Advances in Biological Chemistry
Vol. 2 No. 4 (2012) , Article ID: 24820 , 6 pages DOI:10.4236/abc.2012.24052
Measuring phosphatidic acid phosphohydrolase (EC 3.1.3.4) activity using two phosphomolybdate-based colorimetric methods
![]()
Southern Regional Research Center, Agricultural Research Service, USDA, New Orleans, USA
Email: *abul.ullah@ars.usda.gov
Received 12 October 2012; revised 15 November 2012; accepted 21 November 2012
Keywords: Phosphatidic Acid Phosphohydrolase; Fatty Acid Metabolism; Diacyl Glycerol; Inorganic Orthophosphate Measurement
ABSTRACT
Phosphatidic acid phosphohydrolase (3-sn-phosphatidate phosphohydrolase, EC 3.1.3.4), also known as PAP, catalyzes the dephosphorylation of phosphatidic acid (PtdOH) to form diacylglycerol (DAG) and inorganic orthophosphate. In eukaryotes, the PAP driven reaction is the committed step in the synthesis of triacylglycerol (TAG). Existing methods for measuring PAP activity rely on the use of radioactive PtdOH. These methods are costly and cumbersome. In this report, we describe a simple assay procedure to measure released inorganic orthophosphate, which is a co-product of the PAP reaction. Each molecule of PtdOH would release one molecule of DAG and one molecule of inorganic orthophosphate (Pi) when subjected to enzymatic breakdown under optimal conditions. Given the published rates of in vitro PAP enzymatic activity from various sources, we proposed that colorimetric determination of released Pi is possible. With this view, we performed in vitro PAP activity assays using freshly isolated enzyme from bitter gourd, Momordica charantia, and measured the released Pi using two spectrophotometric methods. Both methods gave about 2.0 to 2.25 ηkat per mg of protein. Thus, it is now possible to perform PAP activity using a simple procedure that uses nonradioactive substrates, provided the sample is dialyzed extensively to lower the intrinsic concentration of free phosphate. The kinetics data presented in this study is comparable to that of other PAP enzymes reported elsewhere, which gives credence to the notion that non-radioactive methods can be used to perform PAP activity.
1. INTRODUCTION
Oil synthesis in oleaginous plants takes place in developing seed with the aid of a host of lipid biosynthetic enzymes of which phosphatidic acid phosphohydrolase (PAP, 3-sn-phosphatidate phosphohydrolase, EC 3.1.3.4) is of paramount importance. This enzyme cleaves the phosphomonoester bond present in phosphatidic acid (PtdOH) yielding diacylglycerol (DAG) and Pi [1,2]. The phosphohydrolase reaction catalyzed by this enzyme is represented in Figure 1.
In the Kennedy pathway of TAG synthesis the formation of DAG is the penultimate step. DAG is not only essential for TAG formation, but is also a substrate in the synthesis of the phospholipids phosphatidylethanolamine and phosphatidylcholine [3,4]. PAP acts as a pivotal biocatalyst in the metabolic flux between the different classes of glycerolipid within endoplasmic reticulum. PAP has also been shown to play an important role in the phospholipase D signaling pathway [5,6]. PAP is present not only in microbes and plants but also in animals; recent studies have shown that human lipin 1 is PAP [7]. Moreover, the lipin 1 deficiency in mouse prevents normal adipose tissue development, which results in lipodystrophy—a disease that results in the loss of body fat and insulin resistance; conversely, lipin 1 promotes obesity and insulin sensitivity [8,9]. Lipin 1, a PAP enzyme,

Figure 1. The schematic representation of enzymatic reaction carried out by phosphatidic acid phosphohydrolase (PAP). Phosphatidic acid yields one molecule of DAG and one molecule of inorganic orthophosphate (Pi) when the phospho-ester linkage is hydrolyzed by PAP.
regulates lipid metabolism in mammalian cells; therefore, the biocatalyst could become a target for drug development in combating fat related diseases in humans.
