Open Journal of Ecology
Vol. 3 No. 2 (2013) , Article ID: 31358 , 11 pages DOI:10.4236/oje.2013.32022
Costs of glucosinolates in Brassica rapa: Are they context dependent?
![]()
1Department of Interdisciplinary Studies, Beacon College, Leesburg, USA; *Corresponding Author: kstowe@beaconcollege.edu
2Department of Biology, University of Evansville, Evansville, USA
3Department of Biology, Vassar College, Poughkeepsie, USA
4Department of Biology, Denison College, Samson Talbot Hall, Granville, USA
Copyright © 2013 Kirk A. Stowe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 13 February 2013; revised 14 March 2013; accepted 16 April 2013
Keywords: Brassica rapa; Plant Defense; Cost of Defense; Benefit of Defense; Temperature; Nutrients
ABSTRACT
Models predicting optimal levels of plant defense against herbivores typically include two assumptions: 1) defense is both beneficial and costly; and 2) the relationship between costs and benefits of a defense is consistent across environments. However, the expression of costs and benefits of defense may be environmentally dependent. We examined lines of Brassica rapa, previously divergently selected for the defensive trait foliar glucosinolate content. In one set of experiments (Experiment #1), plants were grown in herbivore-free and herbivore-present environments to investigate the costs and benefits of this defense. In a second set of experiments (Experiment #2), plants were grown at two nutrient levels and two temperatures to examine the environmental context of costs of defense. In Experiment #1, increased levels of damage resulted in decreased flower production and plants from high glucosinolate lines received less damage than those from low glucosinolate lines, suggesting a benefit of this defense. In this experiment no cost of defense was detected. In Experiment #2, nutrients had a significant positive effect on flower production at 23˚C, but not at 32˚C. No significant effects of glucosinolate line nor interaction between nutrient environment and glucosinolate line were detected at 23˚C, suggesting that no cost of defense occurred at this lower temperature. Similarly, no significant nutrient environment by glucosenolate line interaction was detected at 32˚C. However, a significant effect of glucosinolate line was observed suggesting that at 32˚C costs were incurred, but nutrient environment had no mitigating effect. While results from Experiment #1 suggested that defense was beneficial, but not costly, results from Experiment #2 suggested that costs of defense were temperature dependent. For species occupying broad geographic ranges, these findings of temperaturedependent costs are especially insightful with regard to the evolution of defense because differing geographic populations are likely to experience differing temperature environments.
1. INTRODUCTION
Herbivore damage usually decreases plant fitness, making damage costly to plants [1-5], but see [6-8], so traits that reduce herbivory should benefit a plant and increase in frequency. However, a trade-off between the cost of defense and its benefits may explain why the evolution of defense against herbivores is constrained [9,10], with selection favoring those plants that maintain an optimal combination of defense versus growth [11]. Such trade-offs can be detected as a negative relationship between levels of defense expressed and plant fitness in an herbivore-free environment [9,10,12,13]. Further, some defenses may deter generalist herbivores while at the same time attract specialist herbivores [14-16], but not always [17]. While costs exist for some species and in some instances, costs may not be universal (for review see [12,18-20]). Failure to detect costs has led authors to suggest that the debate over the existence of costs of defense should be shifted to focus on the conditions under which costs of defense are expressed [12,18,20,21].
Investment in defensive traits may be dramatically affected by nutrient environment [22-26]. For example, reference [26] found that various species grown at high nutrients expressed greater deterrence to herbivores, while light treatment had no consistent effect. Although these studies suggest that defenses may be less costly when more resources are available for production of those defenses, reference [21] found that defense was more costly under high nutrient conditions (when allocation to defense might compete directly with allocation to growth). Thus, costs of defense generally increase under stressful conditions [11,18,24,27-30], but not always [21,31]. In addition, whether differing environments alter the benefits of reduced herbivore damage has not commonly been explored, but see [25].
Environmental factors other than nutrient environment may also influence the expression of defense costs [21, 27-30,32-36]. Temperature can influence plant growth [37], but the influence of temperature on costs of defense has been poorly explored, but see [38-41]. Because plant species often grow across a wide geographical range, populations may experience differing temperature regimes. Turnover rates of defensive chemicals should be greater for populations in habitats with higher temperatures [42]. The cost of defense would therefore increase at higher temperatures because more resources would have to be allocated to rebuilding defensive compounds [42]. In addition, if increasing temperature disrupts either photosynthetic rate or nutrient uptake [43], higher temperatures would limit the pool of resources available to defense and growth/reproduction.
In this study, the costs and benefits of foliar glucosenolates were examined using lines of a rapid cycling variety of Brassica rapa that had previously been artificially divergently selected for either high or low foliar glucosinolate content [13]. Glucosinolates are a nitrogen and sulfur-based group of secondary compounds found in the Brassicaceae [42,44,45]. Greater investment in energy may be expected for plant lines with higher concentrations of glucosinolates given the energy required to rebuild these molecules [42]. In this system, foliar glucosinolates, have been shown to reduce herbivore damage [16,17,45-47] and can be used as a measure of investment in one defensive trait even when no herbivore treatment is imposed. Thus, this system is likely to detect benefits and costs of this defense.
In one experiment (Experiment #1), we evaluated: 1) whether increasing levels of herbivory resulted in decreased plant fitness; 2) whether a benefit to high foliar glucosinolate content existed in the presence of herbivores; and 3) whether a cost existed in the absence of herbivores. In a second experiment (Experiment #2), we examined how stress, in terms of nutrients and temperature, affected the expression of costs of foliar glucosenolates in those same lines. More specifically, we examined: 4) whether a greater cost of defense existed at a higher temperature; and 5) whether a greater cost of defense existed under low nutrient conditions.
2. METHODS
Study species: Brassica rapa (Brassicaceae), commonly known as field mustard, is an annual plant that was introduced to the United States from Eurasia and now exists in naturalized populations throughout North America [48,49]. With such a broad geographic range, different populations of this species experience very different mean annual temperatures (e.g., a population in Juneau AK would experience a mean July temperature of 13˚C, while a population in Phoenix AZ would experience a temperature of 34˚C [50]). In addition, B. rapa is often found in disturbed habitats [49], so differing populations may also experience a variety of nutrient environments.
This study used a rapid cycling variety of B. rapa (CRGC #1-1, Aaa). This variety has a short generation time, high fertility, no seed dormancy, and the ability to grow and reproduce under fluorescent lighting [51]. Replicate lines of rapid cycling B. rapa were used in this study. Each replicate line was generated by divergently selecting for either low or high foliar content of glucosenolates over three generations [13]. Reverse-phase liquid chromatography was used to determine foliar concentration of glucosinolates of the first true leaf 14 days after emergence of the 100 plants contained in each replicate line. In the selection process, 20 of the 100 individuals with the highest content of glucosinolates and 20 with the lowest were selected and used to produce each successive generation for the high (HGL) and low (LGL) glucosinolate lines, respectively. Because B. rapa flowers are hermaphroditic and self-incompatible, artificial selection was successfully achieved through controlled pollination. Selection was discontinued after three generations. After the third generation, seeds from these lines were bulked for use in this experiment. While the concentration of glucosinolates was not analyzed in the current experiment, the HGLs differed significantly from the LGLs after the third generation of selection (mean = 14.2 ± 0.9 (±SE) mg glucosinolates per gram of leaf material and mean = 5.5 ± 0.5 mg/g, respectively; ANOVA: F2,3 = 28.29, P < 0.05; [13]).
Trichoplusia ni larvae (Lepidoptera: Noctuidae) were used in Experiment #1 of this study. T. ni often feeds on species from families as different as Apiaceae and Brassicaceae [52]. As a polyphagous herbivore, T. ni was ideal for this study because it feeds on B. rapa, but has been found to be deterred by higher concentrations of glucosinolates [17]. T. ni adults emerging from pupae obtained from a laboratory culture maintained at the Biological Control Laboratory of the United States Department of Agriculture-Agricultural Research Station, Columbia, Missouri, USA, were used to generate a colony of T. ni at Denison University, in Granville, OH. Larvae were reared on glucosinolate-free artificial diet obtained from the Biological Control Laboratory [53].
