Journal of Water Resource and Protection
Vol. 2 No. 6 (2010) , Article ID: 2117 , 5 pages DOI:10.4236/jwarp.2010.26068
Observation on Poterioochromonas sp. (Chrysophyte)
1State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology Chinese
Academy of Sciences, Wuhan, China
2CCCC Second Harbor Consultants Co. Ltd, Wuhan, China
E-mail: guoshj@gmail.com, lrsong@ihb.ac.cn
Received March 10, 2010; revised April 25, 2010; accepted April 30, 2010
Keywords: Microstructure, Ultrastructure, Poterioochromonas sp., Microcystis Aeruginosa
ABSTRACT
Poterrioochromonas sp., isolated from Microcystis cultures in 2002, was described with LM, SEM, TEM. The grazing characteristics of this strain were also observed in laboratory experiments. The results showed that this strain has the representative features of the genus except for the lorica, and the most conspicuous feature of Poterioochromonas sp. was about the chromatophores.
1. Introduction
Golden alga is an important component of plankton in the water ecology. Some species have characteristics of plant nutrition, such as chromatophore (chloroplast) or special product of assimilation (chrysolaminaran, et al.). So they are called as “chrysophytes” by botanists. However, some species have two flagella and can swim, and depend predominantly on phagotrophic nutrition without chromatophore which characterizes the phagotrophic nutrition. Thus some zoologists term them as “chrysomonads”, which is an important part of protozoa. Additionally, some species have both the characteristics of the plant nutrition (photosynthesis) and the phagotrophic nutrition, i.e., they are mixotrophy. Although osmotrophy (dissolved) and phagotrophy (particulate), is observed in many algae, it is the use of particulate food that has generated most interest and to which the term mixotrophy usually applies [1].
Since Pringsheim [2] first reported that a Ochromonas ingested small algae, the phenomenon of ingestion of phytoplankters by chrysomonads has been widely recognized. Some genus of chrysomonads are capable of grazing blue-green algae (Anacystis and Microcystis) and green algae (Chlorella, Chlamydomonas and Carteria), and diatom (Achnanthes) [3-6]. These observations suggest that the ingestion of algae by mixotrophic chrysomonads is common. Our research group got a species of golden alga, isolated from Microcystis cultures in 2002. This alga could grow not only by ingestion and digestion of Microcystis, but also in phototrophic condition at the same time, which is described as mixotrophy. Mixotrophic algae are common in most aquatic ecosystems and, when numerically dominant, they depend significantly on phagotrophy [7,8]. Because of their small size and high metabolic rate they may also be important in the regeneration of nutrients [9], and thus an understanding of their nutritional characteristics is significant.
The golden alga we got was identified as Poterioochromonas sp., a strain that is phylogenetically close to Poterioochromonas malhamensis (99% similarity) by 18s rDNA (GenBank Accession No.AY699607) [10]. In this paper, we investigated the growth and ingestion characteristics of this strain, including the biological morphological features under different growth conditions.
2. Materials and Methods
Poterioochromonas sp. was isolated from the mass culture of Microcystis aeruginosa in our lab in 2002. A clone culture was established by picking up single cells with micropipettes.
1) Cultures were grown in a flask with sterile BG-11 medium at 22℃ under an illumination of ca. 25 μmol photons·m-2·s-1 with a photoperiod of 12 h : 12 h (Light: Dark) from daylight fluorescent lamps.
2) Feeding Poterioochromonas sp. in a relative low ratio of 3:1 (prey : predator) every two days for a week, the initial condition of the predator is 106 mL-1.
3) Inoculating low densities (ca. 103 mL-1) of Poterioochromonas sp. into the cultures containing approximately 106 or 107 mL-1 M. aeruginosa FACHB469.
We often sampled and observed the organism with an olympus CX 41 light microscope (LM).
2.1. Surface and Ultrastructure of Poterioochromonas sp.
For SEM (Scanning electron microscope), cells were fixed for 2 hours in 2.5% glutaraldehyde at room temperature, one drop of cells were placed on little glass slides, which coated with 0.1% poly-L-Lysine, dried for 30 min, and subsequently washed three times (10 min each) in 0.1 M phosphate buffer, pH 7.0. After three 10- min rinse in ultrapure water, samples were dehydrated through 50%, 70%, 80%, 90%, 95% and 100% ethanol (5 min each stage) and then 1:1 (ethanol : isoamyl acetate) for 10 min at room temperature, and used the critical point drying in a HITACHI HCP-2 apparatus after replaced by pure isoamyl acetate. The slides containing the algae were then mounted on stubs and coated with gold in a GIKO ID-3 sputter coater. Coated specimens were examined with HITACHI S-3000N SEM.
