Pharmacology & Pharmacy
Vol.4 No.1(2013), Article ID:27511,7 pages DOI:10.4236/pp.2013.41013
Vernonia amygdalina-Induced Growth Arrest and Apoptosis of Breast Cancer (MCF-7) Cells
![]()
1Cellomics and Toxicogenomics Research Laboratory, NIH-RCMI Center for Environmental Health, Jackson State University, Jackson, USA; 2Laboratory of Cellular Signalling, Phytoceuticals, Cancer Prevention and Therapies, College of Science, Engineering and Technology, Jackson State University, Jackson, USA.
Email: *paul.b.tchounwou@jsums.edu
Received November 6th, 2012; revised December 9th, 2012; accepted January 12th, 2013
Keywords: Vernonia amygdalina; MCF-7 Cells; Apoptosis; Necrosis; Cellometer Imaging
ABSTRACT
Breast cancer is the second leading cause of cancer-related deaths of women in the United States. Fortunately, the mortality rate from breast cancer has decreased in recent years due to an increased emphasis on early detection and more effective treatments. Although great advancements have been made in the treatment and control of cancer progression, significant deficiencies and room for improvement remain. The central objective of this research was to further determine the in vitro mechanisms of Vernonia amygdalina (VA) leaf extracts as an anticancer candidate for the treatment of breast cancer. To achieve our objective, MCF-7 cells were treated with different concentrations of VA for 24 hand 48 h. Cell viability, live and dead cells were determined by the means of trypan blue exclusion test. Live and dead cells were further evaluated by propidium iodine (PI) assay using the Cellometer Vision. Cell apoptosis was measured by flow cytometry assessment using annexin V/PI kit. Data obtained from the trypan blue test demonstrated that VA treatment reduces cell viability in a concentrationand time-dependent manner. Result of the PI assay showed a gradual increase in the population of necrotic cells (fluorescence positive cells) in VA-treated cells compared to the control cells (fluorescence negative cells). Treatment of these cancer cells (MCF-7) for 48 h at concentrations ranging from 250 μg/mL to 1000 μg/mL caused early signs of apoptosis resulting from phosphatidylserine externalization as judged by annexin V assay. We observed a strong concentration-response relationship with regard to VA exposure and annexin V/PI positive cells. In summary, our finding demonstrates that VA-induced cytotoxicity and apoptosis in MCF-7 cells involve phosphatidylserine externalization accompanied by secondary necrotic cell death. With previous findings in our laboratory, the data generated in the present study confirms that VA is a valuable botanical therapeutic agent for the treatment of breast cancer.
1. Introduction
Breast cancer is the second leading cause of cancer-related deaths of women in the United States. Several strategies have been developed over the past decade to improve the treatment outcome of patients with breast cancer. However, many conventional drugs are sometimes inadequate and can have serious adverse effects. It is therefore necessary to search for alternative drugs for the treatment of breast cancer and to replace currently used drugs of doubtful efficacy and safety. Hence, novel natural products or new chemotherapeutic agents are needed to improve cancer treatment outcome. Vernonia amygdalina (family of asteraceae) is a valuable medicinal plant that is widesprea d in East and West Africa [1, 2]. It is known as bitter leaf and may be used as active anticancer agent [3], anti-bacteria, anti-malaria, and antiparasites [4]. This plant contains complex active components that are pharmacologically useful. The roots and the leaves are used in ethnomedicine to treat fever, hiccups, kidney problems, and stomach discomfort [5,6]. The stem and root divested of the bark are used as chewsticks in many West African countries including Cameroon, Ghana, Nigeria, and neighboring countries. Cameroonians usually grow Vernonia amygdalina (VA) primary for therapeutic uses against stomach discomfort, vomiting, diarrhea, and intestinal illnesses. Interestingly, these ailments may stop in less than few hours and/or to a day after oral intake. These vegetables are locally available, affordable, and easily accessible to either the consumers and/or patients. It has a bitter taste and patients seem to believe that the bitterness of VA neutralizes or kills the disease agent in their body system. Iwalokun reported that VA leaf extract enhanced the prophylactic and therapeutic efficacy of chloroquine against Plasmodium berghei malaria in mice [7]. Pharmacological studies have shown that the leaf extracts of this plant have both hypoglycaemic and hypolipidaemic properties in experimental animals and so could be used in managing diabetes mellitus [8]. A previous study by Izevbigie has demonstrated that low concentrations (µg/mL) of watersoluble leaf extracts potently retarded the proliferative activities of estrogen receptor positive (ER+) human breast cancer (MCF-7) cells in vitro in a dose-dependent manner [3]. Other studies have shown that VA-treatment modulates phase 1 and phase 2 gene expression in MCF- 7 cells in a dose and time-dependent fashion [9].
Breast cancer continues to represent the largest cause of mortality in the world especially among women. Recent reports from our laboratory using the MTT and comet assays have demonstrated that in vitro VA treatment reduces cellular viability and induces minimal DNA damage in tumor cells [10]. Although published studies indicate that VA has medicinal properties effective against many diseases, the molecular mechanisms under which this herbal medicine exerts its therapeutic effect in cancer cells remain largely unknown. Therefore, the central objective of this research was to use MCF-7 cells as test model to determine the mechanisms of action of VA as an anticancer candidate for the treatment of breast cancer.