Experiment #1 (Benefits and costs of defense): Artificial selection provided contrasting differences in chemical defense in this experiment, but a single paired set of contrasting lines could be due to inconsistent differences. Therefore, each HGL was paired with one of the two LGLs to create two replicate tests (i.e., independent tests in order to avoid outcomes caused by anomalous differences). In the first experiment, seeds from the LGLs and HGLs were planted individually in 3.1 cm square peat pots filled with Jiffy-Mix growth medium. The pots were labeled and randomly arranged. To avoid confounding effects of germination date, the experiment was started three days following seed germination. At that time, 360 plants were randomly chosen (90 plants from each LGL and HGL replicate test). Three plants from an LGL and three plants from the matching HGL were potted into 13.9 cm diameter circular pots resulting in 30 pots for each of the two replicate tests. Thus, after three days the lines were growing under competition.
Each of the six plants in every pot was fertilized once with 5 ml (6.28 g/L) of Peters All-Purpose 20-20-20 N-P-K fertilizer (31.4 mg fertilizer/plant). Pots were randomly arranged on the growing table and watered daily. To decrease position effects, pot position was randomized weekly. Plants were kept in an environment with continuous light provided by florescent lights suspended one meter above the plants. Temperature was maintained between 22.5˚C and 24.5˚C, with relative humidity ranging from 38% - 40%.
Ten days after germination the leaf area of each plant was measured using a transparent piece of graph paper (0.04 cm2 grids). A single, third instar, T. ni larva was introduced into 15 of the 30 pots for each replicate test to create an environment in which herbivores were present in order to evaluate the benefit of foliar glucosinolate content. The pots were covered with bridal veil to contain the larvae. The 15 pots for each replicate that had no herbivore introduced were designated as the herbivore-free environment in which the costs of production of glucosinolates could be measured; these pots were also covered with bridal veil to control for any shading effect of the bridal veil. Twelve days after germination (two days after the introduction of herbivores), the damaged and undamaged leaf area for every plant in the herbivore-present environment was measured. Because herbivory levels were low, an additional fourth instar larva was added to each herbivore-present treatment to increase herbivory. Two days later (14 days following germination), after removing the bridal veil and the T. ni larvae, the damaged and undamaged leaf areas were measured. Proportion leaf area damaged was calculated (leaf area damaged/total leaf area). Plants were harvested 60 days after germination (the typical generation time of this rapid cycling variety of B. rapa), and the number of flowers produced by each plant was recorded as an estimate of plant fitness. This is a sufficient measure of female fitness in this species, because those individuals that produce more flowers also tend to produce more fruits (Stowe, unpublished data). Further, floral display has also been shown to affect fitness in other species [54-57].
Experiment #2 (Environmental influences on costs of defense): The results from Experiment #1 supported the existence of a benefit of foliar glucosinolate production, but the results differed from those of a previous study with regard to the cost of defense [13]. Temperature environment differed greatly between the studies; the study by reference [13] was performed at a higher temperature (~29˚C) compared to Experiment #1 (~23˚C). Light and nutrient competition may have also differed because plants were placed in single larger pots in Experiment #1.
Five hundred thirty seeds were individually planted in 3.1 cm peat pots and grown in herbivore-free growth chambers with constant light. The pots were filled with moist soil, and the seeds were placed in the middle of the pot and covered with a light layer of soil. To limit any effect of germination time, only plants that had germinated after five days were used in this study. Of the 530 seeds planted, 273 plants met the germination criteria and were used in the study. Plants were randomized and placed in plastic trays to prevent water drainage. To test the effect of temperature on the expression of costs of defense, plants were placed in growth chambers either in the low (23˚C; n = 134) or high (32˚C; n = 139) temperature treatment.
Under these conditions, the effect of resource availability on the cost of defense was also evaluated. Half of the plants in each temperature treatment were randomly assigned to the low nutrient treatment and half were assigned to the high nutrient treatment. Nutrient levels were generated by provisioning each plant with 5 ml of General Purpose Peter’s Growth Formula 20-20-20 at a concentration of 6.28 g/L for low (31.4 mg/plant) and 12.56 g/L for high (62.8 mg/plant) nutrient treatments. Thus, each nutrient treatment was tested in two temperature treatments (23˚C and 32˚C). Plants in each growth chamber were randomized weekly to decrease position effects. In addition, to reduce inherent differences between growth chambers, the growth chambers were switched weekly (i.e., if a growth chamber was used for the high temperature treatment during one week, it was used for the low temperature treatment the following week). Plants were watered two times a day by filling the bottom of the plastic trays with 1.3 cm of water. Sixty days after germination total flower number produced for each plant was recorded and used as an estimate of the female component of plant fitness. This was the end of flowering time and most plants had already senesced (Hochwender pers. observation).
Statistical Analyses
Experiment #1 (Costs and benefits of defense): In this experiment each pot was independent from every other pot; however, the six plants within each pot were not independent due to common environment. Therefore, mean values for the three plants of each glucosinolate line within each pot were used as the unit of analysis. Three plants had to be excluded from the statistical analyses (two due to broken leaves, and one because it produced no leaves). Mean flower number for each glucosinolate line within each pot was calculated for plants within the herbivore-present environment and the herbivore-free environment separately.
To determine if a relationship existed between the amount of herbivore damage and flower production in this variety of B. rapa (i.e., was there a benefit of defense), we examined the effect of mean proportion leaf area damaged on mean number of flowers produced. An ANCOVA was conducted, using flower number as the dependent variable, and proportion leaf area damaged, glucosinolate line (LGL, HGL), and their interaction as the response variables [58]. Glucosinolate line was considered a fixed effect.
To evaluate the effect of glucosinolate line on herbivore damage, the difference in mean proportion leaf area damaged for each pot was calculated as mean proportion leaf area damaged of the HGL minus mean proportion leaf area damaged of the LGL (HGL-LGL). A negative value would indicate that plants of the LGL received more damage than plants of the HGL (i.e., that the higher foliar concentration of glucosinolates deterred herbivory). To avoid problems related to normality, a Wilcoxon signed-rank (i.e., a nonparametric, paired t-test) was used [58]. Separate analyses were performed for each replicate test here and to address each of the following questions.
To determine whether glucosinolate line affected plant fitness, mean number of flowers produced per plant per pot was calculated for each line both in the presence and absence of herbivory. To evaluate the effect of glucosenolate line on plant fitness, the difference in mean number of flowers produced per plant for each pot was calculated (HGL-LGL). In the presence of herbivores, a positive value would indicate that a benefit of defense was detected. In the absence of herbivores, a negative value would indicate a cost of defense. A Wilcoxon signed-rank test [58] was used on the differences in mean number of flowers produced in both the herbivore-free and herbivore-present treatments.
Experiment #2 (Environmental influences on costs of defense): In this experiment, each growth chamber represented a different environment, so the results from the 23˚C growth chamber were analyzed separately from the results from the 32˚C growth chamber. For each temperature, a two-factor ANOVA was used to evaluate the effect of glucosinolate line, nutrient treatment, and their interaction on total flower production. To remain consistent with the Experiment #1, the two replicate tests were run in the same manner using the same paired rapid-cycling B. rapa selection lines, with each replicate test including a high and low glucosinolate line. Nutrient treatment and glucosinolate line were considered fixed effects. A significant effect of glucosinolate line would suggest that plants investing in glucosinolates incurred a cost, depending on the direction of the effect. A significant effect of nutrient treatment would only suggest that nutrient addition influenced flower production. However, an interaction between glucosinolate line and nutrient treatment would suggest that the expression of costs of defense was altered by nutrient environment.