In the biochemical scheme for TAG assembly in developing seeds of oleaginous plants, the formation of DAG from phosphatidic acid by removal of Pi is considered a crucial step [10]. If PAP does not function optimally, it could create a bottleneck for TAG synthesis. Therefore, it follows that the knowledge base of general lipid metabolism will benefit from a more thorough understanding of the enzymatic properties of PAP.
Both pharmaceutical and basic plant science research would benefit from a large-scale search for inhibitors and activators of PAP. This search necessitates a sensitive and speedy enzymatic assay. The radioactive assays [11,12] that are currently being used to measure PAP activity are not only expensive but also not conducive to highthroughput screening of potential molecules that control phosphohydrolase activity. Apart from the fact that handling of radionuclide associated with the assay makes the measurements a bit cumbersome, the procedure requires a chloroform-methanol-water phase partition to separate water-soluble 32Pi from chloroform-soluble [32P]PtdOH or thin layer chromatography (TLC) separation of DAG from the substrate, PtdOH [11,12].
A non-radioactive enzyme assay for a pure Mg2+- dependent PAP was reported in which a malachite green-molybdate reagent was used to measure the released Pi. The reagent produced a colored complex with Pi that could be measured using a spectrophotometer at 660 nm [13]. However, the reported colorimetric method is not suitable for use when cell extracts or crude PAP preparation are being assayed due to a high Pi background. The method was reported to be suitable for measureing only purified PAP1 activity where Pi background was substantially low.
The colorimetric methods that we are reporting here, however, could be used to measure PAP activity even in crude samples, provided a rapid dialysis step is incorporated to remove inorganic orthophosphate from the protein extracts prior to carrying out the assay. The method was successfully used in our laboratory to measure PAP activity in protein extracts of the developing cotyledons of three cucurbits, namely, Momordica charantia, Luffa cylindrica, and Lagenaria siceraria. However, we report here the results of the experiments performed with Momordica charantia cotyledon extracts.
2. EXPERIMENTAL PROCEDURES
2.1. Source of PAP
Bitter gourd, Momordica charantia (Indian type variety) was purchased from an oriental grocery in the New Orleans, Louisiana area. The fruits were at a mid-maturity stage, typically about 6 inch in length.
2.2. Extraction of Soluble PAP from Momordica charantia Developing Cotyledons
The seeds (5.0 g) were obtained from the seed cavity of Momordica charantia fruit and washed with 0.9% ice cold saline solution. The outer coverings of the seeds were removed at room temperature using a scalpel and the cotyledons were removed manually. All subsequent operations were carried out at 4˚C.
A 5.0 mL aliquot of extraction buffer (50 mM acetate, 150 mM sodium chloride and 10 mM MgCl2, pH 5.0) was added to the seeds and homogenized using a Tekmar Tissumizer MarkII (Cincinnati, OH) tissue disruptor sequentially at low, medium and high speed for 30 sec at each speed while keeping the sample in an ice bath. The homogenate was allowed to cool on ice for 1 min between bursts. The sample was then centrifuged at 20,000 × g for 30 min using Sorvall RC-5B centrifuge (Miami, FL). The pellet, containing unbroken cotyledons and other tissue debris, was discarded.
The resulting supernatant was dialyzed overnight against imidazole buffer (50 mM imidazole, 1 mM MgCl2, pH 6.0) with three buffer changes of 500 mL each. The resulting supernatant became cloudy after dialysis due to isoelectric precipitation of proteins, which was removed by centrifugation as mentioned above. The supernatant containing 1.69 mg protein per ml, which was estimated by using Coomassie Plus™ protein assay reagent (Thermo Scientific, Rockford, IL), was used for all subsequent PAP activity measurement and characterization.
2.3. Measurement of PAP-Catalyzed Pi Release
Two methods were used to measure ηmoles of Pi released by Momordica charantia PAP from the substrate, dioleoyl-phosphatidate (1,2-dioleoyl-sn-glycero-3-phosphate, sodium salt), which was obtained from Avanti Polar Lipids, Inc. (Alabaster, Alabama). These methods were designated as molybdenum blue or MB method [14, 15] and ammonium molybdate-based acetone-molybdateacid or AMA method [16,17]. The enzyme unit, kat, is defined as mole substrate converted per sec.
