Journal of Water Resource and Protection
Vol. 2 No. 3 (2010) , Article ID: 1500 , 9 pages DOI:10.4236/jwarp.2010.23032
Comparison of Alkaline Treatment of Lead Contaminated Wastewater Using Lime and Sodium Hydroxide
1Department of Civil Engineering & Chairman, Center for Sustainable Technologies,
Indian Institute of Science, Bangalore, India
2Department of Civil Engineering, BMS College of Engineering, Bangalore, India
E-mail: msrao@civil.iisc.ernet.in
Received July 13, 2009; revised August 20, 2010; accepted August 26, 2010
Keywords: Acid, Alkali Agents, Alkaline Treatment, Battery, Lead, Speciation, TDS
ABSTRACT
A lead-acid storage battery manufacturing industry in India produces several thousand liters of lead contaminated acidic wastewater on a daily basis and uses hydrated lime to render the lead-contaminated acidic wastewater alkaline (pH = 8.0). Alkaline treatment of the acidic wastewater with lime though a cost-effective method, generates copious amount of lead-contaminated gypsum sludge. Other alkali agents such as sodium hydroxide, sodium carbonate and dolomite are also used for alkali treatment of the acid wastewaters. The present paper compares the relative efficiency of hydrated lime and 0.05 M to 1 M NaOH solutions with respect to 1) amounts of sludge produced, 2) immobilization of the soluble lead in the acidic wastewater (AWW) and 3) increase in TDS (total dissolved solids) levels upon treatment of AWW with NaOH solutions and lime. The study also performs equilibrium speciation upon alkaline treatment of AWW with lime and NaOH (sodium hydroxide) solutions using the Visual MINTEQ program to understand the chemical reactions occurring during treatment process.
1. Introduction
A lead-acid storage battery manufacturing industry in India produces several thousand liters of lead contaminated acidic wastewater on a daily basis. The acidic wastewater (AWW) contains lead concentration of 4.24 mg/L and has pH of 0.96. Macchi et al. [1] report similar ranges of pH (1.6 and 2.9) but higher lead concentrations (5–15 mg/L) in acidic wastewaters of lead-acid storage battery units in Italy. Neutralization of the lead-contaminated acidic wastewaters are achieved by adjusting their pH values to a desirable range of 5.5 to 9.5 using alkaline agents such as, sodium hydroxide, sodium carbonate, lime and dolomite [2]. The lead-acid storage battery manufacturing industry in India uses hydrated lime to treat the lead-contaminated acidic wastewater to pH 8.0. Alkaline treatment of acidic wastewater by lime addition occurs according to the reaction:
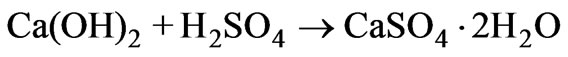 (1)
(1)
Alkaline treatment with lime also precipitates the soluble lead in the acidic wastewater as hydroxides, carbonates and sulfates that is incorporated in the gypsum sludge. Guided by the minimum solubility of lead compounds in the alkaline pH (8-10) range [3] the lead-acid battery manufacturing industry in India chose to treat their acidic wastewater to this pH (8.0).
Alkaline treatment of the acidic wastewater with lime though a cost-effective method, generates copious amount of sludge material. For example, calculations show that treatment of 1000 liters of acidic wastewater produces 100 kg of moist sludge. As mentioned earlier, pH of acidic wastewaters are also rendered alkaline by addition of sodium hydroxide, sodium carbonate, and dolomite [2]. Sodium hydroxide solution neutralizes the acidic wastewater according to the reaction:
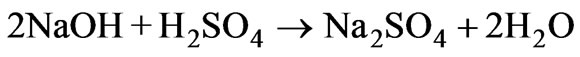 (2)
(2)
The advantage of employing sodium hydroxide solution is that the neutralization reaction (reaction 2) leads to minimum sludge formation as the reaction product Na2SO4 (sodium sulfate) is water-soluble. Macchi et al. [1] observe that neutralization of acidic battery wastewater using sodium hydroxide solution has an added advantage as the resident ferric ions in the battery wastewater precipitate (as hydroxides) and scavenge the lead ions from the wastewater. A possible disadvantage of neutralizing the acid wastewater with sodium hydroxide solution is an increase in total dissolved solids (TDS) concentration of the treated water owing to the formation of soluble sodium sulfate. Increase in TDS levels of the treated water is a concern as MOEF (Ministry of Environment and Forests) regulations [4] state that TDS levels of treated leachate for disposal on land or inland surface water bodies should not exceed 2100 mg/L.
Earlier studies have not considered the relative impacts of lime and sodium hydroxide neutralization of lead-contaminated acidic wastewater on the amounts of sludge produced, lead removal efficiency and TDS levels of the treated wastewater. This paper hence compares the relative efficiency of lime and sodium hydroxide solutions with respect to 1) amounts of sludge produced upon alkaline treatment (pH=8) of AWW, 2) immobilization of available lead in AWW upon alkaline treatment and 3) increase in TDS levels of AWW upon alkaline treatment. Ion speciation studies are performed using the Visual MINTEQ program to obtain insight into changes in hydrogen ion concentration and equilibrium concentrations of aqueous and solid phases formed during the alkaline treatment process.
2. Materials and Methods
2.1. Wastewater Sample and Chemicals
Lead contaminated acidic wastewater (AWW) was supplied by the lead-acid battery industry in India. Laboratory reagent grade hydrated lime and sodium hydroxide were used in the AWW neutralization studies.