3. RESULTS
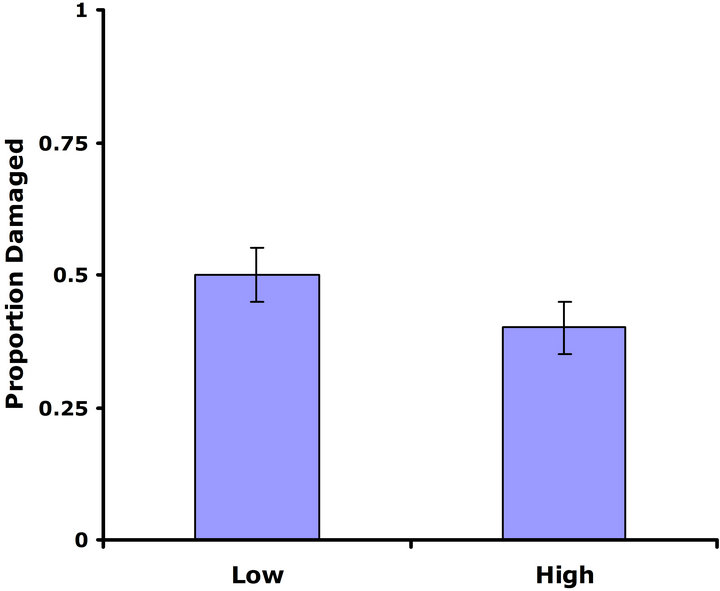
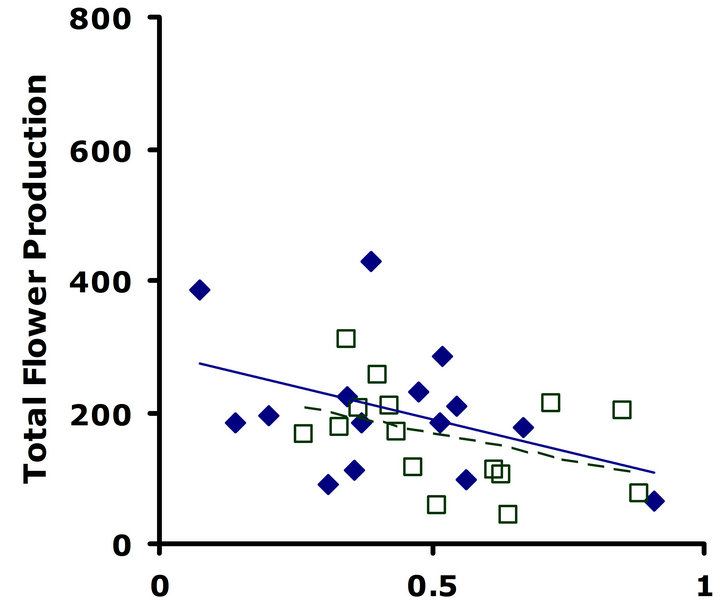
Experiment #1 (Costs and benefits of defense) Plants from the LGL received approximately ten percent more damage than plants from the HGL in both replicate tests (Figures 1(a) and (b)). Plants in the HGL incurred significantly less damage than plants in the LGL for the second replicate test (Wilcoxon signed rankdf=14 = −39; P = 0.026). Although patterns were similar for both replicate tests, no significant difference was detected for the first replicate test (Wilcoxon signed rankdf=14 = −20; P = 0.28). Still, flower production did significantly decrease with increasing damage both in replicate tests (F1,28 = 6.9; P = 0.01; r2 = 0.198; F1,28 = 4.3; P = 0.05; r2 = 0.133) (Figures 2(a) and (b)).
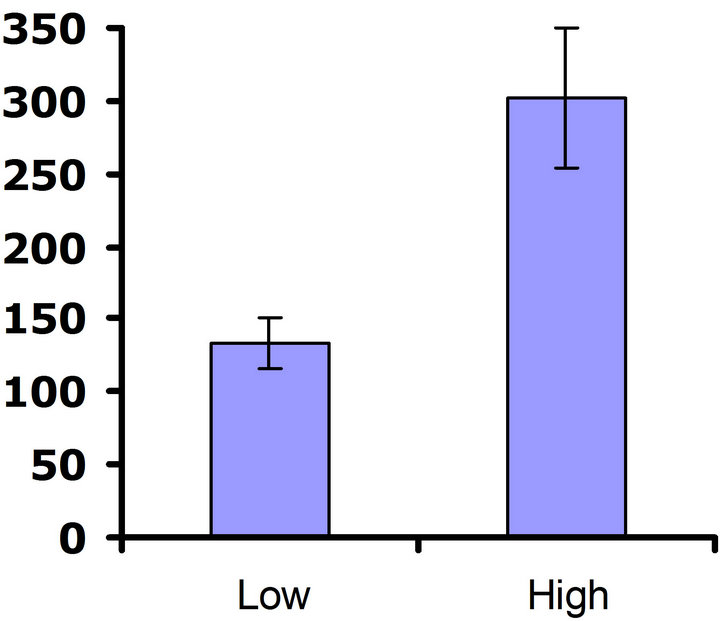
When exposed to herbivores, (Figure 3(a)), differences in flower production were not significant in the first replicate test (Wilcoxon signed rankdf=14 = 23; P = 0.21), but differences in flower production were significant in the second replicate test (Wilcoxon signed rankdf=14 = 51; P = 0.002) (Figure 3(b)). Thus, a benefit of higher glucosinolates was detected in the presence of herbivores, but only in one of the two replicate tests. In the herbivore-free environment, flower production was not significantly different between glucosinolate lines for
 (a)
(a)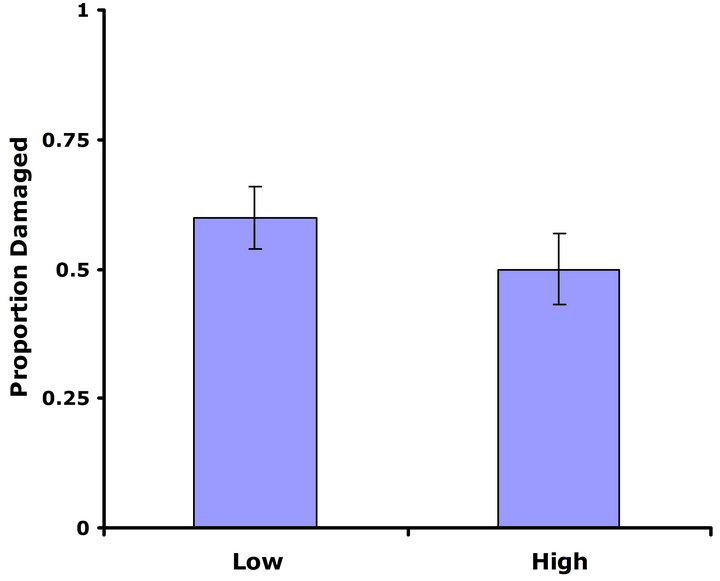 (b)
(b)
Figure 1. Mean proportion leaf area damaged by Trichoplusia ni larvae for both high and low glucosinolate lines. Error bars represent ±1SE. (a) Replicate test one; no significant difference was detected between glucosinolate lines (Wilcoxon signed rankdf=14 = −20; P = 0.28). (b) Replicate testtwo; plants in the HGL incurred significantly less damage than plants in the LGL(Wilcoxon signed rankdf=14 = −39; P = 0.026).
either replicate test (Wilcoxon signed rankdf=14 = 29; P = 0.11 and Wilcoxon signed rankdf=14 = 4; P = 0.85 for replicate tests one and two, respectively) (Figures 3(c) and (d)), so no cost was detected for having higher investment in defense.