For the AMA method, the reaction was stopped by the addition of 2 mL AMA reagent [acetone, 10 mM ammonium molybdate, 5 N sulfuric acid mixture, 2:1:1 (vol/ vol/vol)] followed by 100 mL of 1 M citrate solution. The resulting turbidity was removed by centrifugation at 12,000 × g for 6 min using Eppendorf Model 5415C centrifuge (Westbury, NY) at room temperature. The absorbance of the yellow color developed was read at 355 nm employing Bio-Rad × Mark Microplate Spectrophotometer (Hercules, CA).
For the MB method , the enzymatic reaction was typi-

Table 1. Summary of Momordica charantia PAP activity measurements using two methods.
cally incubated at 37˚C for 30 min after which it was terminated by the addition of 2 mL freshly prepared MB reagent [1 M sulfuric acid, 2.5% ammonium heptamolybdate, and 10% ascorbic acid, (3:1:1, vol/vol/vol)]. The mixture was incubated at 50˚C for 20 min for color development. The resulting turbidity of the final mixture was then removed by centrifugation as described for the AMA method above. The absorbance of the blue color was read at 820 nm using the microplate spectrophotometer described above. For both methods, control reactions contained all the components of the PAP assay, but the reactions were terminated at zero time. The controls were used to zero the absorbance of the plate reader.
3. RESULTS
3.1. Measurement of PAP Activity
The phospho-hydrolytic activity of PAP from Momordica charantia that results in the cleavage of PA into DAG and Pi was determined by both AMA and MB methods. A triplicate activity measurements gave 2.25 ηkat or 135 ηmoles Pi liberated per min per mg of protein for the MB method and 2.07 ηkat or 124 ηmoles Pi liberated per min per mg of protein for the AMA method at pH 6.0 and 37˚C. Thus, the MB method measured 8.6% more activity than the AMA method. When incubations were carried out for 30 min, one of the products of PAP-catalyzed reaction, Pi, was generated in sufficient amounts (315 ηmoles under AMA method and 102 ηmoles under MB method), to allow for spectrophotometric quantitation. Table 1 summarizes the results as determined by both methods. The detection range of the AMA method was about three times higher than the MB method; therefore, less enzymes were used in the MB method. The AMA method offered the advantage of being simpler than the MB method, because no post-reaction treatment was needed to develop the color.
3.2. Pi Measurement versus PAP Concentration
To show that release of Pi from PtdOH increases as a function of PAP concentration, experiments were performed with variable amounts of soluble protein from Momordica charantia cotyledons. The results are shown in Figure 2 for the AMA method and Figure 3 for the MB method. About 37 ηmoles of Pi was released in 30 min at pH 6.0˚C and 37˚C when PAP assay was conducted with 5 µL of crude extract. This value increased to 244 ηmoles when 40 µL of crude extract was assayed for identical lengths of time at the same pH and temperature (Figure 2).
Likewise, in the MB method, 26 ηmoles of Pi was released from the substrate in 30 minutes at pH 6.0 and 37˚C when the assay was conducted with 4 µL of crude extract. This value increased to 67.9 ηmoles when 10 µL of crude extract was assayed for identical time at the same pH and temperature (Figure 3). When the assay was conducted for 30 min with 10 µL of crude extract at 37˚C, both the method gave a similar value, which is 68 ηmoles of Pi released from the substrate. Data analysis revealed that approximately three times more PAP activity can be assayed using the AMA method compared to the MB method (Figures 2 and 3). This is due solely to the sensitivity of the two reagents for binding Pi. In the case of the AMA method, 1 O.D. at 355 nm represents about 360 ηmoles of Pi, whereas, for the MB method, 1 O.D. at 820 nm represents approximately 120 ηmoles of Pi.