For TEM (Transmission Electron Microscopy), Poterioochromonas cells were harvested by gentle centrifugation. Cells were washed 2 times with PBS (pH = 7.0, 0.1 M), fixed with 2.5% glutaraldehyde, and then put in 1% OsO4 for 2 hours at room temperature. After graded ethanol dehydration, samples were embedded in EPOXY epon-812 and polymerized at 70℃ for 8 hours. Sections were cut, stained with uranyl acetate and lead citrate, and then examined with a HITACHI H-600 TEM.
3. Results
The general appearance of Poterioochromonas sp., as observed with LM, is illustrated in Figure 1 and Figure 2.

Figure 1. Photomicrographs of Poterioochromonas sp. in autotrophy.
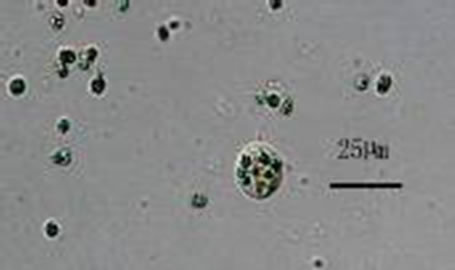
Figure 2. Photomicrographs of Poterioochromonas sp. in mixotrophy.
We found that the autotrophic cells, with two unequal long flagella and two yellow-green chromatophores, are spherical to elongate, and approximately 5-10 µm in di-ameter. We did not see any eye-spot. They could not only photosynthesize with chromatophores, but also efficiently digest the preys. And the volume of the predator could become larger when there were prey(s) in the cell. The diameter of the largest cells can reach 25 µm. The size and color of the chromatophores would change a lot during the Poterioochromonas cell ingested and digested preys. We only noted that the cells reproduce asexually by binary division, remaining motile, although it is reported that some chrysophyte can reproduce sexually. We did not see any lorica or scale outside the cell with LM.
From the pictures of the SEM, we noticed two kinds of cells: sphere or ellipse, as Figure 3 shown.
The Poterioochromonas cells bear two heterodynamic flagella: pleuronematic flagellum with hair-like appendages (mastigonemes, 1.25 µm) and acronematic flagellum without any appendage (Figure 4). The two flagella are unequal in length: the longer is almost two times the body length; the shorter is half of the body’s.
From the pictures of TEM, we observed that the cells are uninucleate, with a fine periplast but no rigid cell wall or spines or scales outside. The two chromatophores were oriented around the nucleus and lacked a pyrenoid. The single Golgi body is anterior to the nucleus and close to the flagellar bases. Mitochondrial cristae are tubular (Figure 5).
When added to the cultures of the Poterioocromonas sp., M. aeruginosa FACHB469 could be swallowed, and transported to a single membrane-bound food vacuole and digested there (Figures 6, 7 and 8) by the predators. The

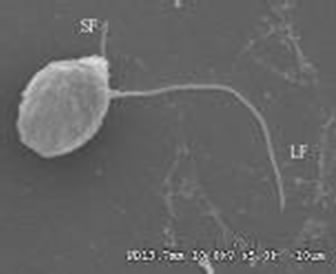
Figures 3-4. Scanning electron micrograph of Poterioochromonas sp.; SF: short flagellum; LF: long flagellum.
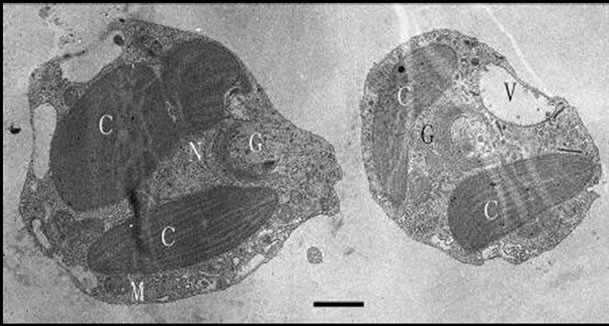
Figure 5. Transmission electron micrograph of Poterioochromonas sp. under autotrophy. C: chromatophore, G: golgi body, M: mitochondrion, N: nucleus, V : vacuole. Bar = 1 µm.