2. Materials and Methods
2.1. Chemicals and Media
Growth medium RPMI 1640 containing 1 mmol/L Lglutamine was purchased from Gibco BRL products (Grand Island, NY). Fetal bovine serum (FBS), phosphate buffered saline (PBS), and MTT assay kits were obtained from Sigma Chemical Company (St. Louis, MO). Annexin V fluorescein isothiocyanale (FITC) kit (annexin V FITC, binding buffer and propidium iodide [PI]) kit were obtained from BD Biosciences (Pharmingen, Becton Dickinson Co., San Diego, CA, USA).
2.2. Vernonia amygdalina Preparation
Vernonia amygdalina (VA) leaves, collected in Benin City, Nigeria, were rinsed with cold, distilled water. The leaves were soaked in cold water (1:1 w/v) overnight at 4˚C before being crushed by a gentle means to a mixture. The mixture was then filtered through clean white gauze to remove particulate matter before further filtration through a 0.45 μm filtration unit for sterilization. The resulting solution was lyophilized (5 g) and stored at −20˚C. This method of VA preparation was previously described by Izevbigie [3].
2.3. Cell Culture
Human breast adenocarcinoma (MCF-7) cells, purchased from the American Type Culture Collection-ATCC (Manassas, VA), were thawed by gentle agitation of their containers (vials) for 2 min in a water bath at 37˚C. After thawing, the content of each vial was transferred to a 75 cm2 tissue culture flask, diluted with RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin, and incubated for 2 to 3 days at 37˚C in a 5% CO2 incubator. The growth medium was changed twice a week. Cells grown to 75% - 85% confluence were washed with phosphate buffer saline (PBS), trypsinized with 3 mL of 0.25% (v) trypsin—0.0.3%/v) EDTA, diluted with fresh medium, and counted using a hemacytometer.
2.4. Cell Treatment and Biochemical Test for Cell Viability Using Trypan Blue Dye
To 900 μL aliquots in three replicates of the cell suspendsion (5 × 105 cells/mL) seeded to 12-well polystyrene tissue culture plates, 100 μL aliquots of stock solutions of VA were added to each well using distilled water as solvent to make-up final concentrations of 250, 500, and 1000 μg/mL of VA, respectively. Control cells received 100 μL of distilled water. Cells were placed in a humidified 5% CO2 incubator at 37˚C for 24 and 48 h, respectively. After incubation, the cell viability was assessed by the trypan blue exclusion test (Life Technologies) using the cellometer vision. Briefly, 10 μL of trypan blue (dye) was added to 100 μL of cell suspension taken out from each sample. Samples were gently mixed and 20 μL of cell suspension was loaded into the Cellometer counting chamber. The Cellometer counting chamber was placed into the Cellometer Vision and both cell concentration and viability were determined using the Cellometer Vision software.
2.5. Biochemical Test for Apoptosis and Necrosis by Flow Cytometry
Annexin V-FITC/PI staining method was used in the present study to identify and quantify apoptotic and/or necrotic death cell. Briefly, 1 × 106 cells/mL was treated with different concentrations of VA for 48 h. Control cells were also made without VA. After treatment, cells were washed in PBS, re-suspended in binding buffer (10 mm Hepes/NaOH pH 7 × 4, 140 mm NaCl, 2 × 5 mm CaCl2), and stained with FITC-conjugated annexin V (Pharmingen, Becton Dickinson Co., San Diego, CA, USA). Then, cells were incubated for 15 min in the dark at room temperature and washed with binding buffer. FITC/PI fluorescence intensity was measured by flow cytometry to differentiate between viable (annexin Vnegative and PI-negative), early apoptotic (annexin Vpositive, PI-negative), and late apoptotic (annexin Vpositive and PI-positive) cells. The extent of apoptosis was quantified as percentage of annexin V-positive cells.
2.6. Biochemical Test for Necrosis by Cellometer Imaging
To confirm that VA-induced cytotoxicity in MCF-7 cells is mediated primarily by necrosis, we also measured cell viability by propidium iodine (PI) staining using the Cellometer Imaging system. Briefly, 1 × 106 cells/mL in culture media were harvested from each well of 12 well plates and washed twice with 5 mL of culture media. Washed cells were re-suspended in 1 mL of culture media. Five (5) μL of PI was added to 100 μL of cell suspension taken out from each sample. Samples were gently mixed and incubated for 20 min at room temperature in dark. Samples were mixed again and 20 μL of each sample was loaded into the Cellometer counting chamber. The Cellometer counting chamber was placed into the Cellometer Vision and both cell concentration and viability were determined with the Cellometer Vision software. This new technique that helps to identify necrotic cell death from live cells was recently described in our laboratory [11].
2.7. Statistical Analysis
Experiments were performed in triplicates. Data were presented as means ± SDs. Where appropriate, one-way ANOVA or Student paired t-test was performed using SAS Software available in the Biostatistics Core Laboratory at Jackson State University. P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Cell Viability
Cells were treated with increasing concentrations of VA for 24 h and 48 h, and viable cells were monitored by trypan blue exclusion test using the Cellometer Vision. Treatment of MCF-7 cells with 250, 500, and 1000 μg/mL VA for 24 h decreased cell viability to approximately 18%, 19%, and 28%, respectively (Figure 1). Exposing MCF-7 cells to 250, 500, and 1000 μg/mL VA for 48 h reduced viable cell counts to 19%, 30%, and 49%, respectively (Figure 1).
