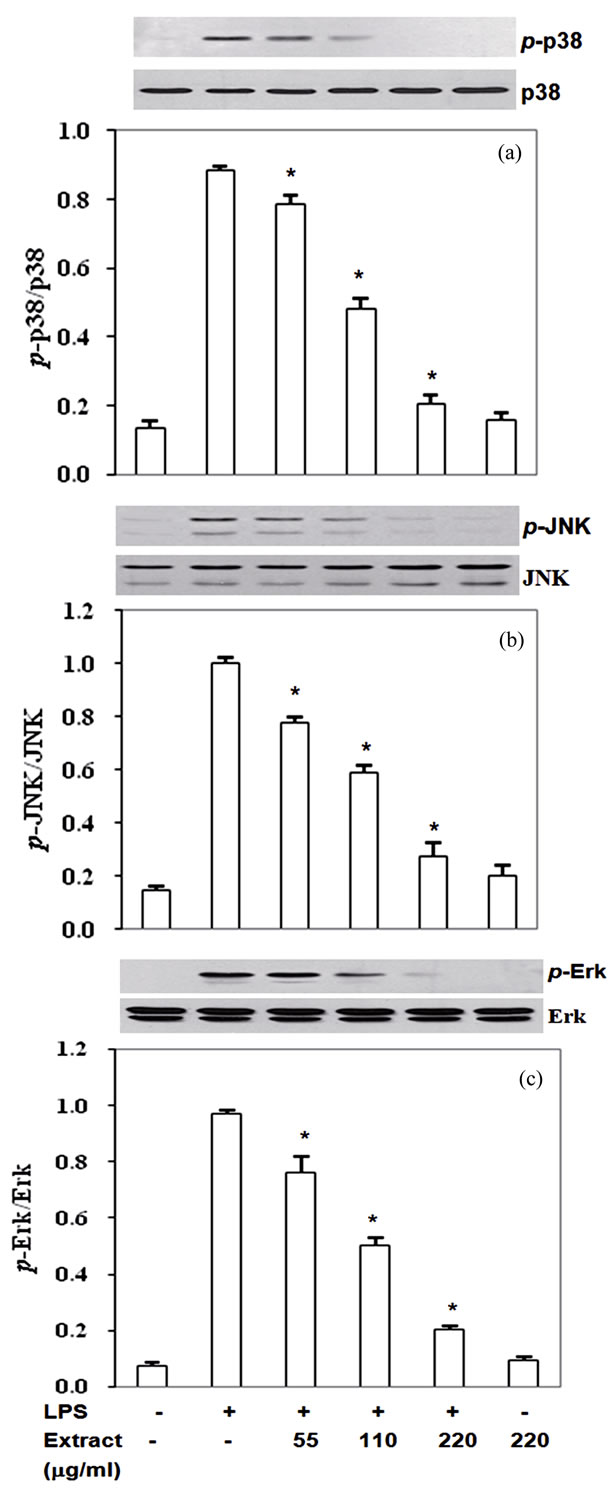
Figure 5. Stir-Fry chicken with green curry extract inactivates LPS-induced MAPK signaling pathway. Results are expressed as ratio of p-JNK, p-p38 and p-Erk1/2 relative to total forms of the proteins. Values are presented as mean ± SD in three independent experiments. *P < 0.05 relative to LPS treatment alone.
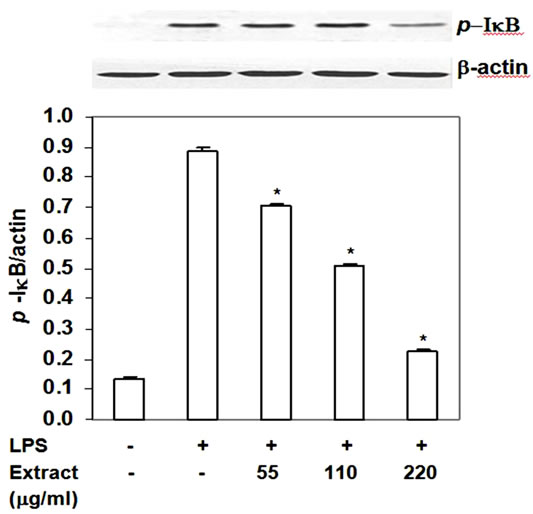
Figure 6. Inhibition of stir-fry chicken with green curry extract on LPS-induced phosphorylation of IkB-a. Results are expressed as ratio of p-IkB-a to b-actin. Values are presented as mean ± SD in three independent experiments. *P < 0.05 relative to LPS treatment alone.