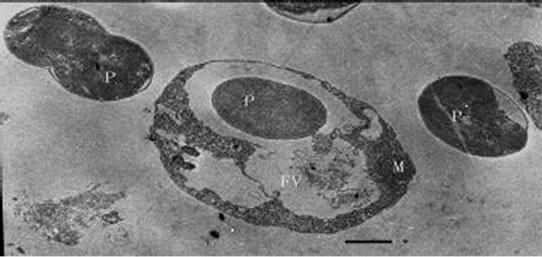
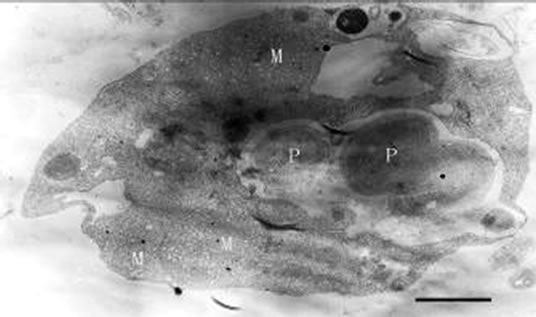
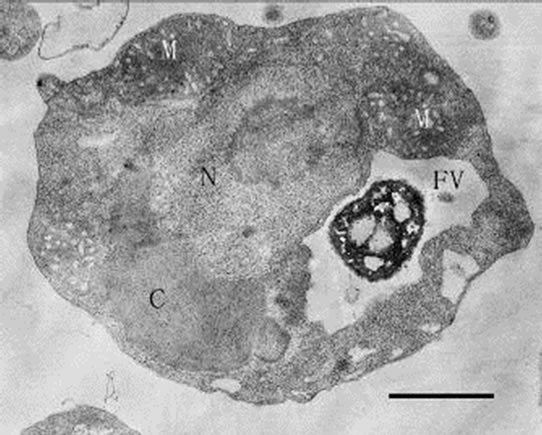
Figures 6-8. Transmission electron micrograph of Poterioochromonas sp.with prey(s). P : prey. Bar = 1 µm.
shape of the predator could be elongated, and the ultrastructure would change a lot. The most conspicuous feature of the predators was about the chromatophore.
When feeding Poterioochromonas sp. in a relative low ratio of 3:1 (prey: predator) every two days for a month, we could see no less than two swelling chromatophores with blurry or clear lamina around the nucleus (Figures 9, 10 and 11). Some times the number of the chromatophores could reach six. At the same time there are many little osmiophilic globules in the interthylakoid spaces of the chromatophores. The predators could still move well and ingest preys.
When inoculating low densities (ca. 103 mL-1) of Poterioochromonas sp. into the cultures containing approximately 106 or 107 mL-1 M. aeruginosa FACHB469, the predators could ingest preys very quickly and grow exponential rapidly. Meanwhile the chromatophores of most predators might become shrunken or missing in the first few days (Figures 12, 13 and 14), and there were also many large osmiophilic globules located in the cytoplasm at the same time (Figure 15). Later when the most preys were nearly eaten off, the predators entered a “stationary growth phase”, and chromatophores of Poterioochromonas sp. could appear again (Figure 16), and the large osmiophilic globules would disappear.
During the process of mixotrophic growth, the volumes of mitochondria become much larger (Figures 8 and 12) than those under autotrophy. We also observed a cell under the division (Figure 17).