The viability measurement was directly determined and provided by the Cellometer Vision. Overall, data obtained from the trypan blue exclusion test demonstrated that VA treatment reduced cell viability in a concentrationand time-dependent manner
3.2. Induction of Apoptosis and Necrosis
Annexin V FITC/PI assay helps to distinguish between

Figure 1. Cytotoxic effect of VA extract to human breast adenocarcinoma (MCF-7) cells. MCF-7 cells were cultured with increasing concentrations of VA extract (250 μg/mL, 500 μg/mL, and 1000 μg/mL) for 24 and 48 h as indicated in the Materials and Methods. Cell viability was determined based on the trypan blue exclusion test. Live cells and dead cells were determined using the cellometer vision software.
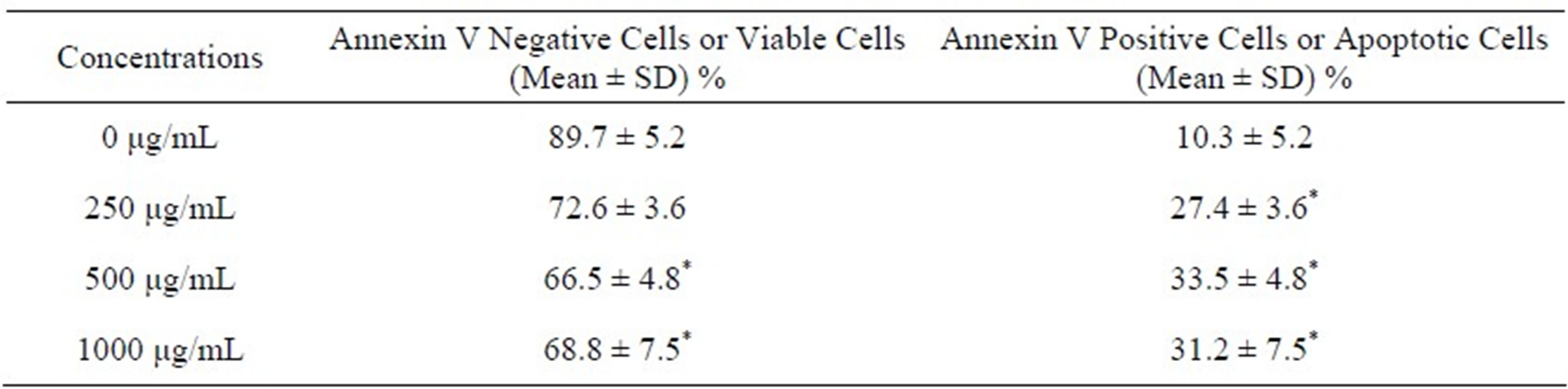
apoptotic and necrotic cell death. Hence, to determine whether VA-induced cytotoxicity in MCF-7 cells is mediated by apoptosis and/or necrosis, we treated the cells for 48 h and measured the annexin V FITC/PI staining responses by flow cytometry analysis. We observed a strong concentration-response relationship with regard to VA exposure and annexin V positive cells (apoptotic and necrotic cells) (Figure 2). Viable cells were negative for both PI and annexin V (Figure 2, quadrant 1); early apoptotic cells were positive for annexin V and negative for PI (Figure 2, quadrant 2); whereas late apoptotic or necrotic cells displayed both high annexin V and PI labeling (Figure 2, quadrant 3); non-viable cells undergoing necrosis were positive for PI and negative for annexin V (Figure 2, quadrant 4). The percentages of annexin V and PI positive cells were (10.3 ± 5.2)%, (27.4 ± 3.6)%, (33.5 ± 4.8)%, and (31.2 ± 7.5)% in 0, 250, 500, and 1000 μg/mL VA, respectively (Table 1).
Cell death by necrosis was further confirmed by the result of propidium iodide assay. The data generated from this assay indicated that VA exposure significantly (P < 0.05) increased the proportion of fluorescence positive cells (necrotic death cells) in a concentrationand time-dependent fashion compared to the control (Figures 3 and 4).