Experiment #2 (Environmental influences on costs of defense) In our set of experiments examining the environmental influences on costs of defense, no cost of foliar glucosinolates was detected at 23˚C in either replicate test (F3,61 = 0.8; P = 0.39 and F3,65 = 0.3; P = 0.56 for replicate test one and two, respectively) (Figures 4(a) and (b)). Nutrient treatment had a significant effect on
 (a)
(a)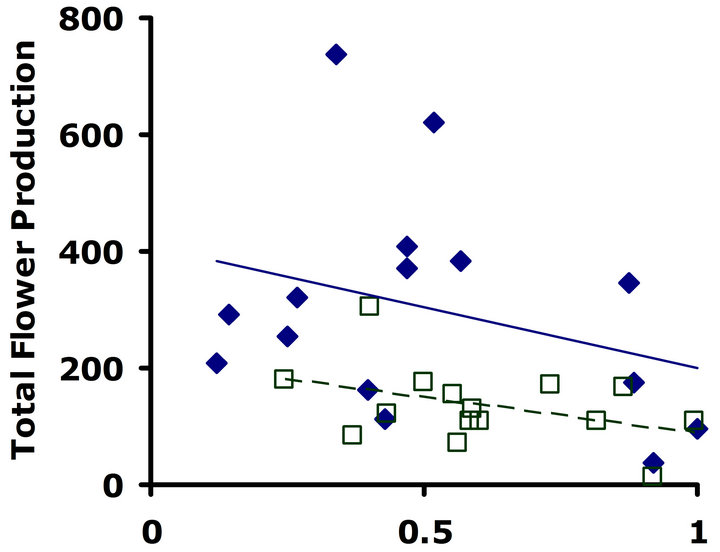 (b)
(b)
Figure 2. Correlations between total flower production and proportion leaf area damaged. Filled diamonds correspond to high glucosinolate lines; open squares correspond to low glucosinolate lines. Solid lines are trend-lines for high glucosinolate lines; dashed lines are trend-lines for low glucosinolate lines. (a) Replicate test one; flower production significantly decreased with increasing damage (F1,28 = 6.9; P = 0.01; r2 = 0.198). (b) Replicate test two; flower production significantly decreased with increasing damage (F1,28 = 4.3; P = 0.05; r2 = 0.133).
flower production in both replicate tests (F3,61 = 10.3; P < 0.002 and F3,65 = 22.5; P < 0.0001 for replicate test one and two, respectively), but no significant interaction between nutrient treatment and glucosinolate line occurred (F3,61 = 2.5; P = 0.12 and F3,65 = 0.3; P = 0.60 for replicate test one and two, respectively). Thus, altering the nutrient environment did not influence the expression of the cost of the content of foliar glucosinolates at 23˚C.
At 32˚C, patterns suggested a cost of glucosinolate production. In replicate test one, a significant difference in flower production was detected (F3,62 = 5.6; P = 0.02) (Figure 4(c)). In replicate test two, a similar pattern was observed, but the trend was non-significant (F3,68 = 3.6; P = 0.06) (Figure 4(d)). Nutrient treatment had a significant effect on flower production in replicate test one at Herbivores Present
 (a)
(a)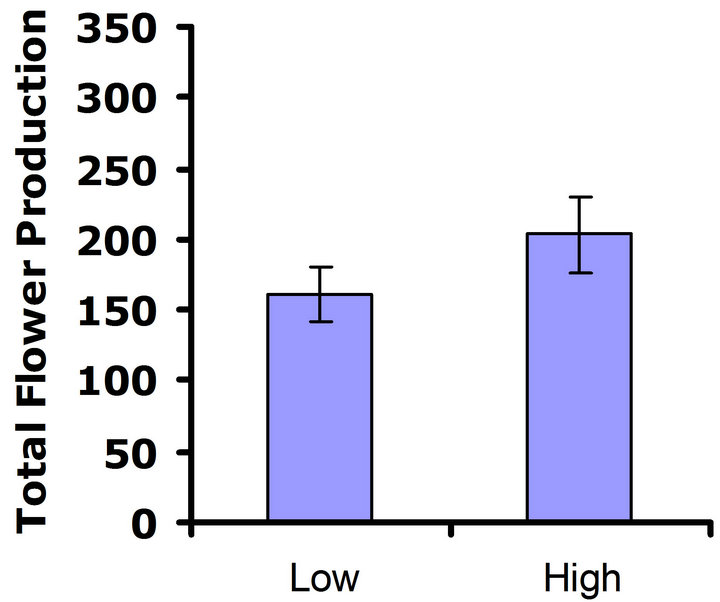 (b)
(b)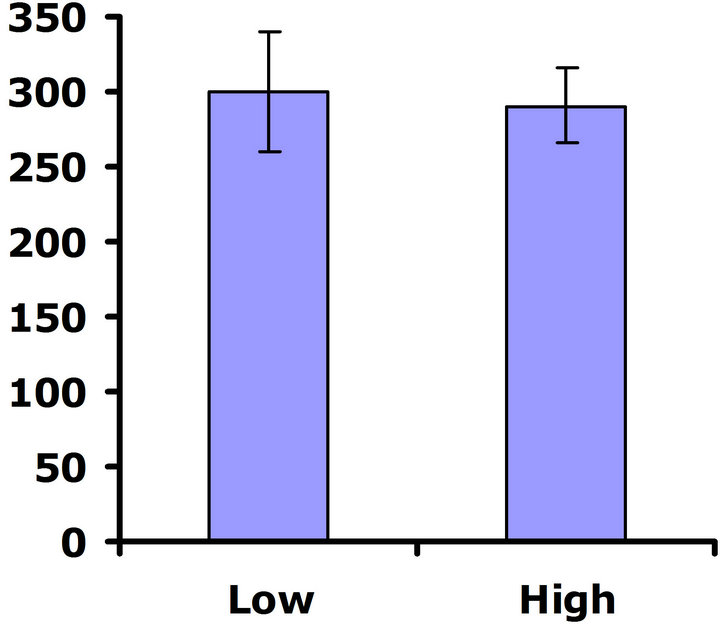 (c)
(c)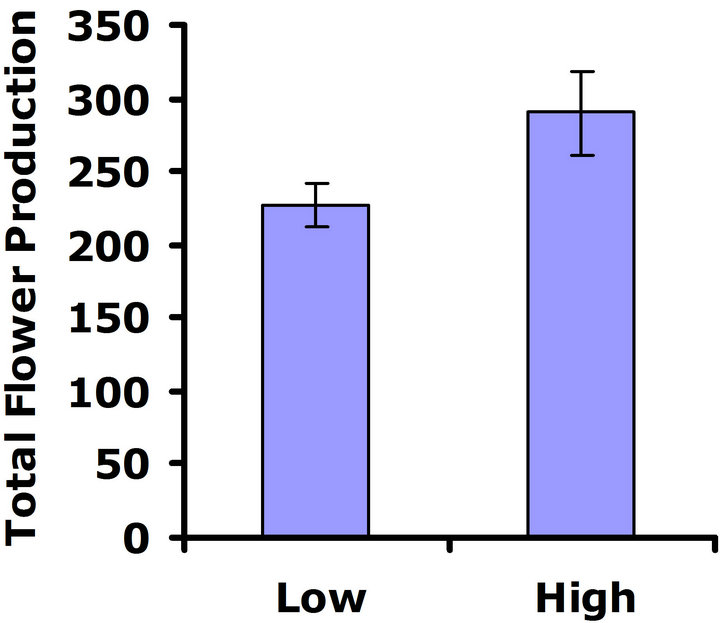 (d)
(d)
Figure 3. Mean number of flowers produced for both high and low glucosinolate lines. Error bars represent ±1SE. In the presence of herbivores: (a) Replicate test one; differences were not significant between glucosinolate lines (Wilcoxon signed rankdf=14 = 23; P = 0.21); (b) Replicate test two; flower production wassignificantly greater in the HGL (Wilcoxon signed rankdf=14 = 51; P = 0.002). In the absence of herbivores: (c) Replicate test one; no significant difference was detected between glucosinolate lines (Wilcoxon signed rankdf=14 = 29; P=0.11); (d) Replicate test two; flower production did not differ significantly between glucosinolate lines (Wilcoxon signed rankdf=14 = 4; P = 0.85).
this higher temperature, (F3,62 = 10.7; P = 0.002), but did not have a significant effect on flower production in replicate test two (F3,68 = 0.3; P = 0.56). No significant interaction between nutrient treatment and glucosinolate line was detected in replicate test one (F3,62 = 0.5; P = 0.49) nor in replicate test two (F3,68 = 0.04; P = 0.84) (Figures 4(c) and (d)). Thus, similar to the 23˚C environment, the nutrient environment did not influence the cost of glucosinolate level at 32˚C.