2.2. Alkaline Treatment Assays
The pH of the acidic wastewater was increased by adding incremental amounts of laboratory reagent grade hydrated lime to separate one-liter batches (of acid wastewater) with manual stirring until the pH of the wastewater stabilized for the given lime addition. Experimental results estimated that addition of 15.5 g of lime per liter of acidic wastewater altered its pH to 8.0. The acidic wastewater was elevated to this pH to simulate the alkaline treatment process of the lead acid battery industry. To estimate the sludge generated by this lime addition process, a separate experiment was performed; 15.5 g of hydrated lime was added to one liter of acidic wastewater (pH = 0.96). The lime-treated solution was filtered and analyzed for lead concentration using atomic absorption spectrophotometer. The resultant sludge was weighed in the moist condition.
The pH of the acidic wastewater was also increased by adding incremental amounts of 1 molar (M), 0.1 M and 0.05 M NaOH solutions to separate, one-liter batches of AWW. The incremental volumetric additions were continued until the acidic wastewater was rendered sufficiently alkaline (pH 10.1 to 10.7). In separate experiments 0.405 L (liter), 3.78 L and 8.2 L of 1 M, 0.1 M and 0.05 M NaOH solutions were added to separate 1 L batches of acidic wastewater; the treated solutions were filtered and the resulting sludge were weighed in the moist condition. Lead concentrations in the filtrates were analyzed using atomic absorption spectrophotometer.
2.3. Equilibrium Ion Speciation Estimation
The study performed equilibrium speciation of AWW upon neutralization by lime and NaOH solutions using the Visual MINTEQ program [http://www.lwr.kth.se/ English/OurSoftware/vminteq/#down load]. Visual MINTEQ is a geochemical program to model aqueous solutions and the interactions of aqueous solutions with hypothesized assemblages of solid phases. It applies the fundamental principles of thermodynamics to solve geochemical equilibria from a set of mass balance equations. The input parameters to the Visual MINTEQ program were the chemical composition of AWW (Table 1), the pH of AWW on addition of lime or 1 M NaOH solution (Figure 1), mass of lime added (incorporated as solid finite phase) and increase in sodium ion concentration from addition of 1 M NaOH solution. Speciation of the lead by Visual MINTEQ program at various lime and 1 M NaOH solution additions provided information on the relative amounts of lead occurring in the soluble and precipitated states during the treatment process.
2.4. Analytical Methods
Lead contaminated acidic wastewater (AWW) supplied by the lead-acid battery industry in India was filtered through 0.45 micron filter. The filtrate was analyzed for lead, iron, calcium, magnesium, sodium and potassium concentrations using atomic absorption spectrophotometer (model-Perkin Elmer Analyst 200). The concentration of, sulfate ions was determined by the turbidity method, while the chloride concentration was determined by ion
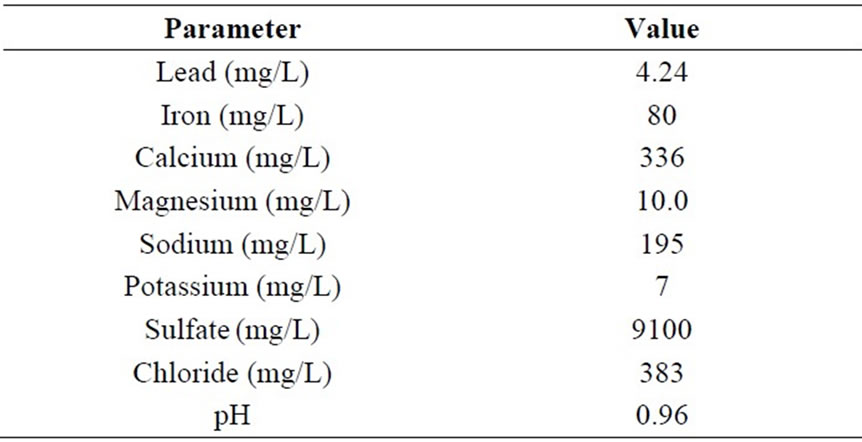
Table 1. Acidic wastewater composition.
meter method using an ionmeter (Orion, 720A) with a chloride ion-selective electrode. The pH and electric conductivity of the acidic wastewater were also determined.
3. Results and Discussion
3.1. Characteristic of Acidic Wastewater
Table 1 presents the chemical composition of the acidic wastewater. The AWW has an acidic pH of 0.96 and soluble lead concentration of 4.24 mg/l. The lead concentration of 4.24 mg/l in the acidic wastewater greatly exceeds the permissible limit (0.1 mg/l) for disposal of treated lead-acid battery effluent into the environment [5]. Besides, containing excess lead concentration, the acidic pH of the acidic wastewater (0.96) classifies the wastewater as a corrosive liquid [6]. High sulfate ion concentrations occur in the acidic wastewater of lead-acid storage battery units (Table 1), as sulfuric acid mainly constitutes the acidic wastewater. Soluble iron (80 ppm) occurs in the acidic wastewater (Table 1) due to corrosion reactions [1]. Speciation by the Visual MINTEQ program indicated hydrogen ion concentration of 0.1096 M for the AWW which is expected for its pH value of 0.96. Further, the speciation results show that bulk of the sulfate concentration in the AWW (0.1 M) occurs as HSO4- (0.08 M) species.