4. Discussion
In the present study, we evaluated the cytotoxic efficacy of Vernonia amygdalina (VA) extracts to MCF-7 cells by the means of trypan blue exclusion test. Our results demonstrated that VA extract significantly (P < 0.05) reduces the viability of MCF-7 cells in concentrationand time-dependent manner (Figure 1). These results were consistent with recent finding in our laboratory
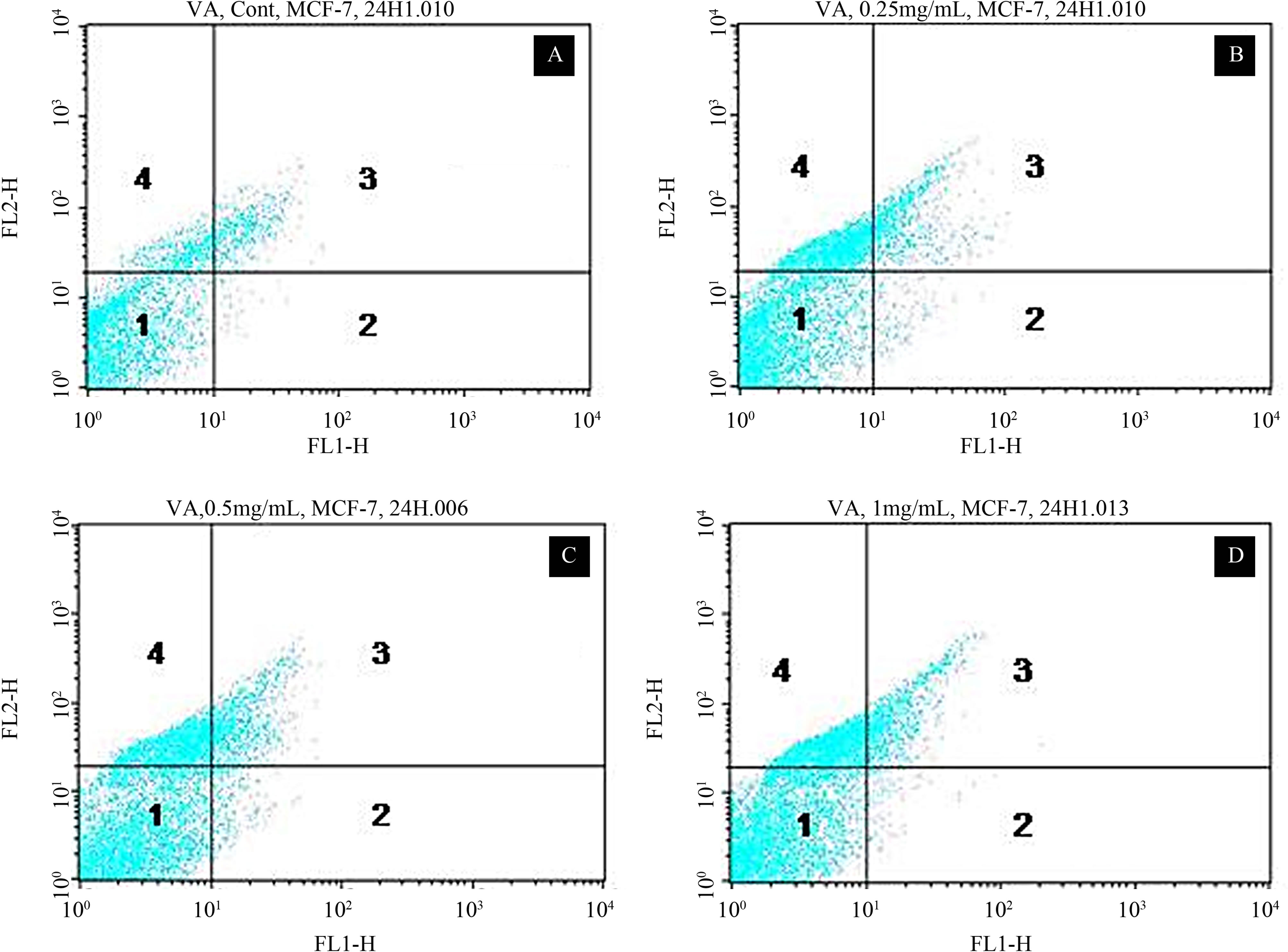
Figure 2. Representative dot plots showing the apoptotic potential of VA in MCF-7 cells upon 48 h of exposure. A = Control Cell (Untreated), B = 250 μg/mL VA, C = 500 μg/mL VA, D = 1000 μg/mL VA, 1 = Live cells or annexin V and PI negative cells, 2 = Early apoptotic cells or annexin V positive cells, 3 = Late apoptotic and necrotic cells or annexin V and PI positive cells, 4 = Necrotic cells or PI positive cells.
Table 1. Summary data of annexin V/PI assay obtained from the flow cytometry. Human adenocarcinoma (MCF-7) cells were cultured in the absence or presence of VA for 48 h as indicated in the Materials and Methods. Values are shown as means ± SDs of 3 replicates per experiment. *P < 0.05 versus compared with control group.