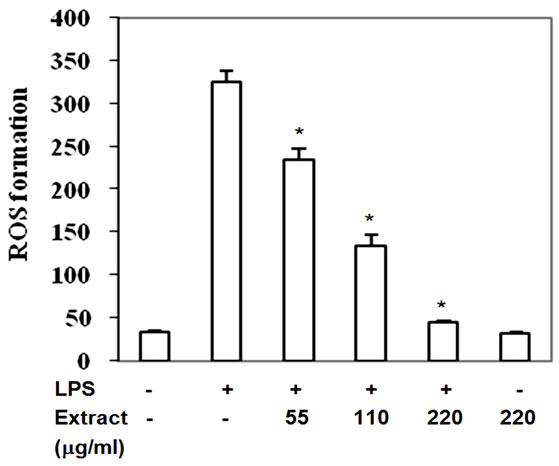
Figure 7. Stir-Fry chicken with green curry extract decreases LPS-induced intracellular ROS accumulation. Values are presented as mean ± SD in three independent experiments. *P < 0.05 relative to LPS treatment alone.
4. Discussion
Phytochemicals present in the ethanol extract of stir-fry chicken with green curry in the present study clearly exhibit anti-inflammatory properties by suppressing production of LPS-induced inflammatory mediators. The extract exerted these activities partly through inactivating the phosphorylation of IkB and MAPK Erk1/2, p38 and JNK. In addition the food extracts also exhibit high capacity to scavenge reactive oxygen species in LPS-induced murine macrophage cells.
Stir-fry chicken with green curry paste is one of the popular Thai cuisines. Its recipe consists of chicken meat, green curry paste, big egg plant fruits, pea egg plant fruits, sweet basil leaves, red chili pepper fruits and kaffer lime leaves. The green curry paste recipe comprised of 9 spices which has been shown to have a strong antiinflammatory activity in the same cell model (unpublished data). The green curry paste contributes only 6.5% of the whole dish composition; we postulated that it may partially exert the anti-inflammatory activity with the other ingredients of the whole dish not to be the main player.
The big egg plant and pea egg plant fruits contribute around 20% of the recipe. Egg plant fruits are rich in phenolic acids such as caffeic acid, chlorogenic acid (5- O-caffeoylquinic acid) and other caffeoyl esters. They are potent free radical scavengers found in plant tissues [12]. The present study found chlorogenic acid at 17.9 ± 1.3 mg/g and caffeic acid at 23.2 ± 0.8 mg/g in the stirfried chicken with green curry extract. Chlorogenic acid has been demonstrated to exhibit anti-inflammatory and antioxidant properties in vitro and in vivo [13-14]. Chlorogenic acid-rich coffee has been recently demonstrated to induce the Nrf2 (nuclear factor-erythroid 2-related factor-2)/antioxidant-response element (ARE) detoxifying pathway in vitro and in vivo [13]. In addition, humans consuming instant coffee at 800 ml/day for 5 days decreased oxidative stress markers 8-isoprostaglandin F2α by 15.3% and 3-nitrotyrosine by 16.1% in urine [14]. Caffeic acid phenethyl ester (CAPE) has been shown to exhibit anti-inflammatory activity in vitro and in vivo [15]. A previous report indicated that CAPE inhibited LPS-induced NO and prostaglandin E2 (PGE2) production and suppressed iNOS and COX-2 via blocking NFkB translocation and DNA-binding in LPS-stimulated RAW 264.7 cells. This effect was mediated through the inhibition of IkB degradation and phosphorylation of p38 and Erk1/2 in part by decreased intracellular ROS. The same study also found that CAPE could rescue C57BL/6 mice from lethal LPS-induced septic shock while their serum TNF-a and IL-1b also decreased [15]. Antioxidant activity of caffeic acid was also showed in t-butyl hydroperoxide-induced oxidative stress in U937 human monocytic cells by blocking glutathione depletion and inhibiting lipid peroxidation [16]. These data supported that chlorogenic and caffeic acids in this food extract may play a role to attenuate LPS-induced enhanced TNFa, COX-2 and iNOS expression by blocking phosphorylation of p38 and Erk1/2 and IkB-a resulting from reduction of ROS in LPS-activated RAW264.7 cells in the present study.