3.3. Measurement of Pi Released by PAP as a Function of Time
To investigate the rate linearity of PAP activity as a function of time, the released Pi was measured by both AMA and MB methods. The results are shown in Figures 4 and 5. Because of the higher sensitivity of Pi detection using the AMA method, 50 µL of enzyme was used
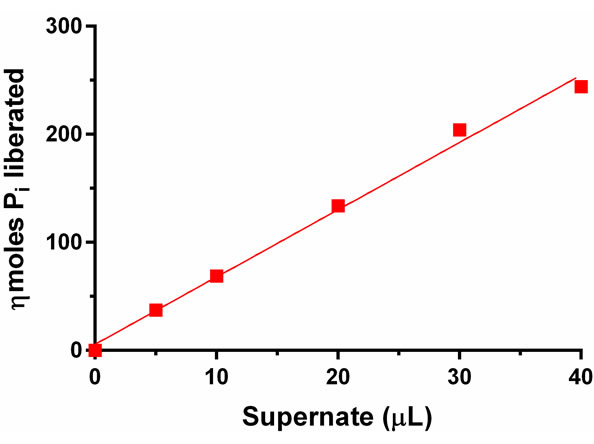
Figure 2. PAP catalyzed liberation of Pi as a function of increasing amounts of supernatant derived from developing cotyledons of Momordica charantia. Liberated Pi was measured by AMA method. The standard deviation was ±5.4 per cent of the average of nkat per mg of protein (n = 6).

Figure 3. PAP catalyzed liberation of Pi as a function of increasing amounts of supernatant derived from developing cotyledons of Momordica charantia. The liberated Pi was measured by MB method. The standard deviation was ±3.8 per cent of the average of nkat per mg (n = 5).
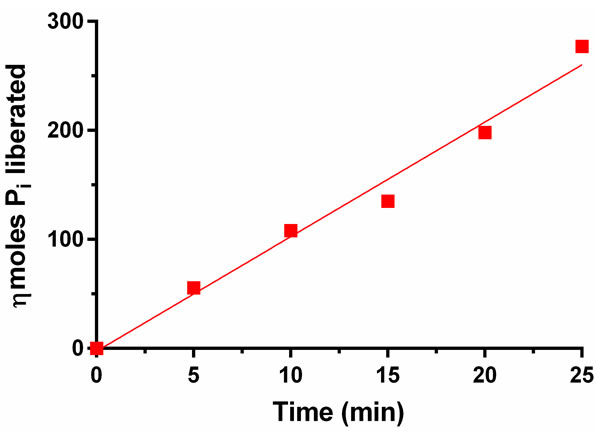
Figure 4. PAP catalyzed liberation of Pi as a function of incubation time. The liberated Pi was measured by AMA method. The standard deviation was ±8.1 per cent of the average of nkat per mg (n = 6).
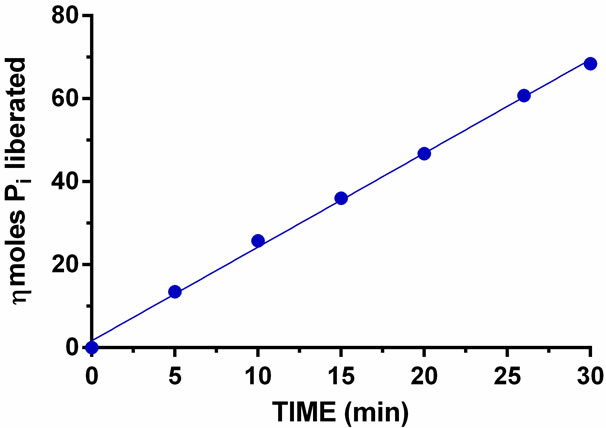
Figure 5. PAP catalyzed liberation of Pi as a function of incubation time. The liberated Pi was measured by MB method. The standard deviation was ±1.9 percent of the average of nkat per mg (n = 6).
whereas, 10 µL sample was used to perform the time course study with the MB method. The enzyme released Pi linearly throughout the 30 min course as determined by both methods; however, the MB method gave a nearly straight line (Figure 5). Under the AMA method, a higher level of Pi could be detected as compared to the MB method, which indicates that the AMA method is more sensitive than the MB method.