showing that VA exerts antiproliferative activity by inhibiting tumor cell growth and inducing minimal DNA damage in MCF-7 cells [10]. These data led us to believe that the leaves of VA plant consumed in many African countries may be relatively toxic if consumed in large quantities, which is very unlikely. For example, the consumption of VA at 0.04 - 0.08 g/mL per body weight did not cause any cytotoxic effects in 5 - 6 weeks of male abino rats [12]. To extrapolate, since the average body weight is about 70 kg, it will require the consumption of 0.028 - 0.056 kg (28 - 56 g) VA per adult person. Based on these data, we believe that the incorporation of VA in diet may help prevent or reduce the risk of breast cancer and other diseases considering the nutritional and therapeutic applications of the plant by almost all African populations. In Cameroon for instance, the leaves of VA
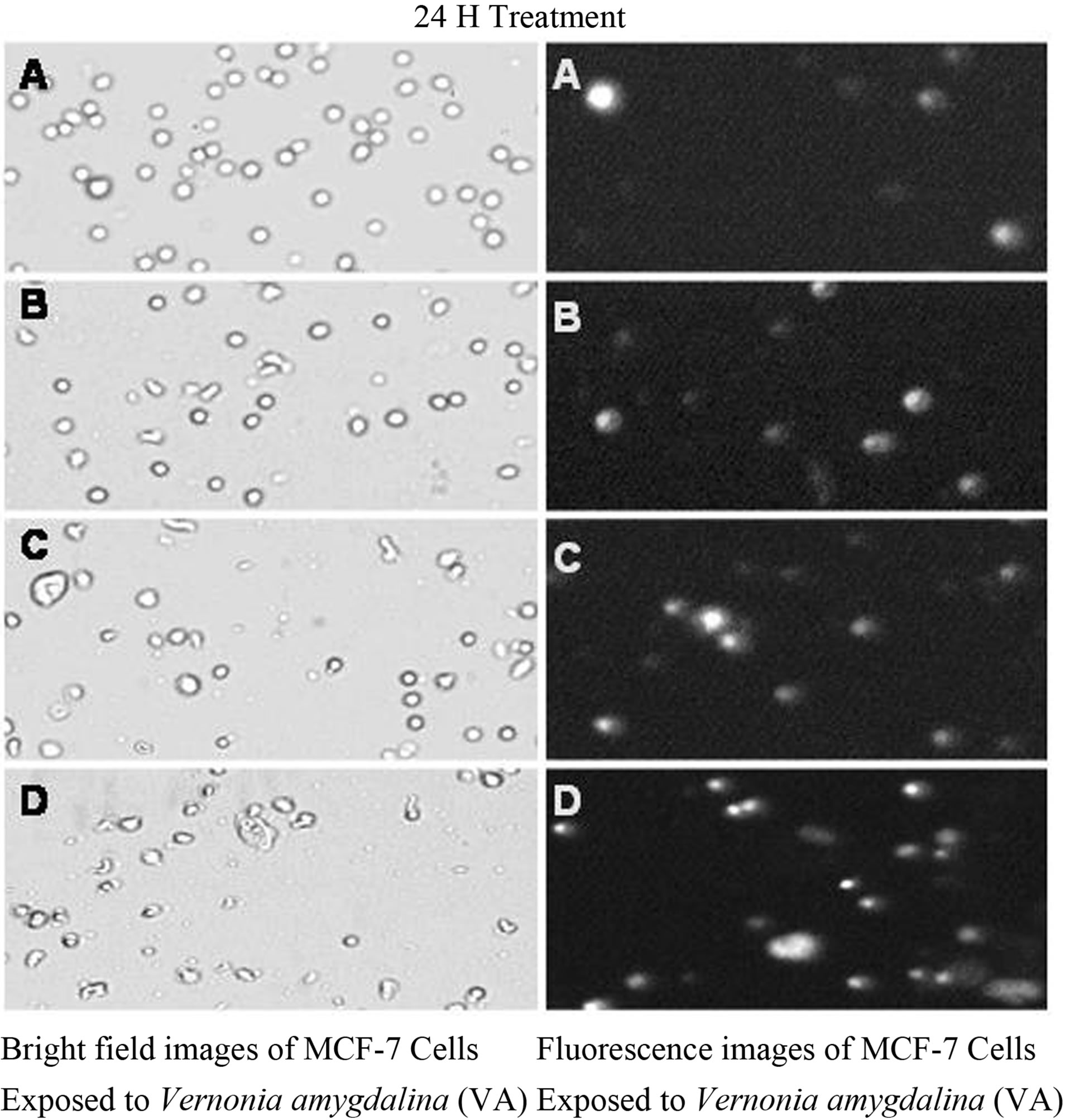
Figure 3. Bright field images (left) and fluorescent images (right) of MCF-7 cells exposed to VA for 24 h. MCF-7 cells were exposed to different concentrations of VA as described in the Materials and Methods.
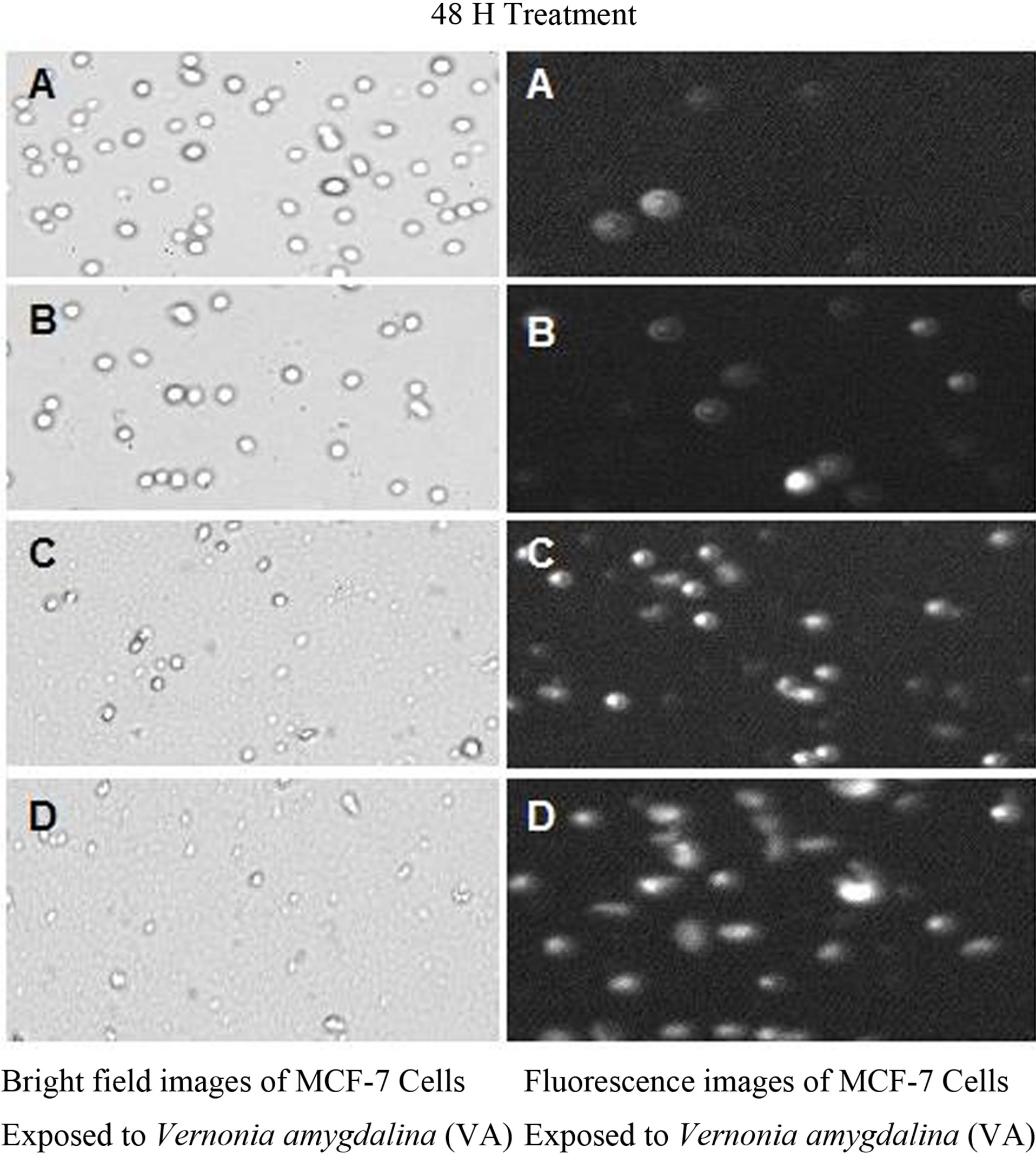
Figure 4. Bright field images (left) and fluorescent images (right) of MCF-7 cells exposed to VA for 48 h. MCF-7 cells were exposed to different concentrations of VA as described in the Materials and Methods.
are the most consumed vegetables (Ndole) during special occasions including marriage, baptism, Christmas, birthday, funeral and sometimes on a daily basis (Figure 5).

Figure 5. Photo of Vernonia amygdalina (VA) leaves taken in Yaounde, Cameroon on May 10, 2012 by Clement Yedjou. Cameroonians usually consume Vernonia amygdalina (VA) for therapeutic uses against stomach discomfort, vomiting, diarrhea, and intestinal illnesses.
Previous studies indicated that VA possesses potent antimalarial and antihelmintic properties [13], and antitumorigenic properties [14]. VA contains many active ingredients including Vernonioiside B and Myricetin (flavonol) [15]. Oral administration of the aqueous leaf extract of VA has been found to relieve pain and lower body temperature [16]. Many studies have documented the beneficial use of VA as a potent botanical agent for the treatment of different diseases [10,14,17-19]. A recent report has also demonstrated that VA leaf extract enhances the prophylactic and therapeutic efficacy of chloroquine against Plasmodium berghei malaria in mice [7].