4. DISCUSSION
Experiment #1 (Costs and benefits of defense)—Our results demonstrated a negative relationship between proportion leaf area damaged by T. ni and flower production in B. rapa; increasing levels of herbivory resulted in decreased plant fitness as measured by flower production. Thus, a cost of herbivore damage occurred. Similarly, most other research has found that damage decreases plant fitness [13], but see [4,6-8]. Further, in our study, plants from the HGLs received less damage by T. ni than plants from the LGLs, similar to the findings of a previ23˚C
 (a)
(a)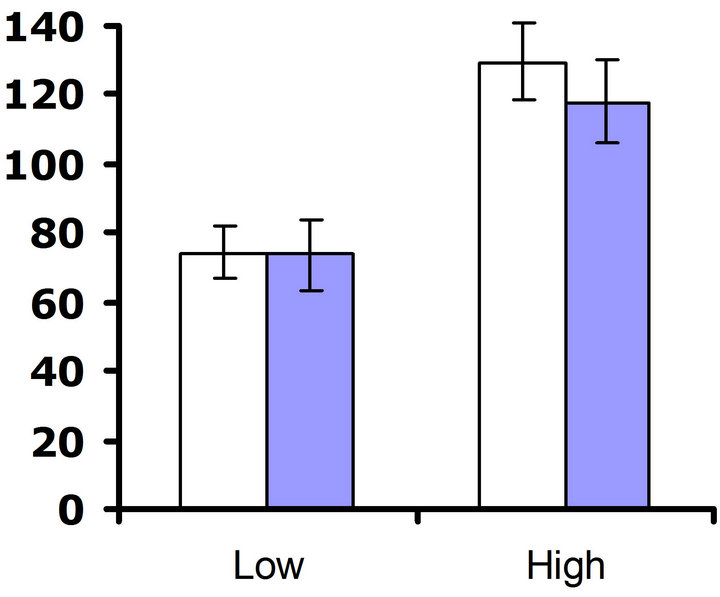 (b)
(b)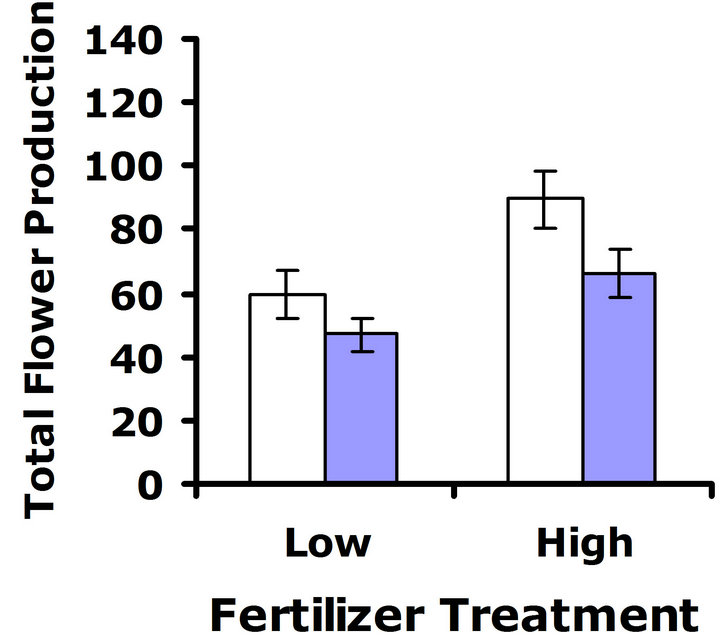 (c)
(c)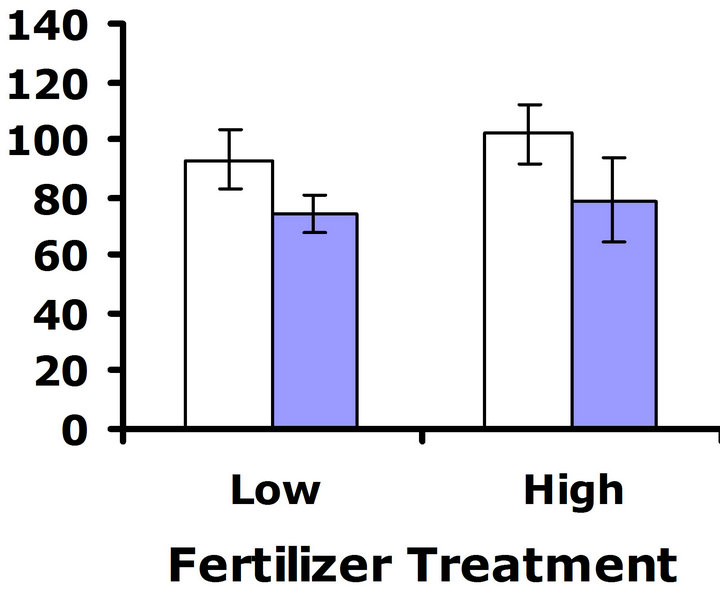 (d)
(d)
Figure 4. Mean number of flowers produced by low and high glucosinolate lines at high and low nutrient treatments. Open bars correspond to LGLs and filled bars correspond to the HGLs. Error bars represent ±1 SE. At 23˚C: (a) Replicate test one; nutrient treatment had a significant effect on flower production (F3,61 = 10.3; P < 0.002); no significant difference between glucosinolate lines was detected (F3,61 = 0.8; P = 0.39); no significant interaction between nutrient treatment and glucosinolate line occurred (F3,61 = 2.5; P = 0.12); (b) Replicate test two; nutrient treatment had a significant effect on flower production (F3,65 = 22.5; P < 0.0001); no significant difference between glucosinolate lines was detected (F3,65 = 0.3; P = 0.56); no significant interaction between nutrient treatment and glucosinolate line occurred (F3,65 = 0.3; P = 0.60). At 32˚C: (c) Replicate test one; nutrient treatment had a significant effect on flower production (F3,62 = 10.7; P = 0.002); a significant difference between glucosinolate lines was detected (F3,62 = 5.6; P = 0.02); no significant interaction between nutrient treatment and glucosinolate line occurred (F3,62 = 0.5; P = 0.49). (d) Replicate test two; nutrient treatment had no significant effect on flower production (F3,68 = 0.3; P = 0.56); no significant difference between glucosinolate lines was detected (F3,68 = 3.6; P = 0.06); no significant interaction between nutrient treatment and glucosinolate line occurred (F3,68 = 0.04; P = 0.84).
ous study using these lines [17]. Because plants receiving less herbivore damage had higher flower production, selection should favor plants in the HGLs (i.e., a greater benefit than cost of defense). While these results suggest that glucosinolates were responsible for our results, plants in Brassicaceae also contain trypsin inhibitors [59,60], which inhibit the digestion of protein by the herbivore, and myrosinases, that breakdown the glucosenolates into toxic compounds [44]. These defensive traits were not measured, but our results could be explained by selection for these other traits in the opposite direction than selection for foliar glucosinolates.
However, we detected a significant benefit of higher defense in only one of the two replicate tests. A significant benefit of defense may not have been detected in one of the two tests because of the limited sample size. However, reference [61] found that plants from the LGLs had greater tolerance to herbivore damage than plants from the HGLs, when using these lines of B. rapa. Greater tolerance to damage for LGLs might counterbalance the reduced damage experienced by HGLs, thereby limiting one’s ability to detect a benefit of defense [62].
Surprisingly, in the replicate test in which a benefit of defense was detected, no cost for this defense was observed. Nor did we detect a significant cost of defense in the other replicate. One would expect that without herbivory, plants with a higher concentration of foliar glucosinolates would be at a disadvantage due to their greater allocation of resources to defenses. Such was the case in a previous study examining these same lines [13]. However, distinct differences in experimental design existed. The plants in the current experiment were grown together in a pot (under intraspecific competition), whereas those in the previous experiment were grown individually (no competition). While competition has been shown to influence both herbivore damage and chemical defense in B. rapa [63] and the related species, B. napus [64], and costs of defense in Arabidopsis thaliana [59], competition had no effect on the magnitude of costs observed in B. rapa [31] nor among lines of Plantago lanceola artificially selected for iridoid glycoside concentration [24]. In fact, reference [31] found greater cost when plants were grown alone (as in Experiment #2). However, the cost of allocation to the inducible defense, proteinase inhibitors, was more apparent in genotypes of Nicotiana attentuata grown under competition [65]. Thus, it appears that predicting the effect of competition on the costs of any particular defense is very complex.
Perhaps the more dramatic difference in experimental design was that plants in this current study were grown at a lower temperature (22.5˚C - 24.5˚C) than those in the previous study (27˚C - 29˚C) [13]. Costs may not be as great at lower temperatures compared to higher temperatures (see Environmental influences of costs of defense below).