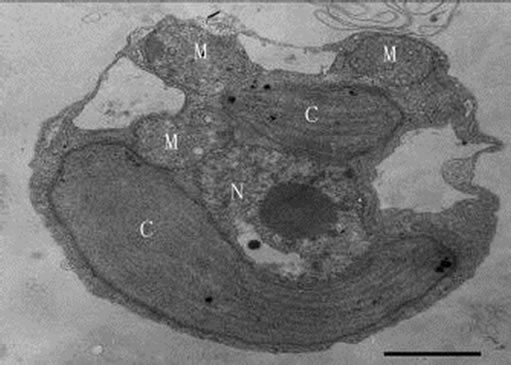
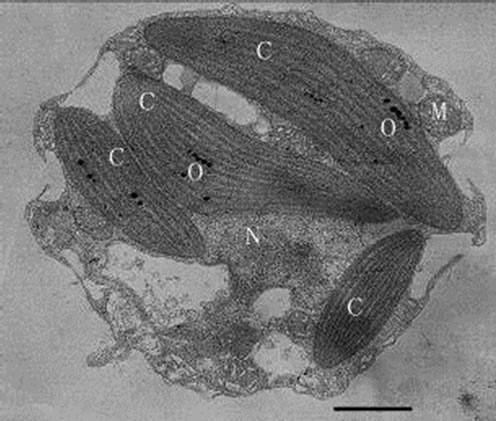
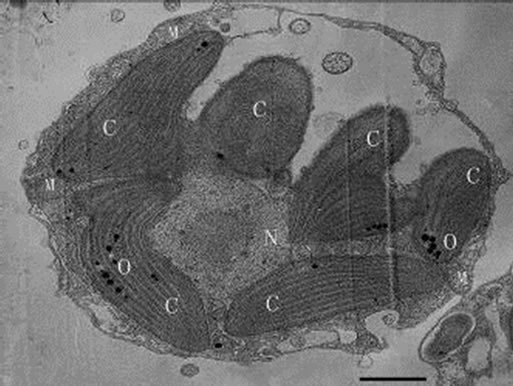
Figures 9-11. Transmission electron micrograph of Poterioochromonas sp. growth with low concentration of preys. O: osmiophilic globule. Bar = 1 µm.
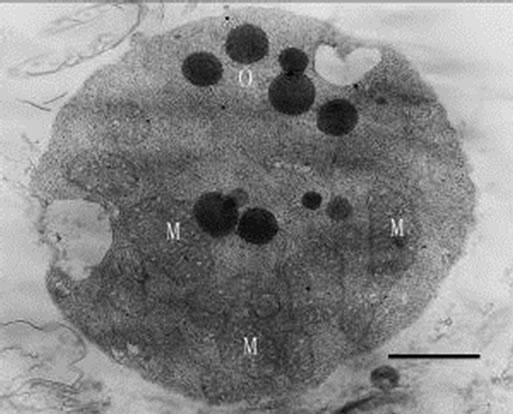
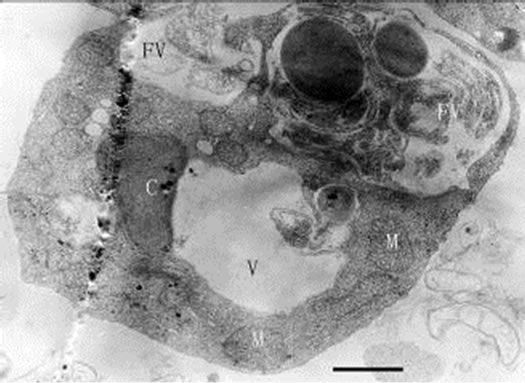
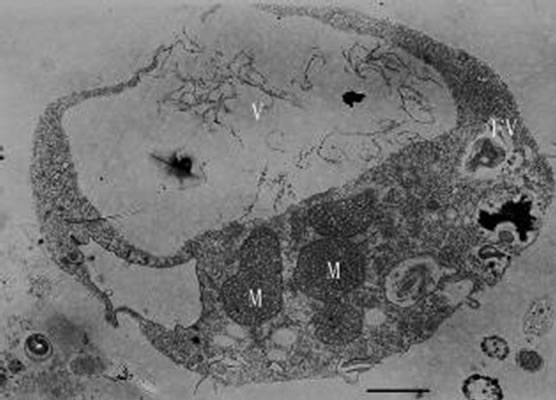
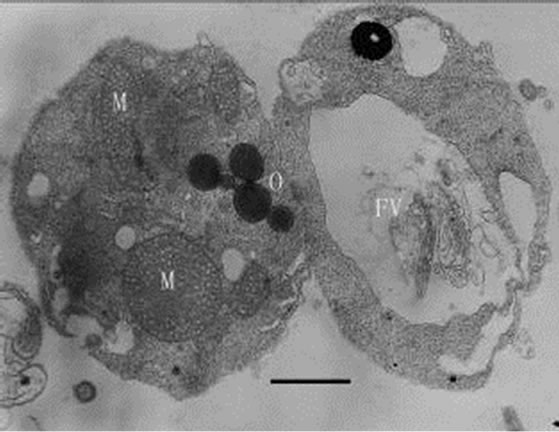
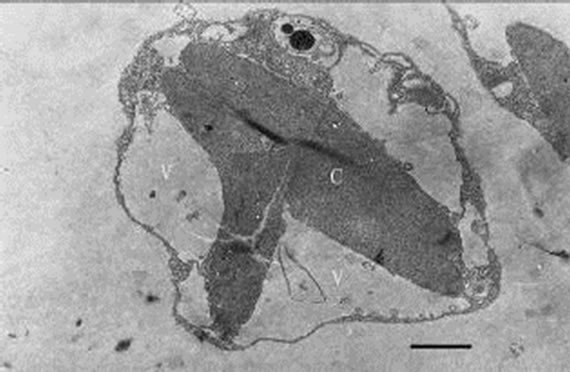
Figures 12-16. Transmission electron micrograph of Poterioochromonas sp. when adding with high concentration of preys. O: osmiophilic globule. Figures 12-15. Sampled within 3 days; Figure 16. Sampled after 7 days. Bar = 1 µm.