To determine whether VA-induced cytotoxicity in MCF-7 cells is mediated by apoptosis and/or necrosis, we measured the Annexin V FITC/PI staining using the flow cytometry analysis. Annexin V FITC/PI assay helps to distinguish between apoptotic and necrotic cell death. Annexin V binds to the membrane phospholipid phosphatidylserine that is located within the plasma membrane of apoptotic cells and PI stains the cellular DNA of those that have a compromised cell membrane [4]. Data generated from the flow cytometry analysis demonstrated that VA induces apoptosis in MCF-7 cells in a dosedependent fashion. The percentage of cells stained with Annexin V (positive cells) and PI (necrotic cells) significantly (P < 0.05) increased with the increasing concentrations of VA (Figure 2). By the means of annexin V/PI assay, we report for the first time in our laboratory that VA induces apoptosis in human breast adenocarcinoma (MCF-7) cells via phosphatidylserine externalization as result of the loss of membrane integrity, a major characteristic of cell death by apoptosis. Rupture of the cellular membrane is one of the crucial criteria used to distinguish necrosis from apoptosis [20]. In the case of the present study, we observed that VA moderately induced annexin V positive cells (apoptotic cells), but the primary pathway of cell death was by necrosis (PI positive cells) as shown in Figures 3 and 4. Thus, it means that the cell membrane was disrupted and the cells died by necrosis at higher concentrations of VA exposure. Consistent with our finding, a previous study by Adjei and Rowinsky demonstrated that antitumor agents such as cisplatin, mitomycin, and actinomycin D induced apoptotic cell death in susceptible cells [21]. Similarly, a recent study in our laboratory indicated that garlic extract (a natural plant) exerts its toxicity efficacy by inducing oxidative stress and apoptosis in human promyelocytic leukemia (HL-60) cells [22]. Botanical compounds have contributed significantly to the discovery of therapeutic drugs against cancers and other diseases. For example, among 79 US. Food and Drug Administration approved anticancer drugs and vaccines between 1983 and 2002; 9 of them were directly from the isolation of natural products and 21 of them were natural product derivatives. In addition, among the 39 synthetic anticancer drugs; 13 of them were based on a pharmacophore originated from natural compounds [23].