3.2. Lime Treatment of AWW
Figure 1 presents the experimentally obtained plot of OH
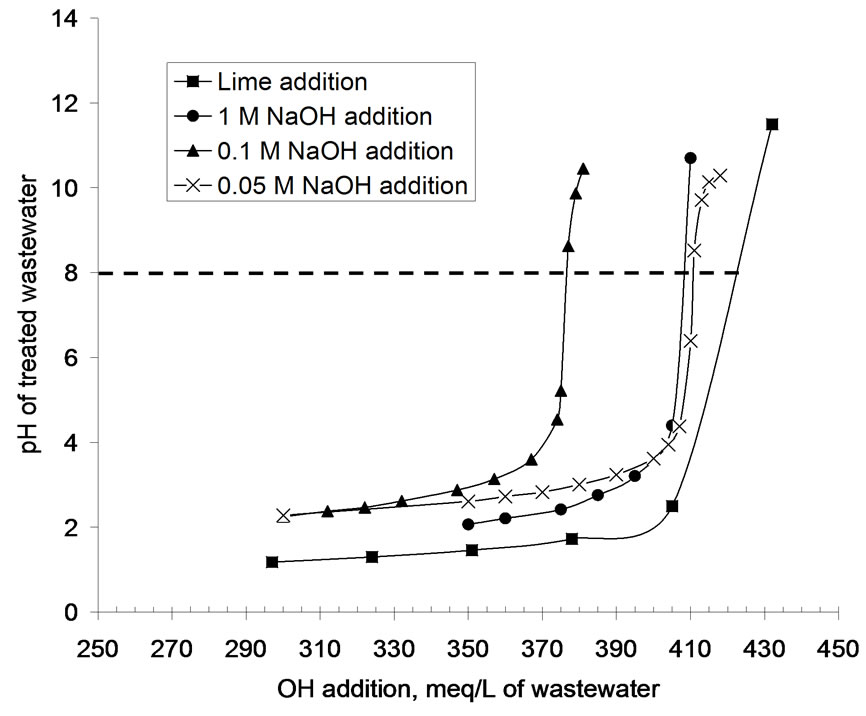
Figure 1. pH of acidic wastewater upon lime and NaOH solution addition.
addition (in meq/L) versus pH of AWW (Figure 1). The OH values in meq/L are obtained by converting mass of lime addition /L of AWW to equivalent OH concentration. The figure shows that hydroxyl ion additions up to 405 meq/liter marginally change the pH of the acidic wastewater. Increasing the hydroxyl ion concentration from 405 to 432 meq/L sharply increased the pH of AWW to 11.5. Addition of 422 meq of OH ions per liter of acidic wastewater is necessary to increase its pH to 8 (Figure 1). Alkaline treatment (pH=8) of 1 liter of AWW with hydrated lime generated 0.102 kg of moist sludge. The prominent peaks at 7.63Å, 4.28Å and 3.07Å in the X-ray diffraction of the air-dried (at 45 0C) sludge (Figure 2)
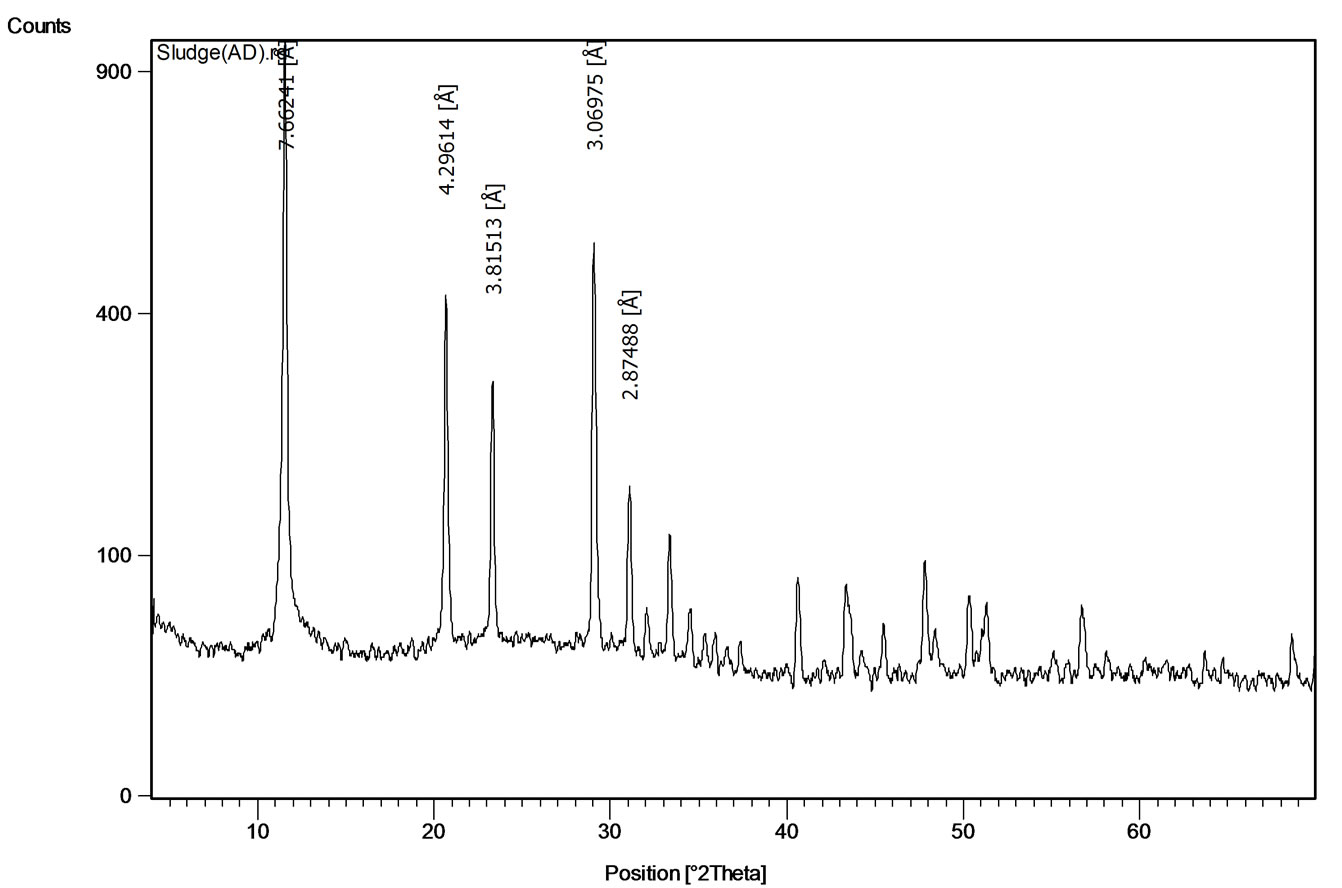
Figure 2. X-ray diffraction pattern of air-dried sludge.