An alternative explanation for not detecting costs is that costs of defense may not be universal [20,66]. References [67,68] found no cost of trichome production (a putative defense) in B. rapa. Furthermore, some studies examining other species have also failed to detected costs of defense [9,10,69-72], but see [21,25,73-75]. Several arguments have been posited suggesting why no costs may be detected [12,61,62]. References [61,62] have suggested that the relationship between defense against and tolerance to herbivore damage may obscure the detection of a cost of defense. While some studies have demonstrated a trade-off between defense and tolerance [61,62,74], others have not [21,75]. Further, reference [12] has also suggested that once defense arises in a population, selection should act to decrease its cost. This argument is similar to the argument presented in studies that have explored the reduction of costs for insecticide resistance in Lucilia cuprina populations [76,77] and virus-resistant populations of Escherichia coli [78,79].
Experiment #2 (Environmental influences on costs of defense)—Nutrient environment did not appear to influence the expression of the costs of defense in either temperature environment. Nutrients may not have been limiting, even in the low nutrient environment. Further, while increased nutrients did increase flower production at 23˚C and in one replicate at 32˚C, flower production was relatively similar across both nutrient regimes and across both temperature environments. In comparison, flower production in the Experiment #1 (the one using larger pots) was twoto three-fold greater. This reduced flower production in the smaller pots in experiment #2 suggests that pot constraints had a greater effect on flower production than did nutrient treatment. Thus, while nutrient limitation has been shown to affect investment in defense [64,80-82], nutrient environment did not appear to affect the expression of the cost of defense. These findings are contrary to the results of reference [21], where nutrients significantly affected the expression of the cost of resistance with costs incurred under high-nutrient conditions.
Similar to Experiment #1, no cost of glucosinolate production was detected at 23˚C in Experiment #2. However, a cost of glucosinolate production was detected in one replicate test at 32˚C; plants from the LGL produced significantly more flowers than plants from the HGL at this temperature. Although not significant, the pattern in the second replicate showed the same trend. This pattern may be due to plants from the HGL investing more in defenses when faced with higher temperatures, i.e., environmental stress. Environmental stress has been shown to increase investment in defensive chemicals [18,27]. Specifically, broccoli grown at higher temperatures invests more in glucosinolate production [40]. Greater investment of limited resources in defense at higher temperatures would magnify defense costs and our ability to detect those costs. While this is similar to what was found in these same lines [13], it may also be due to the fact that individuals were grown alone [31]. Alternatively, turnover of glucosinolates may occur at a faster rate at higher temperatures because catabolic and anabolic processes typically increase with temperature [43]. So, more resources might have to be diverted from growth and reproduction to the maintenance of foliar glucosinolate content [42]. In addition, myrosinases are required to enzymatically alter glucosinolates into toxic isothiocyantes. Myrosinases are kept isolated from the glucosinolates in cellular compartments until damage is caused [83]. Because these cellular compartments also require maintenance, they may also require more resources for maintenance at higher temperatures. Moreover, temperature can affect plant respiration, transpiretion, and photosynthetic rates, as well as nutrient uptake [43,84], which may create a situation where resources are even more limiting, making investment in defense more costly. However, plants in this experiment were grown in individual pots and produced fewer flower that those in experiment #1. This might suggest that they were under more stress and the ability to detect costs might be higher. Yet, we only detected costs at the higher temperature treatment. Whether due to inherent costs at different temperatures or due to complex effects of temperature on plant physiology and growth, plant defense may be more costly at higher temperatures.
Our results suggest that the expression of costs and benefits of defense can be quite variable and complex. Detecting costs and benefits may be contingent upon the environment within which the individual is grown. While some effort has been spent examining how temperature affects investment in defense [32,40], little information exists concerning the effects of temperature on the expression of benefits and costs of defense. Both spatial and temporal variation in temperature may influence the cost to benefit ratio of defense within and across habitats. These temperature differences may maintain genetic variation for constitutive investment in defense for plant populations. Temperature variation may also favor individuals that show plasticity in the investment in defense [85, 86], with plants investing more in defense during the milder parts of the growing season or when established in cooler habitats. In any case, more attention should focus on how costs of chemical defense might be influenced by temperature because global temperatures are increasing. Our study suggests that benefits of glucosenolate production are not universal and that greater costs of constituitive glucosinolate production are incurred at higher temperatures, but the general importance of increasing temperature as a constraint on plant evolution of defense against herbivores remains unknown.
5. ACKNOWLEDGEMENTS
We are thankful to Denison University and Vassar College for allowing us to use their facilities for our research. A. Aldridge helped with data processing and analysis at UE. We thank the associate editor and all reviewers for valuable guidance during the review process. This research was partially supported by NSF grant DEB 0127369 to CGH.
![]()
![]()
REFERENCES
- Marquis, R.J. (1984) Leaf herbivores decrease fitness of a tropical plant. Science, 226, 537-539. doi:10.1126/science.226.4674.537
- Marquis, R.J. (1992) The selective impact of herbivores. In: Fritz, R.S. and Simms, E.L., Eds., Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics, The University of Chicago Press, Chicago, 310- 325.
- Fritz, R.S., Hochwender, C.G., Lewkiewicz, D.A., Bothwells, S. and Orians, C.M. (2001) Seedling herbivory by slugs in a willow hybrid system: Developmental changes in damage, chemical defense, and plant performance. Oecologia, 129, 87-97. doi:10.1007/s004420100703
- Hochwender, C.G., Sork, V.L. and Marquis, R.J. (2003) Fitness consequences of herbivory on Quercus alba. American Midland Naturalist, 150, 246-253. doi:10.1674/0003-0031(2003)150[0246:FCOHOQ]2.0.CO;2
- Kettenring, K.M., Weekley, C.W. and Menges, E.S. (2009) Herbivory delays flowering time and reduces fecundity of Liatris ohlingerae (Asteraceae), an endangered, endemic plant of the Florida scrub. The Journal of the Torrey Botanical Society, 136, 350-362. doi:10.3159/08-RA-113.1
- Paige, K.N. and Whitham, T.G. (1987) Overcompensation in response to mammalian herbivory: The advantage of being eaten. The American Naturalist, 129, 407-416. doi:10.1086/284645
- Becklin, K.M. and Kirkpatrick, H.E. (2006) Compensation through rosette formation: The response of scarlet gilia (Ipomosis aggregate: Polemoniaceae) to mammalian herbivory. Canadian Journal of Botany, 84, 1298-1303. doi:10.1139/b06-099
- Huttunen, L., Miemela, P., Peltola, H., Heiska, S., Rousi, M. and Kellomäki, S. (2007) Is a defoliated silver birch seedling able to overcompensate the growth under changing climate? Environmental and Experimental Botany, 60, 227-238. doi:10.1016/j.envexpbot.2006.10.010
- Simms, E.L. and Rausher, M.D. (1987) Costs and benefits of plant defense to herbivory. The American Naturalist, 130, 570-581. doi:10.1086/284731
- Simms, E.L. and Rausher, M.D. (1989) The evolution of resistance to herbivory in Ipomoea purpurea II. Natural selection by insects and cost of defense. Evolution, 43, 575-585. doi:10.2307/2409060
- Herms, D.A. and Mattson, W.J. (1992) The dilemma of plants: To grow or defend. Quarterly Review of Biology, 67, 283-335. doi:10.1086/417659
- Simms, E.L. (1992) Costs of plant resistance to herbivory. In: Fritz, R.S. and Simms, E.L., Eds., Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics, The University of Chicago Press, Chicago, 392- 425.
- Stowe, K.A. and Marquis, R.J. (2011) Costs of Defense: Correlated responses to divergent selection for foliar glucosinolate content in Brassica rapa. Ecology and Evolution, 25, 763-775. doi:10.1007/s10682-010-9443-9
- Chew, F.S. and Renwick J.A.A. (1994) Host plant choice in Pieris butterflies. In: Carde, R.T. and Bell, W.B., Eds., Chemical Ecology of Insects II, Chapman and Hall, New York, 214-238.