Apart from big egg plant, the recipe also contains 4% of pea egg plant (S. torvum) fruits and sweet basil O. basilicum. The ethanol extracts from pea egg plant and sweet basil have been previously shown to suppress LPSinduced NO and TNF-a produced by RAW264.7 cells [17]. Treatment with S. torvum (100 mg/kg and 300 mg/kg) significantly increased the antioxidant defense enzyme levels of superoxide dismutase (SOD) and catalase (CAT) in kidney of doxorubicin-induced rats [18]. Thus, both vegetables may partly contribute to reduce NO, TNF-a and also play a role on reduction of ROS in this food extract.
Regarding the flavonoids in the extract, quercetin is the predominant flavonoid found. A previous study reported that pretreatment with quercetin significantly inhibited NO production, and iNOS, TNF-a, IL-1b, and IL-6 expression in the same cell model by inhibiting phosphorylation of Erk1/2 and p-38 MAPK via stabilizing the NF-kB/IkB complex [19]. Recently, the antioxidant capacity of quercetin was also examined comparing with curcumin on ROS and NO production in LPSstimulated human THP-1 monocytic leukemia cells [20]. Quercetin had a higher reduction potential and higher total antioxidant capacity compared with curcumin and also had a better efficiency to decrease LPS-induced ROS and NO production. Thus, either quercetin itself or synergistically acted with other bioactive compounds present in the extract to suppress proinflammatory genes expression via inactivation of IkB as shown in the present results.
Although the stir-fry chicken with green curry extract contains small content of b-carotene, lutein, luteolin and apigenin, such compounds may act together with other compounds in the extract to inhibit anti-inflammatory gene expression. A previous study demonstrated that 10 mM of pure b-carotene inhibited proinflammatory genes expression in LPS-induced RAW264.7 cells by suppressing redox-based NF-κB activation [21]. Another study found that lutein suppressed LPSand H2O2-induced phosphatidylinositol 3-kinase activity, NF-kB– inducing kinase, and Akt phosphorylation, which are upstream of IKK activation [22]. A previous study also showed that pretreatment with luteolin inhibited LPSstimulated TNF-a and IL-6 secretions by attenuating phosphorylation of protein kinase B (Akt) and subsequently inactivation of NF-kB in RAW264.7 cells [23]. Pretreatment of RAW264.7 macrophage-like cells with luteolin dose-dependently inhibited LPS-induced radical formation and expression of COX-2 protein and PGE2 formation [24]. Apigenin also previously showed to suppress LPS-induced expression of iNOS and COX-2 by blocking activation of NF-kB in RAW264.7 cells [25]. These data support the possible role of b-carotene, lutein, luteolin and apigenin to attenuate the proinflammatory mediator expression and ROS accumulation by inactivation of JNK and IkB in LPS-activated RAW264.7 cells in the present study.
5. Conclusions
The observed activities in this study suggest the mode of actions of active compounds derived from the whole diet to prevent inflammation which may help to use this food to reduce clinical complication causing from inflammatory mediators in some inflammatory associated disorders. However, these in vitro doses and activities cannot apply directly to those of in vivo. Because dietary phytochemicals in plants have to be metabolized by phase I and II detoxification system during absorption prior to transport through blood circulation and finally deliver to target tissues to exert their functions. Further studies have to be explored to reveal the potential effects of stirfry chicken with green curry to alleviate the pathological complication associated with inflammatory response in vivo.
6. Acknowledgements
This study was supported by Mahidol University. The authors thank Dr. Harold Furr for his helpful commentary during manuscript preparation. They also appreciate the assistance of Dr. Sitima Jittinandana and Dr. Aikkarach Kettawan with preparation of the curry paste.