reveal the presence of gypsum mineral (calcium sulfate dihydrate, CaSO4.2H2O).
The speciation results show that alkaline treatment of AWW with lime reduces its initial hydrogen ion concentration from 0.1096 M to 4.35 × 10-12 M. Correspondingly the HSO4- concentration reduced from 0.08 M to 2.25 × 10-13 M and the calcium ion concentration increased from 0.074 M to 0.16 M (Figure 3). The increase in calcium ion concentration and reduction in hydrogen ion concentration upon lime treatment of AWW is explained
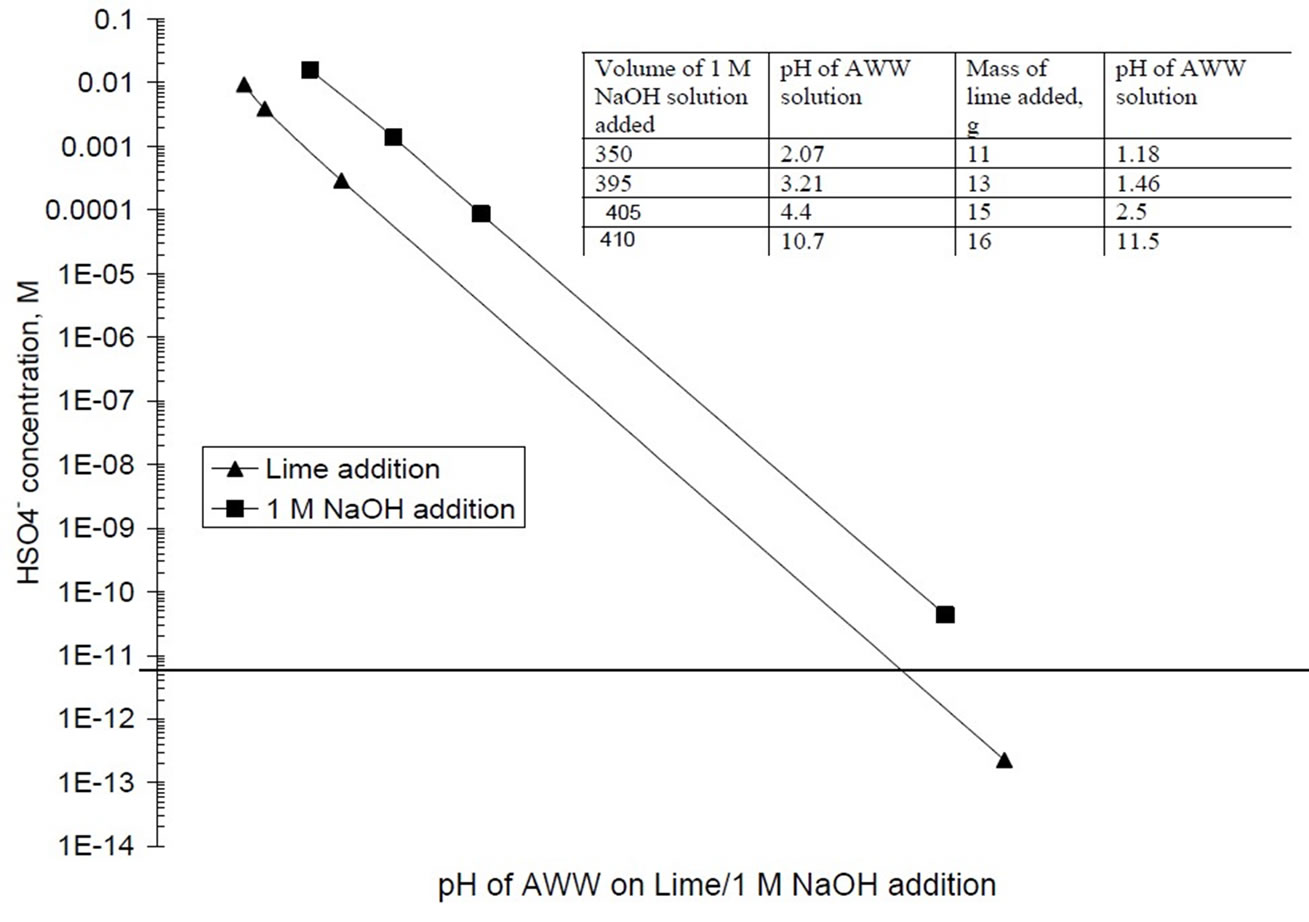
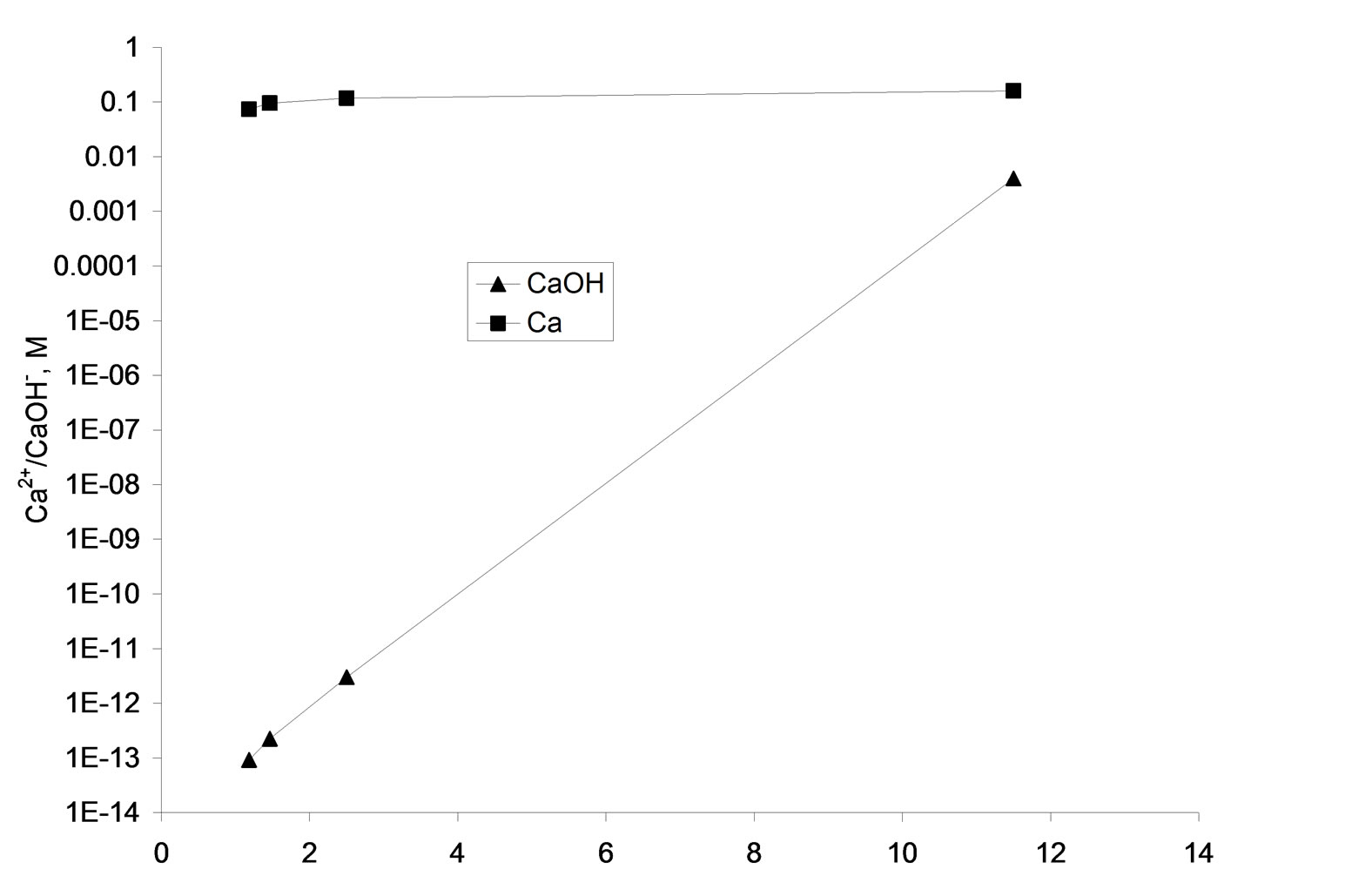
Figure 3. Variations in ion concentrations upon AWW neutralization with lime and 1 M NaOH solution-visual MINTEQ results.
from the following reactions. Lime dissociates into calcium and hydroxyl ions (reaction 4); the dissociated hydroxyl ions reduce the hydrogen ions with the formation of H2O (reaction 5). Increasing addition of lime to AWW shifts the equilibrium to the right enhancing the hydroxyl ion concentration.