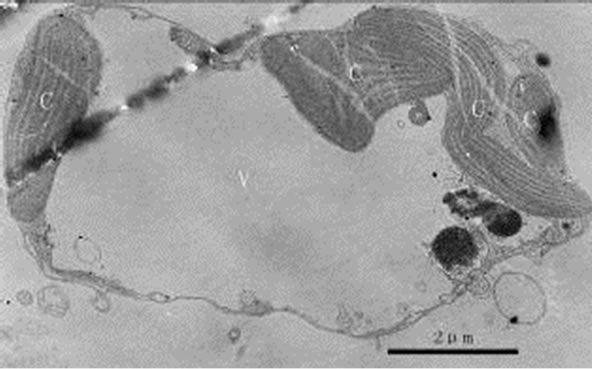
Figure 17. Transmission electron micrograph of Poterioochromonas sp. in division. Bar = 2 µm.
4. Discussion
Mixotrophy in algal flagellates is an interesting phenomenon from both cytological and ecological viewpoints [6]. However, as we know, there are few reports about such chrysomonas fed with algae in China. We isolated a strain Poterioochromonas and got the clone culture in 2002 [10]. We studied many morphological features and characteristics of this golden alga though we are not sure which species it was.
First, the most conspicuous feature of Poterioochromonas sp. was about the chromatophores. The morphology and number of chromatophore changed a lot during the mixotrophic growth. When the concentration of preys was relatively low, the number of chromatophores would increased obviously, and when much plentiful, the chromatophores would be shrunken or disappeared in the “exponential growth phase”, while they would be observed again when the predators entered a “stationary growth phase” (Figures 9-14 and 16). Based on references [1,11], the reason for the shrunken and disappearance of chromotaphore appears to be the result of rapid mixotrophic growth as the flagellate division rate exceeds the assemblage of the cellular organelle, such as chromotaphore. The recovery of chromotaphore may be attributed to the effects of preys limitation and subsequent decrease in the predator growth rate and gave enough time for the assemblage of chromotaphore.
During the changes of chromatophores, osmiophilic globules changed similarly. The globules apparently formed from breakdown products of the chromophore membranes as well as from pigments synthesized during growth, and they are a reservoir of energy-rich components in the cell [12,13]. The appearance of osmiophilic materials seems to be the product of the digesting preys and the disorganized chromophore of the predator.
The giant mitochondria might indicate the metabolic utilization of the preys, which might provide more energy for the predators.
Additionally, from the preys’ view, after being swallowed for a period of time, the prey(s) in the Poterioochromonas disappeared, it suggested that the predators not only ingest but also digest the prey organisms.
We could not observe any lorica outside Poterioochromonas sp. with TEM and LM, which was the special structure for the genus and difficult to identify [14]. More information should be explored to conclude whether this strain has lorica or not.
5. Conclusions
In conclusions, we investigated the morphological features and growth characteristics of Poterioochromonas sp., the results showed that the strain has the representative features of the genus except for the lorica. Mixotrophic chrysomonads can be found in many kinds of water bodied, including oligotrophic environments [15,16], mesotrophic and eutrophic waters [11,17]. It is an ecology strategy to control the harmful algae by using mixotrophy. Although we have done some research in the laboratory, there are many problems required further investigation before putting Poterrioochromonas sp. in the field.
REFERENCES
- D. A. Holen, “Effects of Prey Abundance and Light Intensity on the Mixotrophic Chrysophyte Poterioochromonas malhamensis from a Mesotrophic Lake,” Freshwater Biology, Vol. 42, No. 3, 1999, pp. 445-455.