To the best of our knowledge, no data was available in the literature regarding the apoptosis or necrosis potential of VA to human cancer cell lines or animal models. Here, we report that the cytotoxic efficacy of VA in MCF-7 cells may be associated at least in part, with apoptosis accompanied by secondary necrotic cell death probably due to the loss of membrane integrity. Apoptosis, a genetically controlled process is characterized by cytoplasmic and nuclear shrinkage, chromatin margination and fragmentation, and breakdown of the cell into multiple spherical bodies that retain membrane integrity [24,25]. In contrast, necrosis is an uncontrolled cell death that is characterized by progressive loss of cytoplasmic membrane integrity, rapid influx of Na+, Ca2+, and water, resulting in cytoplasmic swelling and nuclear pyknosis [26-28]. The latter feature leads to cellular fragmentation and release of lysosomal and granular contents into the surrounding extracellular space, with subsequent inflamemation [24,25].
5. Conclusion
In this study, the cell viability of MCF-7 cells exposed to Vernonia amygdalina (VA) was assessed by trypan blue exclusion assay and PI staining using the Cellometer Vision. We demonstrated that VA extract significantly (P < 0.05) reduces the viability of MCF-7 cells in a concentrationand time-dependent manner. We also evaluated in the present study the use of flow cytometry for apoptosis/necrosis detection, by studying the concentrationresponse effect of VA-induced cell death in MCF-7 cells labeled with annexin V-FITC (apoptotic) and propidium iodide (necrotic). Necrotic dead cells were further revealed by Cellometer imaging, showing a gradual increase in necrotic cell death (fluorescence positive cells) in VA-treated cells compared to the control (fluorescence negative cells). These data also revealed a gradual migration of the DNA from the head region into the tail region in VA-treated cells compared to the control cells. The extent of DNA damage increased proportionately with increasing doses of VA. In summary, our data indicate that the toxicity of VA extract to human breast adenocarcinoma (MCF-7) cells is associated with apoptotic and secondary necrotic cell death resulting from phosphatedylserine externalization due to loss of membrane integrity. Although the preclinical and therapeutic significance of our study remains to be established, our data provide a basis for further studies on the mechanisms of action and the potential for using VA as therapeutic agent in the treatment of breast cancer.
6. Acknowledgements
The research described in this publication was made possible by a grant from the National Institutes of Health (Grants No. 5G12RR013459 and 8G12MD007581), through the RCMI-Center for 
 Environmental Health at Jackson State University. A poster based on this paper was presented at the Advances in Breast Cancer Research: Genetics, Biology, and Clinical Applications Special Conference, held at the Fairmont Hotel in San Francisco, CA. October 12-15, 2011.
Environmental Health at Jackson State University. A poster based on this paper was presented at the Advances in Breast Cancer Research: Genetics, Biology, and Clinical Applications Special Conference, held at the Fairmont Hotel in San Francisco, CA. October 12-15, 2011.
REFERENCES
- J. R. Ainslie, “List of Plants Used in Native Medicine in Nigeria,” Imperial Forestry Institute, Oxford, 1973, p. 42.
- H. M. Burkill, “The Useful Plants of West Tropical Africa,” 2nd Edition, Royal Botanical Gardens, Kew, Vol. 1, 1985.
- E. B. Izevbigie, “Discovery of Water-Soluble Anticancer Agents (Edotides) from a Vegetable Found in Benin City, Nigeria,” Experimental Biology and Medicine, Vol. 228, No. 3, 2003, pp. 293-298.
- A. Tadesse, A. Gebre-Hiwot, K. Asres, M. Djote and D. Frommel, “The in Vitro Activity of Vernonia amygdalina on Leishmania aethiopica,” Ethiopian Medical Journal, Vol. 31, 1993, pp. 183-189.
- A. M. Hamowia and A. M. Saffaf, “Pharmacological Studies on Vernonia amygdalina (Del) and Tithonia Diversifolia (Gray),” Journal of Veterinary Medicine, Vol. 42, No. 2, 1994, pp. 91-97.
- B. A. Iwalokun, “Enhanced Antimalarial Effects of Chloroquine by Aqueous Vernonia amygdalina Leaf Extract in Mice Infected with Chloroquine Resistant and Sensitive. Plasmodium berghei Strains,” African Health Sciences, Vol. 8, No. 1, 2008, pp. 25-35.
- A. Akah and C. I. Okafor, “Hypoglycaemic Effect of Vernonia Amygdalina Del, in Experimental Rabbits,” Planta Medica, Vol. 1, 1992, pp. 6-10.
- C. G. Yedjou, E. Izevbigie and P. B. Tchounwou, “Preclinical Assessment of Vernonia Amygdalina Leaf Extracts as DNA Damaging Anti-Cancer Agent in the Management of Breast Cancer,” International Journal of Environmental Research and Public Health, Vol. 5, No. 5, 2008, pp. 337-341. doi:10.3390/ijerph5050337
- C. B. Howard, J. Stevens, E. B. Izevbigie, A. Walker and O. McDaniel, “Time and Dose-Dependent Modulation of Phase 1 and Phase 2 Gene Expression in Response to Treatment of MCF-7 Cells with a Natural Anti-Cancer Agent,” Cell and Molecular Biology, Vol. 49, No. 7, 2003, pp. 1057-1065.