4. DISCUSSION
In this communication we reported two colorimetric methods to measure PAP activity. The enzyme liberated DAG and inorganic orthophosphate (Pi) when the phosphomonoester bond present in phosphatidic acid was hydrolyzed. The liberated Pi reacted with molybdate under acidic conditions to absorb at 355 nm in presence of acetone and at 820 nm in presence of ascorbic acid. Table 1 summarizes the activity measurements performed by Momordica charantia phosphatidic acid phosphohydrolase as determined by both AMA and MB methods. Additional data were included in the table to reflect the detection range of each method, the heating requirement for color generation in the MB method, and the amounts of sample needed to perform the PAP assay for each method.
While both methods could measure released Pi resulting from phosphohydrolase activity, the AMA method was able to measure up to 500 ηmoles of Pi, compared to 150 ηmoles for the MB method (Table 1). In this respect the AMA method was deemed to be superior to the MB method. Furthermore, the MB method requires an additional 20 min, 50˚C incubation step after the addition of color-developing reagent (sulfuric acid-ammonium hepta molybdate-ascorbic acid); this step is not necessary when using the AMA method.
A HPLC-fluorescence detection method for the determination of phosphatidic acid phosphohydrolase activity was reported in which the unreacted substrate was separated from the product by HPLC and the latter was detected by fluorimetry [18]. The method is not as simple or cost-effective as the one we are reporting in this communication.
The colorimetric method we described here was found to be as sensitive as other colorimetric [19,20] and radioactive methods [21] in determining low ηmole quantities of either Pi or DAG generated by PAP.
In summary, we described here two colorimetric methods to measure Pi released by PAP from 1,2-dioleoyl-sn-glycero-3-phosphate, a commonly used representtative form of PtdOH. The level of activity as determined by these methods compare favorably with radioactive PAP activity assay methods [21,22]. Therefore, non-radioactive phosphatidic acid could be used as the substrate in place of its radioactive counterpart to measure this important catalytic reaction. The method reported here is also substantially faster, as the cumbersome TLC separation and scintillation counting steps common to the radioactive methods are avoided. The use of non-radioactive PtdOH will allow for analysis of a wider range of potential PtdOH substrates compared to the limited number of commercially available radiolabeled PtdOH species. Furthermore, the colorimetric procedure we detailed here is also a substantially more cost-effective than the radioactive methods.
5. CONCLUSION
In this article, we described two simple spectrophotometric methods to measure inorganic orthophosphate, which is a co-product of phosphatidic acid phosphohydrolase (PAP) reaction. Each molecule of PtdOH, the substrate, would release one molecule of diacylglycerol (DAG) and one molecule of inorganic orthophosphate (Pi) when subjected to enzymatic breakdown under optimal conditions. Given the published rates of in vitro PAP enzymatic activity from various sources, we proposed that colorimetric determination of released Pi is possible. With this view, we performed in vitro PAP activity assays using freshly isolated enzyme from bitter gourd, Momordica charantia, and measured the released Pi using two spectrophotometric methods. Both methods gave about 2.0 to 2.25 ηkat per mg of protein. Thus, it is now possible to perform PAP activity assays using a simple procedure that uses nonradioactive substrates, provided the sample is dialyzed extensively to lower the intrinsic concentration of free phosphate. The kinetics data presented in this study is comparable to that of other PAP enzymes reported elsewhere, which gives credence to the notion that non-radioactive methods can also be used to perform PAP activity, facilely.
![]()
![]()
REFERENCES
- Smith, S.W., Weiss, S.B. and Kennedy, E.P. (1957) The enzymatic dephosphorylation of phosphatidic acids. Journal of Biological Chemistry, 228, 915-922.
- Carman, J.M. (1997) Phosphatidate phosphatases and diacylglycerol pyrophosphate phosphatases in Saccharomyces cerevisiae and Escherichia coli. Biochimica et Biophysica Acta, 1348, 45-55. doi:10.1016/S0005-2760(97)00095-7
- Kocsi, M.G. and Weselake, R.J. (1996) Phosphatidate phosphatases of mammals, yeast, and higher plants. Lipids, 31, 785-802. doi:10.1007/BF02522974
- Nanjundan, M. and Possmayer, F. (2003) Pulmonary phosphatidic acid phosphatase and lipid phosphate phosphohydrolase. American Journal of Physiology Lung Cell Molecular Physiology, 284, L1-L23.