- E. G. Pringsheim, “On the Nutrition of Ochromonas,” Quarterly Journal of Microscopical Science, Vol. 93, No. 21, 1952, pp. 71-96.
- R. J. Daley, G. P. Morris and S. R. Brown, “Phagotrophic Ingestion of a Blue-Green Alga by Ochromonas,” Journal of Protozool, Vol. 20, No. 1, 1973. pp. 58-61.
- G. T. Cole and M. J. Wynne, “Endocytosis of Microcystis aeruginosa by Ochromonas danica,” Journal of Phycology, Vol. 10, No. 4, 1974, pp. 397-410.
- M. E. Boraas, K. W. Estep, P. W. Johnson and J. M. Sieburth, “Phagotrophic Phototrophs: The Ecological Significance of Mixotrophy,” Journal of Protozoology, Vol. 35, No. 2, 1988, pp. 249-252.
- X. M. Zhang, M. M. Watanabe and I. Inouye, “Light and Electron Microscopy of Grazing by Poterioochromonas Malhanmensis (Chrysophyceae) on a Range of Phytoplankton Taxa,” Journal of Phycology, Vol. 32, No. 1, 1996, pp. 37-46.
- D. F. Bird and J. Kalff, “Algal Phagotrophy: Regulating Factors and Importance Relative to Photosynthesis in Dinobryon (Chrysophyceae),” Limnology and Oceanography, Vol. 32, No. 2,1987, pp. 277-284.
- U.-G. Berninger, D. A. Caron and R. W. Sanders, “Mixotrophic Algae in Three Ice-Covered Lakes of the Pocono Mountains,” U.S.A. Freshwater Biology, Vol. 28, No. 2, 1992, pp. 263-272.
- K. O. Rothhaupt, “Nutrient Turnover by Freshwater Bacterivorous Flagellates: Differences between a HeteroTrophic and Mixotrophic Chrysophyte,” Aquatic Microbial Ecology, Vol. 12, No. 1, 1997, pp. 65-70.
- D. Y. Ou, L. R. Song, N. Q. Gan and W. Chen, “Effect of Microcystins on and Toxin Degradation by Poterioochromonas sp.,” Environmental Toxicology, Vol. 20, No. 3, 2005, pp. 373-380.
- R. W. Sanders, U. G. Berninger, E. L. Lim, P. F. Kemp and D. A. Caron, “Heterotrophic and Mixotrophic Nanoplankton Predation on Picoplankton in the Sargasso Sea and on Georges Bank,” Marine Ecology Progress Series, Vol. 192, No. 1, 2000, pp. 103-118.
- N. W. Withers and F. T. Haxo, “Isolation and Characterization of Carotenoid-Rich Lipid Globules from Peridinium foliaceum,” Plant Physiology, Vol. 62, No. 1, 1978, pp. 36-39.
- B. Camara, P. Hugueney, F. Bouvier, M. Kuntz and R. Monéger, “Biochemistry and Molecular Biology of Chromoplast Development,” International Review of Cytology, Vol. 163, No. 1, 1995, pp. 175-247.
- R. W. Sanders, K. G. Porter and D. A. Caron, “Relationship between Phototrophy and Phagotrophy in the Mixotrophic Chrysophyte Poterioochromonas malhamensis,” Microbial Ecology, Vol. 19, 1990, pp. 97-109.
- I. Domaizon, S. Viboud and D. Fontvieille, “Taxon Specific and Seasonal Variations in Flagellates Grazing on Heterotrophic Bacteria in the Oligotrophic Lake Annecy—Importance of Mixotrophy,” FEMS Microbiology Ecology, Vol. 46, No. 3, 2003, pp. 3117-3329.
- A. Katechakis and H. Stibor, “The Mixotroph Ochromonas tuberculata may Invade and Suppress Specialist Phagoand Phototroph Plankton Communities Depending on Nutrient Conditions,” Oecologia, Vol. 148, No. 4, 2006, pp. 692-701.
- J. Comte, S. Jacquet, S. Viboud, D. Fontvieille, A. Millery, G. Paolini and I. Domaizon, “Microbial Community Structure and Dynamics in the Largest Natural French Lake (Lake Bourget),” Microbial Ecology, Vol. 52, No. 1, 2006, pp. 72-78.
NOTES
This paper sponsored by National Hi-Tech Research and Development Program of China (2005AA60101005).