- Huang, X., Renwick, J.A.A., and Chew, F. (1995) Oviposition stimulants and deterrents control acceptance of Alliaria petiole by Pieris rapae and P. napi oleracea. Chemoecology, 6, 79-87. doi:10.1007/BF01259436
- Siemens, D.H. and Mitchell-Olds, T. (1996) Glucosinolates and herbivory by specialists (Coleoptera: Chrysomelidae. Lepidoptera: Plutellidae): Consequences of concentration and induced resistance. Environmental Entomology, 25, 1344-1353.
- Stowe, K.A. (1998) Realized defense of artificially selected line of Brassica rapa: Effects of quantitative genetic variation in foliar glucosinolate content. Environmental Entomology, 27, 1166-1174.
- Bergelson, J. and Purrington, C.B. (1996) Surveying patterns in the cost of resistance in plants. The American Naturalist, 14, 536-558. doi:10.1086/285938
- Purrington, C.B. (2000) Costs of resistance. Current Opinion in Plant Biology, 3, 305-308. doi:10.1016/S1369-5266(00)00085-6
- Korchieva, J. (2002) Meta-analysis of sources of variation in fitness costs of plant antiherbivore defense. Ecology, 83, 176-190. doi:10.1890/0012-9658(2002)083[0176:MAOSOV]2.0.CO;2
- Stevens, M.T., Waller, D.M. and Lindroth, R.L. (2007) Resistance and tolerance in Populus tremuloides: Genetic variation, costs, and environmental dependency. Ecology and Evolution, 21, 829-847. doi:10.1007/s10682-006-9154-4
- Coley, P.D., Bryant, J.P. and Chapin III, F.S. (1985) Resource availability and plant antiherbivore defense. Science, 230, 895-899. doi:10.1126/science.230.4728.895
- Asare, E. and Scarisbrick, D.H. (1995) Rate of nitrogen and sulphur fertilizers on yield, yield components and seed quality of oilseed rape (Brassica napus L.). Field Crop Research, 44, 41-46. doi:10.1016/0378-4290(95)00051-7
- Marak, H.B., Biere, A. and van Damme, J.M.M. (2003) Fitness costs of chemical defense in Plantago lanceolata L.: Effects of nutrient and competition stress. Evolution, 57, 2519-2530.
- Donaldson, J.R. and Lindroth, R.L. (2007) Genetics, environment, and their interaction determine efficacy of chemical defense in trembling aspen. Ecology, 88, 729- 739. doi:10.1890/06-0064
- Hendriks, R.J.J., Luijten, L. and van Groenendael, J.M. (2009) Context-dependent defence in terrestrial plants: The effects of light and nutrient availability on plant resistance against herbivory. Entomologia Experimentalis et Applicata, 131, 233-242. doi:10.1111/j.1570-7458.2009.00852.x
- Hirata, K., Asada, M., Yarani, E., Miyamoto, K. and Miura, Y. (1993) Affects of near-ultraviolet light on alkaloid production in Catharanthus roseues plants. Planta Medica, 59, 46-50.
- Dixon, R.A. and Paiva, N.L. (1995) Stress-induced phenylpropanoid metabolism. Plant Cell, 7, 1085-1097.
- Haugen, R., Steffes, L., Wolf, J., Brown, P., Matzner, S. and Siemens, D.H. (2008) Evolution of drought tolerance and defense: Dependence of tradeoffs on mechanism, environment and defense switching. Oikos, 117, 231-244. doi:10.1111/j.2007.0030-1299.16111.x
- Ramirez, C.C. and Verdugo, J.A. (2009) Water availability affects tolerance and resistance to aphids but not the trade-off between the two. Ecological Research, 24, 881- 888. doi:10.1007/s11284-008-0565-2
- Siemens, D.H., Garner, S.H. and Mitchell-Olds, T. (2002) Cost of defense in the context of plant competition: Brassica rapa may grow and defend. Ecology, 83, 505-517.
- Gershenzon, J. (1984) Changes in the levels of plant secondary metabolites under water and nutrient stress. Recent Advances in Phytochemistry, 18, 273-320.
- Hare, J.D., Elle, E. and van Dam N.M. (2003) Costs of glandular trichomes in Datura wrightii: A three year study. Evolution, 57, 793-805.
- Hare, J.D. and Elle, E. (2004) Survival and seed production of sticky and velvety Datura wrightii in the field: A five year study. Ecology, 85, 615-622. doi:10.1890/03-3069
- Francescangeli, N., Sangiacomo, M.A. and Marti, H.R. (2007) Vegetative and reproductive plasticity of broccoli at three levels of incident photosynthetically active radiation. Spanish Journal of Agricultural Research, 5, 389- 401.
- Zhang, H., Schonhof, I., Krumbein, A., Gutezeit, B., Li, L., Stützel, H. and Schreiner, M. (2008) Water supply and growing season influence glucosinolate concentration and composition in turnip root (Brassica rapa ssp. Rapifera L.). Journal of Plant Nutrition and Soil Science, 171, 255-265. doi:10.1002/jpln.200700079
- Qaderi, M.M., Kurepin, L.V. and Reid, D.M. (2006) Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiologia Plantarum, 128, 710-721. doi:10.1111/j.1399-3054.2006.00804.x
- Lincoln, D.E. and Langenheim, J.H. (1978) Effect of light and temperature on monoterpenoid yield and composition. Biochemical Systematics and Ecology, 6, 21-32. doi:10.1016/0305-1978(78)90021-2
- Plowman, A.B. and Richards, A.J. (1997) The effect of light and temperature on competition between atrzine 80, 583-590. doi:10.1006/anbo.1997.0496
- Schonhof, I., Blaring, H.-P., Krumbein, A., Claußen, W. and Schreiner, W. (2007) Effect of temperature increase under low radiation conditions on phytochemicals and ascorbic acid in greenhouse grown broccoli. Agriculture, Ecosystems, and Environment, 119, susceptible and resistant Brassica rapa. Annals of Botany, 103-111. doi:10.1016/j.agee.2006.06.018
- Jahangir, M., Abdel-Farid, I.B., Kim, H.K., Choi, Y.H. and Verpoorte, R. (2009) Healthy and unhealthy plants: The effects of stress on the metabolism of Brassicaceae. Environmental and Experimental Botany, 67, 23-33. doi:10.1016/j.envexpbot.2009.06.007
- Grubb, C.D. and Abel, S. (2006) Glucosinolate metabolism and its control. Trends in Plant Science, 11, 89-100. doi:10.1016/j.tplants.2005.12.006
- Salisbury, F.B. and Ross, C.W. (1985) Plant physiology. Wadsworth Publishing Co., Belmont.
- Louda, S.M. and Mole, S. (1991) Glucosinolates: Chemistry and ecology. In: Rosenthal, G.A. and Berenbaum, M.R., Eds., Herbivores, Their Interactions with Secondary Plant Metabolites, Academic Press, San Diego, 123- 163. doi:10.1016/B978-0-12-597183-6.50009-7
- Louda, S.M. (1987) Variation in methylglucosinolate and insect damage to Cleome serrulata (Capparaceae) along a natural soil moisture gradient. Journal of Chemical Ecology, 1, 569-581. doi:10.1016/B978-0-12-597183-6.50009-7
- Mauricio, R., Rausher, M.D. and Burdick, D.S. (1997) Variation in the defense strategies of plants: Are resistance and tolerance mutually exclusive? Ecology, 78, 1301-1311. doi:10.1890/0012-9658(1997)078[1301:VITDSO]2.0.CO;2
- Van Dam, N.M., Tygat, T.O.G. and Kirkland, J.A. (2009) Root and shoot glucosinolates: A comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochemistry Reviews, 8, 171-186. doi:10.1007/s11101-008-9101-9
- Steyermark, J.A. (1963) Flora of Missouri. The Iowa State University Press, Ames.
- USDA, NRCS (2010) PLANTS Profile: Brassica rapa L. http://plants.usda.gov/java/profile?symbol=BRRA
- United States Census Bureau. (2006) Daily mean temperature. http://www.allcountries.org/uscensus/408_normal_daily_mean_temperature_selected_cities.html
- Williams, P.H. and Hill, C.B. (1986) Rapid-cycling populations of Brassica. Science, 232, 1385-1389. doi:10.1126/science.232.4756.1385
- Borror, D.J., Triplehorn, C.A. and Johnson, N.F. (1989) An introduction to the study of insects. Saunders, Philadelphia.