- Exton, J.H. (1990) Signaling through phosphatidylcholine breakdown. Journal of Biological Chemistry, 265, 1-4.
- Billah, M.M. and Anthes, J.C. (1990) The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochemical Journal, 269, 281-291.
- Hans, G.S., Wu, W.I. and Carman, G.M. (2006) The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. Journal of Biological Chemistry, 281, 9210-9218.
- Peterfy, M., Phan, J., Xu, P. and Reue, K. (2001) Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nature Genetics, 27, 121-124. doi:10.1038/83685
- Phan, J. and Reue, K. (2005) Lipin, a lypodystrophy and obesity gene. Cell Metabolism, 1, 73-83. doi:10.1016/j.cmet.2004.12.002
- Carman, G. and Han, G.S. (2009) Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. Journal of Biological Chemistry, 284, 2593-2597. doi:10.1074/jbc.R800059200
- Carman, G.M. and Lin, Y.P. (1991) Phosphatidate phosphatase from yeast. Methods in Enzymology, 197, 548- 553. doi:10.1016/0076-6879(91)97182-X
- Martin, A., Gomez-Munoz, A., Jamal, Z. and Brindley, D.N. (1991) Characterization and assay of phosphatidate phosphatase. Methods in Enzymology, 197, 553-563. doi:10.1016/0076-6879(91)97183-Y
- Habriluk, T., Lozy, F., Siniossoglou, S. and Carman, G.M. (2008) Colorimetric determination of pure Mg2+-dependent phosphatidate phosphatase activity. Analytical Biochemistry, 373, 392-394. doi:10.1016/j.ab.2007.08.037
- Chen, P.S., Toribara, J.T.Y. and Warner, H. (1956) Microdetermination of phosphorus. Analytical Chemistry, 28, 1756-1758. doi:10.1021/ac60119a033
- Weaver, J.D., Ullah, A.H.J., Sethumadhavan, K., Mullaney, E. J. and Lei, X.G. (2009) Impact of assay conditions on activity estimate and kinetics: Comparison of Aspergillus niger PhyA and Escherichia coli AppA2 phytases. Journal of Agricultural and Food Chemistry, 57, 5315-5320. doi:10.1021/jf900261n
- Heinonen, J.K. and Lahti, R. J. (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Analytical Biochemistry, 113, 313-317. doi:10.1016/0003-2697(81)90082-8
- Ullah, A.H.J., Sethumadhavan, K. and Mullaney, E.J. (2005) Monitoring of unfolding and refolding in fungal phytase (PhyA) by dynamic light scattering. Biochemical Biophysical Research Communications, 327, 993-998. doi:10.1016/j.bbrc.2004.12.111
- Burgdorf, C., Prey, A., Richardt, G. and Kurz, T. (2008) A HPLC-fluorescence detection method for determination of phosphatidic acid phosphohydrolase activity: Application in human myocardium. Analytical Biochemistry, 374, 291-297. doi:10.1016/j.ab.2007.10.039
- Moore, T.S., Lord, J.M., Kagawa, T. and Beevers, H. (1973) Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiology, 52, 50-53. doi:10.1104/pp.52.1.50
- Ichihara, K., Norikura, S. and Fujii, S. (1989) Microsomal phosphatidate phosphatase in maturing safflower seeds. Plant Physiology, 90, 413-419. doi:10.1104/pp.90.2.413
- Pearce, M.L. and Slabas, A.R. (1989) Phosphatidate phosphatase from avocado (Persea Americana)—Purification, substrate specificity and possible metabolic implications for the Kennedy pathway and cell signaling in plants. Plant Journal, 14, 555-564. doi:10.1046/j.1365-313X.1998.00152.x
- Eastmond, P.J., Quettier, A.-L., Kroon, J.T.M., Craddock, C., Adams, N. and Slabas, A.R. (2010) Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell, 22, 2796-2811.
NOTES
*Corresponding author.