REFERENCES
- B. B. Aggarwal, “Nuclear Factor-[Kappa] B: The Enemy within,” Cancer Cell, Vol. 6, No. 3, 2004, pp. 203-208. doi:10.1016/j.ccr.2004.09.003
- M. Guha and N. Mackman, “LPS Induction of Gene Expression in Human Monocytes,” Cellular Signalling, Vol. 13, No. 2, 2001, pp. 85-94. doi: 10.1016/S0898-6568(00)00149-2
- S. I. Grivennikov and M. Karin, “Inflammatory Cytokines in Cancer: Tumour Necrosis Factor and Interleukin 6 Take the Stage,” Annals of the Rheumatic Diseases, Vol. 70, 2011, pp.104-108. doi:10.1136/ard.2010.140145
- A. Scalbert, C. Andres-Lacueva, M. Arita, P. Kroon, C. Manach and M. Urpi-Sarda, “Databases on food phytochemicals and their health promoting effects,” Journal of Agricultural and Food Chemistry, Vol. 59, No. 9, 2011, pp. 4331-4348. doi:10.1021/jf200591d
- B. B. Aggarwal, M. E. Van Kuiken, L. H. Iyer, K. B. Harikumar and B. Sung, “Molecular Targets of Nutraceuticals Derived from Dietary Spices: Potential Role in Suppression of Inflammation and Tumorigenesis,” Experimental Biology and Medicine, Vol. 234, No. 8, 2009, pp. 825-849. doi:10.1158/1078-0432.CCR-08-0149
- H. Wu-Yang, C. Yi-Zhong and Z. Yanbo, “Natural Phenolic Compounds from Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention,” Nutrition & Cancer, Vol. 62, No. 1, 2011, pp. 1-20. doi:10.1080/01635580903191585
- H. M. Merken and G. R. Beecher, “Liquid ChromaTographic Method for the Separation and Quantification of Prominent Flavonoid Aglycones,” Journal of Chromatography A, Vol. 897, No.1-2, 2000, pp.177-184. doi:10.1016/S0021-9673(00)00826-8
- V. Vichai and K. Kirtikara, “Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening,” Nature Protocol, Vol. 1, No. 3, 2006, pp. 1112-1116. doi: 10.1038/nprot.2006.179
- L. C. Green, D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok and S. R. Tannenbaum, “Analysis of Nitrate, Nitrite, and [15N] Nitrate in Biological Fluids,” Analytical Biochemistry, Vol. 126, 1982, pp. 131-138. doi:10.1016/0003-2697(82)90118-X
- S. Tuntipopipat, C. Muangnoi, P. Chingsuwanrote, M. Parengam, P. Chantravisut and S. Charoenkiatkul, “AntiInflammatory Activities of Red Curry Paste Extract on Lipopolysaccharide-Activated Murine Macrophage Cell Line,” Nutrition, Vol. 27, No. 4, 2011, pp. 479-487. doi:10.1016/j.nut.2010.04.009
- L. F. de Souza, F. Barreto, E. G. da Silva, M. E. Andrades, E. L. M. Guimares and G. A. Behr, “Regulation of LPS Stimulated ROS Production in Peritoneal Macrophages from Alloxan-Induced Diabetic Rats: Involvement of High Glucose and PPAR [Gamma],” Life Science, Vol. 81, 2007, pp. 153-159. doi:10.1016/j.lfs.2007.04.035
- N. Nakatani, S.-I. Kayano, H. Kikuzaki, K. Sumino, K. Katagiri and T. Mitani, “Identification, Quantitative deTermination, and Antioxidative Activities of Chlorogenic Acid Isomers in Prune (Prunus domestica L.),” Journal of Agricultural and Food Chemistry, Vol. 48, No. 11, 2000, pp. 5512-5516. doi:10.1021/jf000422s
- U. Boettler, N. Volz, G. Pahlke, N. Teller, C. Kotyczka and V. Somoza, “Coffees Rich in Chlorogenic Acid or NMethylpyridinium Induce Chemopreventive Phase II-Enzymes via the Nrf2/ARE Pathway in Vitro and in Vivo,” Molecular Nutrition & Food Research, Vol. 55, No. 5, 2011, pp. 798-802. doi:10.1002/mnfr.201100115
- C. Hoelzl, S. Knasmüller, K.-H. Wagner, L. Elbling, W. Huber and N. Kager, “Instant Coffee with High Chlorogenic Acid Levels Protects Humans against Oxidative Damage of Macromolecules,” Molecular Nutrition & Food Research, Vol. 54, No. 12, 2010, pp. 1722-1733. doi:10.1002/mnfr.201000048