- Shorey, H.H. and Hale, R.L. (1965) Mass-rearing of the larvae of nine Noctuid species on a simple artificial medium. Journal of Economic Entomology, 58, 522-524.
- Conner, J.K., Rush, S. and Jennetten, P. (1996) Measurements of natural selection on floral traits in wild radish (Raphanus raphanistrum). I. Selection through lifetime female fitness. Evolution, 50, 1127-1136. doi:10.2307/2410653
- Strauss, S., Conner, J.K. and Rush, S.L. (1996) Foliar herbivory affects floral characters and plant attractiveness to pollinators: Implications for male and female fitness. The American Naturalist, 147, 1098-1107. doi:10.1086/285896
- Strauss, S.Y., Siemens, D.H., Decher, M.B. and MitchellOlds, T. (1999) Ecological costs of plant resistance to herbivores in the currency of pollination. Evolution, 53, 1105-1113. doi:10.2307/2640815
- Strauss, S., Rudgers, J.A., Lau, J.A. and Irwin, R.E. (2002) Direct costs of resistance to herbivory. Trends in Ecology and Evolution, 17, 278-285. doi:10.1016/S0169-5347(02)02483-7
- JMP Version 5 (2005) SAS Institute Inc., Cary.
- Cipollini, D.F. (2002) Variation in the expression of chemical defenses in Alliaria petiole (Brassicaeae) in the field and common garden. American Journal of Botany, 89, 1422-1430. doi:10.3732/ajb.89.9.1422
- [61] Cipollini, D.F., Busch, J.W., Stowe, K.A., Simms, E.L. and Bergelson, J. (2003) Genetic variation and relationships of constitutive and herbivore-induced glucosinolates, trypsin inhibitors, and herbivore resistance in Brassica rapa. Journal of Chemical Ecology, 29, 285-302. doi:10.1023/A:1022673726325
- [62] Stowe, K.A. (1998) Experimental evolution of resistance in Brassica rapa: Correlated response of tolerance in lines selected for glucosinolate content. Evolution, 52, 703-712. doi:10.2307/2411265
- [63] Simms, E.L. and Triplett, J. (1994) Costs and benefits of plant responses to disease: Resistance and tolerance. Evolution, 48, 1973-1985. doi:10.2307/2410521
- [64] Lankau, R.A. and Strauss, S.Y. (2008) Community complexity drives patterns of natural selection on a chemical defense of Brassica rapa. The American Naturalist, 171, 150-161. doi:10.1086/524959
- [65] Cipollini, D.F. and Bergelson, J. (2002) Interspecific competition affects growth and herbivore damage of Brassica napus in the field. Plant Ecology, 162, 227-231. doi:10.1023/A:1020377627529
- [66] Glawe, G.A., Zavala, J.A., Kessler, A., van Dam, N.M. and Baldwin, I.T. (2003) Ecological costs and benefits correlated with trypsin inhibitor production in Nicotiana attentuata. Ecology, 84, 79-90. doi:10.1890/0012-9658(2003)084[0079:ECABCW]2.0.CO;2
- [67] Nuñez-Faran, J., Fornoni, J. and Valverde, P.L. (2007) The evolution of resistance and tolerance to herbivores. Annual Review of Ecology, Evolution, and Systematics, 38, 541-566. doi:10.1146/annurev.ecolsys.38.091206.095822
- [68] Agren, J. and Schemske, D.W. (1993) The cost of defense against herbivores: An experimental study of trichome production in Brassica rapa. The American Naturalist, 141, 338-350. doi:10.1086/285477
- [69] Agren, J. and Schemske, D.W. (1994) Evolution of trichome number in a naturalized population of Brassica rapa. The American Naturalist, 143, 1-13. doi:10.1086/285593
- [70] Windle, P.N. and Franz, E.H. (1979) The effects of insect parasitism on plant competition: Greenbugs and barley. Ecology, 60, 521-529. doi:10.2307/1936072
- [71] Cheplick, G.P., Clay, K. and Marks, S. (1989) Interactions between infection by endophytic fungi and nutrient limitation in grasses Lolium perenne and Festuca arundinaceae. New Phytologist, 111, 89-97. doi:10.1111/j.1469-8137.1989.tb04222.x
- [72] Rousi, M. (1988) Resistance breeding against voles in birch: Possibilities for increasing resistance by provenance transfers. European and Mediterranean Plant Protection Organization Bulletin, 18, 257-263.
- [73] Rousi, M. (1989) Susceptibility of winter-dormant Pinus sylvestris families to vole damage. Scandinavian Journal of Forest Research, 4, 149-161. doi:10.1080/02827588909382554
- [74] Sagers, C.L. and Coley, P.D. (1995) Benefits and costs of defense in a neotropical shrub. Ecology, 76, 1835-1843. doi:10.2307/1940715
- [75] Preisser, E.L., Gibson, S.E., Adler, L.S. and Lewis, E.E. (2007) Underground herbivory and the cost of constitutive defense in tobacco. International Journal of Ecology, 31, 210-215.
- [76] Ivey, C.T., Carr, D.E. and Eubanks, M.D. (2009) Genetic variation and constraints on the evolution of defense against spittlebug (Philaenus spumarius) herbivory in Mimulus guttatus. Heredity, 10, 303-311. doi:10.1038/hdy.2008.122
- [77] McKenzie, J.A., Whitten, M.J., and Adena, M.A. (1982) The effect of genetic background on the fitness of diazinon resistance genotypes of the Australian sheep blowfly, Lucilia cuprina. Heredity, 49, 1-9. doi:10.1038/hdy.1982.60
- [78] McKenzie, J.A. and Purvis, A. (1984) Chromosomal localization of fitness modifiers of diazinon resistance genotypes of the Australian sheep blowfly, Lucilia cuprina. Heredity, 53, 625-634. doi:10.1038/hdy.1984.120
- [79] Lenski, R.E. (1988) Experimental studies of pleiotropy and epistasis in Escherichia coli. I. Variation in competitive fitness among mutants resistant to virus T4. Evolution, 42, 425-432. doi:10.2307/2409028
- [80] Lenski, R.E. (1988) Experimental studies of pleiotropy and epistasis in Escherichia coli. II. Compensation for maladaptive effects associated with resistance to virus T4. Evolution, 42, 433-440. doi:10.2307/2409029
- [81] Bezemer, T.M., Jones, T.H. and Newington, J.E. (2000) Effect of carbon dioxide and nutrient fertilization on phenolic content in Poa annua L. Biochemical Systematics and Ecology, 28, 839-846. doi:10.1016/S0305-1978(99)00130-1
- [82] Coviella, C.E., Stipanovic, R.D. and Trumble, J.T. (2002) Plant allocation to defensive compounds: Interactions between elevated CO2 and nitrogen in transgenic cotton plants. Journal of Experimental Botany, 53, 323-331. doi:10.1093/jexbot/53.367.323
- [83] Prudic, K.L., Oliver, J.C. and Bowers, D.M. (2005) Soil nutrient effects on ovipositon preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia, 143, 578-587. doi:10.1007/s00442-005-0008-5
- [84] Kliebenstein, D.J., Kroymann, J. and Mitchell-Olds, T. (2005) The glucosinolate-myrosinase system in an ecological and evolutionary context. Current Opinion in Plant Biology, 8, 263-271. doi:10.1016/j.pbi.2005.03.002
- [85] Campbell, N.A. and Reece, J.B. (2005) Biology. Benjamin Cummings, San Francisco.
- [86] Agerbirk, N., Olsen, C.E. and Neilsen, J.K. (2001) Seasonal variation in leaf glucosinolate and insect resistance in two types of Barbarea vulgaris spp. arcuata. Phytochemistry, 58, 91-100. doi:10.1016/S0031-9422(01)00151-0
- [87] Gols, R., Raaijmakers, C.E., van Dam, N.M., Dicke, M., Bukovinsky, T. and Harvey, J.A. (2007). Temporal changes affect plant chemistry and tritrophic interactions. Basic and Applied Ecology, 8, 421-433. doi:10.1016/j.baae.2006.09.005