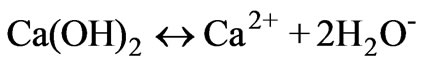 (4)
(4)
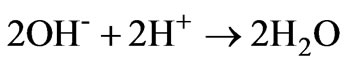 (5)
(5)
The sharp increase in CaOHconcentration (Figure 3) from 9.2 × 10-14 M at pH 1.18 (lime addition = 11 g/liter) to 3.99 × 10-3 M at pH 11.5 (lime addition 16 g/Liter) is attributed to the enhanced formation CaOH- in the strongly alkaline pH medium according to reaction:
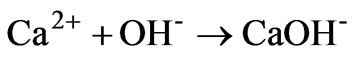 (6)
(6)
The speciation results show that the HSO4- concentration decreases from 0.009 M to 2.25 × 10-13 on increasing the pH of the AWW from 1.18 to 11.5 (Figure 3). Decrease in HSO4- concentration is attributed to the reduction of bisulfate to sulfate (reaction 7) and combination of calcium and sulfate ions to form CaSO4 (aq):
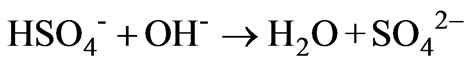 (7)
(7)
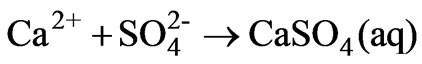 (8)
(8)
Speciation results showed that alkaline treatment (pH=8) of AWW with lime leads to the formation of 0.0052 M to 0.0054 M CaSO4 (aq) species.
The lead ion concentration in lime-treated AWW (pH=8) correspond to 0.11 mg/L which marginally exceeds the permissible limit (0.1 mg/L) for disposal of treated effluent into environment [5]. The initial lead concentration in AWW corresponds to 4.24 mg/L or 2.046 × 10-5 M. Speciation of the lead by Visual MINTEQ program at various lime additions provided information on the relative amounts of lead occurring in the soluble and precipitated forms during the lime treatment process. Figure 4 plots the soluble lead content as function of pH on lime addition. Lime addition up to 15 g/L causes the entire lead to remain in soluble form (pH of treated effluent = 2.5). Addition of 15.7 g/L increases the AWW pH to 8 and causes near complete (99.48 %) precipitation of lead.
Figure 5 shows that 0.215 kg of moist sludge is generated upon alkaline treatment (pH=8) of AWW (volume = 1L) by lime addition. Figure 6 shows that the TDS level of lime-treated AWW (pH 8) corresponds to 2100 mg/L which meets the TDS limit (2100 mg/L) recommended for disposal of treated leachate to land, inland surface water or public sewers [4].
3.3. Sodium Hydroxide Treatment of Acidic Wastewater
Figure 1 includes the experimentally obtained plots of OH addition (in meq/L) versus pH of AWW (Figure 1) for volumetric additions of 1M, 0.1 M and 0.05 M
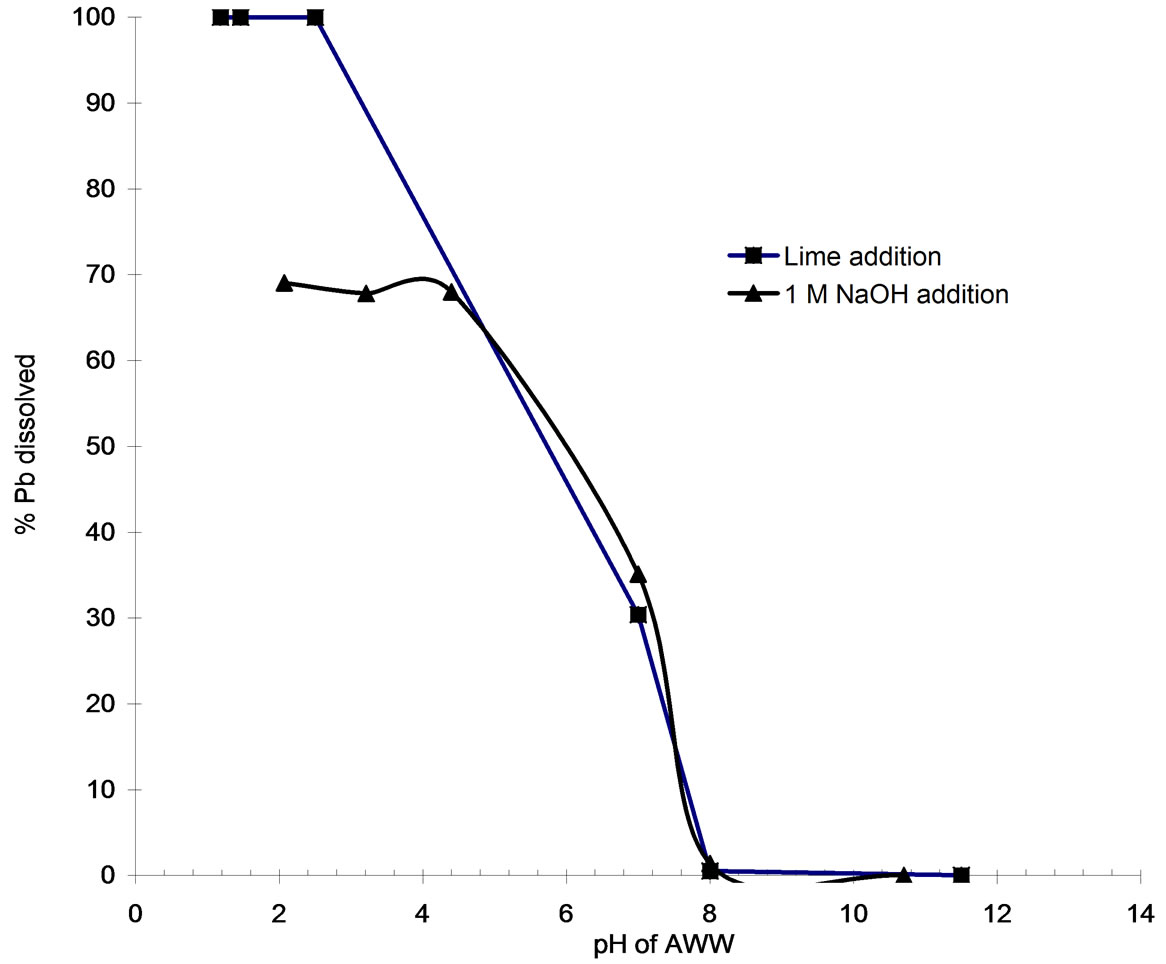
Figure 4. Variations of soluble lead concentrations as a function of pH upon AWW neutralization with lime and 1 M NaOH solution.