- W.-K. Jung, I. Choi, D.-Y. Lee, S.-S. Yea, Y.-H. Choi and M.-M. Kim, “Caffeic Acid Phenethyl Ester Protects Mice from Lethal Endotoxin Shock and Inhibits Lipopoly-Saccharide-Induced Cyclooxygenase-2 and Inducible Nitric Oxide Synthase Expression in RAW 264.7 Macrophages via the p38/ERK and NF-[kappa]B Pathways,” The International Journal of Biochemistry & Cell Biology, Vol. 40, No. 1, 2008, pp. 2572-2582. doi:10.1016/j.biocel.2008.05.005
- M. Nardini, P. Pisu, V. Gentili, F. Natella, M. Di and F. Piccolella, “Effect of Caffeic Acid on Tert-Butyl Hydroper-Oxide-Induced Oxidative Stress in U937,” Free Radical Biology and Medicine, Vol. 25, No. 9, 1998, pp. 1098-1105. doi:10.1016/S0891-5849(98)00180-4
- S. Tuntipopipat, C. Muangnoi and M. L. Failla. “AntiInflammatory Activities of Extracts of Thai Spices and Herbs with Lipopolysaccharide-Activated RAW 264.7 Murine Macrophages,” Journal of Medicinal Food, Vol. 12, No.6, 2009, pp. 1213-1220. doi:10.1089/jmf.2009.1118
- M. Mohan, S. Kamble, P. Gadhi and S. Kasture, “Protective Effect of Solanum Torvum on Doxorubicin-Induced Nephrotoxicity in Rats,” Food and Chemical Toxicology, Vol.48, No.1, 2010, pp. 436-440. doi:10.1016/j.fct.2009.10.042
- S.-Y. Cho, S.-J. Park, M.-J. Kwon, T.-S. Jeong, S.-H. Bok and W.-Y. Choi, “Quercetin Suppresses Proinflammatory Cytokines Production through MAP Kinases and NF-kB Pathway in Lipopolysaccharide-Stimulated Macrophage,” Molecular and Cellular Biochemistry, Vol. 243, No. 1, 2003, pp. 153-160. doi:10.1023/A:1021624520740
- M. Zhang, S. G. Swarts, L. Yin, C. Liu, Y. Tian, Y. Cao, et al., “Antioxidant Properties of Quercetin,” Advances in Experimental Medicine and Biology, Vol 701, Part 10, 2011, pp. 283-289. doi: 10.1007/978-1-4419-7756-4
- S. K. Bai, S. J. Lee, H. J. Na, K. S. Ha, J. A. Han, H. Lee, Y. G. Kwon, C. K. Chung and Y. M. Kim, “Beta-Carotene Inhibits Inflammatory Gene Expression in Lipopolysaccha-Ride-Stimulated Macrophages by Suppressing Redox-Based NF-kB Activation,” Experimental and Molecular Medicine, Vol. 37, No. 4, 2005, pp. 323-334
- J.-H. Kim, H.-J. Na, C.-K. Kim, J.-Y. Kim, K.-S. Ha and H. Lee, “The Non-Provitamin A Carotenoid, Lutein, Inhib-Itsnf-[Kappa] B-Dependent Gene Expression through Redox-Based Regulation of the Phosphatidylinositol 3- Kinase/PTEN/Akt and NF-[Kappa]B-Inducing Kinase Pathways: Role of H2O2 in NF-[Kappa]B Activation,” Free Radical Biology and Medicine, Vol. 45, No. 6, 2008, pp. 885-896. doi:10.1016/j.freeradbiomed.2008.06.019
- A. Xagorari, A. Papapetropoulos, A. Mauromatis, M. Economou, T. Fotsis and C. Roussos, “Luteolin Inhibits an Endotoxin-Stimulated Phosphorylation Cascade and Proinflammatory Cytokine Production in Macrophages,” Journal of Pharmacology and Experimental Therapeutics, Vol. 296, No. 1, 2001, pp. 181-187.
- G. K. Harris, Y. Qian, S. S. Leonard, D. C. Sbarra and X. Shi, “Luteolin and Chrysin Differentially Inhibit Cyclooxy-Genase-2 Expression and Scavenge Reactive Oxygen Species but Similarly Inhibit Prostaglandin-E2 Formation in RAW 264.7 Cells,” The Journal of Nutrition, Vol. 136, No. 6, 2006, pp. 1517-1521.
- Y.-C. Liang, Y.-T. Huang, S.-H. Tsai, D. Tsai, S.-Y. Lin-Shiau, C.-F. Chen and J.-K. Lin, “Suppression of inDucible Cyclooxygenase and Inducible Nitric Oxide Synthase by Apigenin and Related Flavonoids in Mouse Macrophages,” Carcinogenesis, Vol. 20, No. 10, 1999, pp. 1945-1952. doi: 10.1093/carcin/20.10.1945