- C. G. Yedjou, M. A. Saeed, M. A. Hossain, W. Dorsey, H. Yu and P. B. Tchounwou, “Basic Apoptotic and Necrotic Cell Death in Human Liver Carcinoma (HepG(2)) Cells Induced by Synthetic Azamacrocycle,” Environmental Toxicology, 2012, in Press. doi:10.1002/tox.21786
- G. Koopman, C. P. M. Reutelingsperger, G. A. M. Kuijten, R. M. J. Keehnen, S. T. Pals and M. H. van Oers Jr., “Annexin-V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis,” Blood, Vol. 84, No. 5, 1994, pp. 1415-1420.
- O. Adaramoye, B. Ogyngbenro, O. Anyaegou and M. Fafunso, “Protective Effects of Extracts of Vernonia Amygdalina, Hisbicus. Sabdaiffa and Vitamin C against Radiation-Induced Liver Damage in Rats,” Journal of Radiation Research, Vol. 49, 2008, pp. 123-131. doi:10.1269/jrr.07062
- A. O. Abosi and B. H. Raseroka, “In Vivo Antimalarial Activity of Vernonia amygdalina,” British Journal of Biomedical Science, Vol. 60, No. 2, 2003, pp. 89-91.
- E. B. Izevbigie, T. L. Bryant and A. Walker, “A Novel Natural Hibitor of Extracellular Signalregulated Kinases and Human Breast Cancer Cell Growth,” Experimental Biology and Medicine, Vol. 229, No. 2, 2004, pp. 163- 169.
- C. Manach, A. Scalbert, C. Morand, C. Remesy and H. Jimenez, “Polyphenols: Food Sources and Bioavailability,” American Journal of Clinical Nutrition, Vol. 79, No. 5, 2004, pp. 727-747.
- A. M. Tekobo, A. O. Onabanjo, O. O. Amole and P. M. Emeka, “Analgesic and Antipyretic Effects of the Aqueous Extract of Vernonia amygdalina,” West African Journal of Pharmacy, Vol. 16, 2002, pp. 68-74.
- S. B. Mbinglo, “Survey on the Production of Bitterleaf Vernonia spp. in Bamenda, N.W. Cameroon,” Student Project Report for Natural Resource Institute, United Kingdom/Dschang University Cameroon, Chatham, 1998.
- C. F. L. Onwuka, A. O. Akinsoyinu and O. O. Tewe, “Feed Value of Some Nigerian Browse Plants: Chemical Composition and in Vitro Digestibility,” East African Agricultural and Forestry Journal, Vol. 54, 1989, pp. 157- 163.
- E. M. K. Aregheore, H. P. S. Makkar and K. Becker, “Feed Value of Some Browse Plants from the Central Zone of Delta State,” Nig. Trop. Sci, Vol. 38, 1998, pp. 97-104.
- H. Kim, S. You, B. Kong, L. Foster, J. Farris and D. Foster, “Necrotic Cell Death by Hydrogen Peroxide in Immortal DF-1 Chicken Embryo Fibroblast Cells Expressing Deregulated MnSOD and Catalase,” Biochimca et Biophysica Acta, Vol. 1540, 2001, pp. 137-146.
- C. G. Yedjou and P. B. Tchounwou, “In Vitro Assessment of Oxidative Stress and Apoptotic Mechanisms of Garlic Extract in the Treatment of Acute Promyelocytic Leukemia,” Journal of Cancer Science and Therapy, Vol. 10, No. 4, 2012, pp. 1948-1956. doi:10.1016/S0167-4889(01)00131-8
- A. A. Adjei and E. K. Rowinsky, “Novel Anticancer Agents in Clinical Development,” Cancer Biology & Therapy, Vol. 2, 2003, pp. 5-15.
- G. M. Cragg, D. J. Newman and K. M. Snader, “Natural Products as Sources of New Drugs over the Period 1981-2002,” Journal of Natural Products, Vol. 66, No. 7, 2003, pp. 1022-1037.
- L. M. Buja, M. L. Eigenbrodt and E. H. Eigenbrodt, “Apoptosis and Necrosis. Basic Types and Mechanisms of Cell Death,” Archives of Pathology & Laboratory Medicine, Vol. 117, No. 2, 1993, pp. 1208-1214. doi:10.1021/np030096l
- G. Majno and I. Joris, “Apoptosis, Oncosis, and Necrosis: An Overview of Cell Death,” American Journal of Pathology, Vol. 146, No. 1, 1995, pp. 3-15.
- A. H. Wyllie, “Death from Inside Out: An Overview,” Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 345, No. 1313, 1994, pp. 237-241.
- M. J. Berridge, P. Lipp and M. D. Bootman, “The Versatility and Universality of Calcium Signaling,” Nature Reviews Molecular Cell Biology, Vol. 1, No. 1, 2000, pp. 11-21. doi:10.1098/rstb.1994.0099
- L. F. Barros, T. Hermosilla and J. Castro, “Necrotic Volume Increase and the Early Physiology of Necrosis,” Comparative Biochemistry and Physiology, Vol. 130, No. 3, 2001, pp. 401-409. doi:10.1038/35036035
NOTES
*Corresponding author.