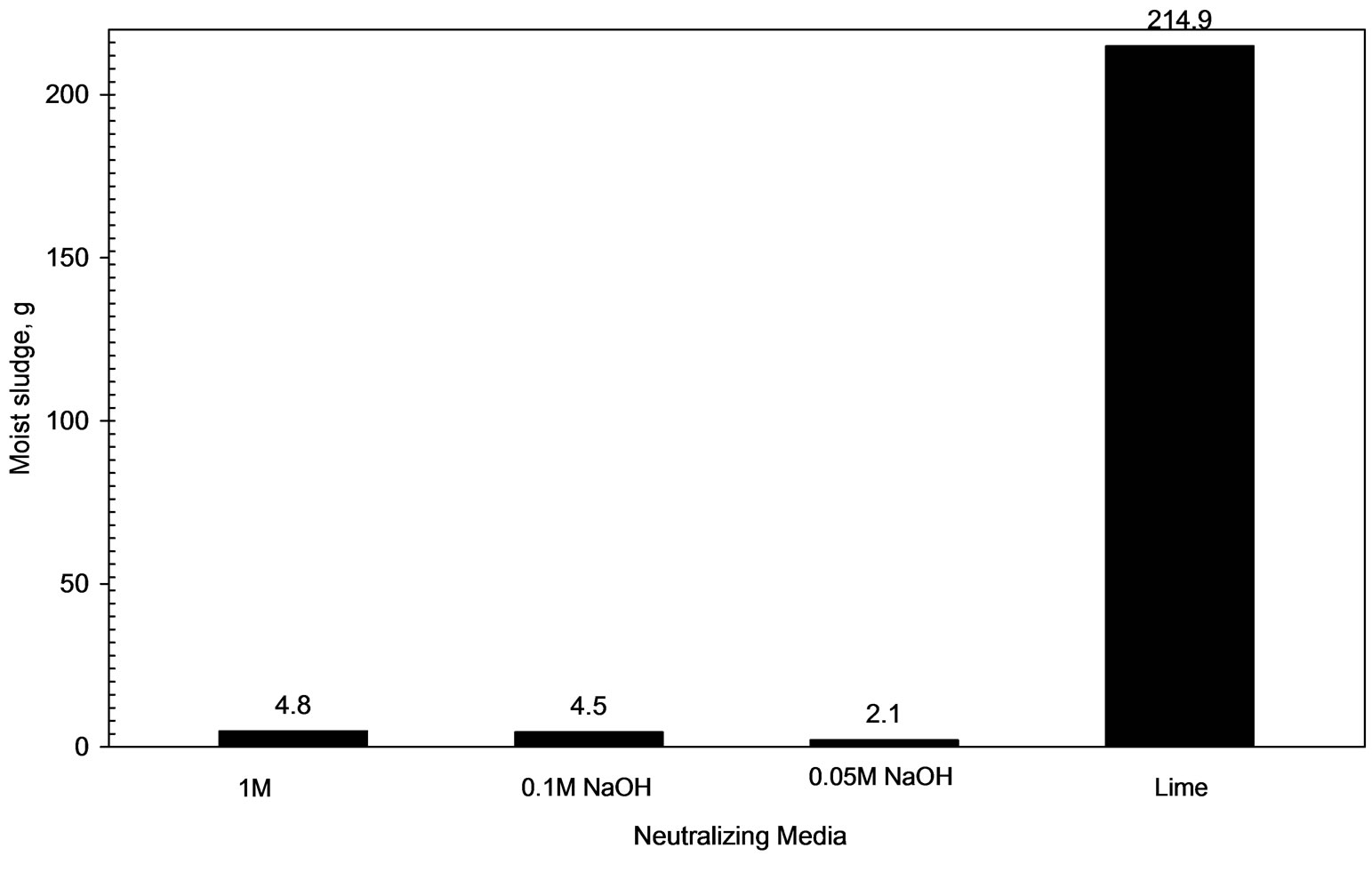
Figure 5. Comparison of moist sludge produced by different alkali agents.
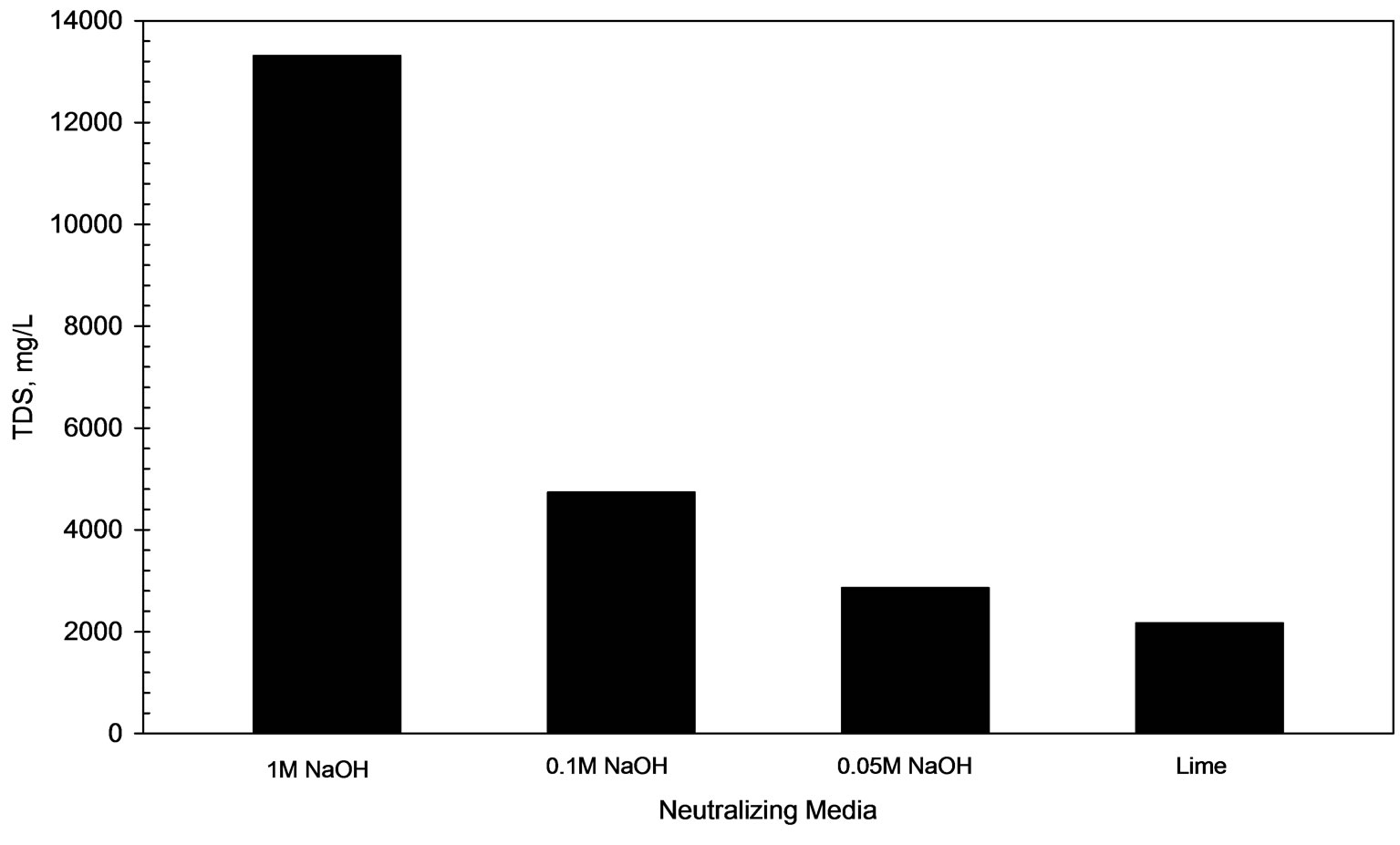
Figure 6. TDS levels in AWW neutralized with different alkali agents.
NaOH solutions. The figures show that the pH of the acidic wastewater responds to NaOH addition in two phases; the rate of increase in pH is gradual up to pH values of 3.6 to 4.4; thereafter rate of increase in pH with addition of NaOH solutions is a rapid process. These plots estimate that 407 meq/L, 377 meq/L and 410 meq/L of hydroxyl ions are required to modify the pH of the acidic wastewater from an initial value of 0.96 to desired value of 8.0 on addition of 1 M, 0.1 M and 0.05 M NaOH solutions.
Visual MITEQ speciation results show that alkaline treatment (pH=8) of AWW with 1 M NaOH solution reduces the initial hydrogen ion concentration from 0.1096 M to 2.72 × 10-11 M and the HSO4- concentration from 0.08 M to 4.32 × 10-11 M (Figure 3); concomitantly, sodium ion concentration increases from 0.33 M to 0.38 M (Figure 7). The increase in sodium ion concentration and reduction in hydrogen ion concentration upon NaOH addition is attributed to the reactions:
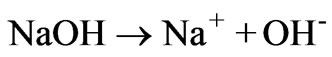 (9)
(9)
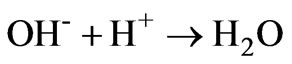 (10)
(10)
Speciation results show that the HSO4- concentration decreases from 0.016 M to 4.31 × 10-11 M on increasing the pH of the AWW from 2.07 to 10.7 (Figure 3). The reduction in HSO4- concentration is attributed to the reaction:
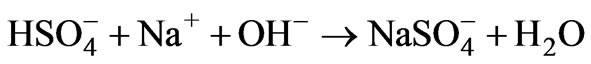 (11)
(11)
The concentration of NaSO4- species varies from 0.026 M at pH 2.07 (350 ml addition) to 0.034 M at pH 10.7 (410 ml addition, Figure 7). Experimental measurements showed that the lead concentration in AWW treated with1 M NaOH solution (0.24 mg/L) does not meet the permissible limit (0.1 mg/l) for disposal of treated effluent into inland surface water [5], while, AWW treated with 0.1 M (lead concentration = 0.07 mg/L) and 0.05 M NaOH (lead concentration = 0.06 mg/L) solutions meet this requirement. The inverse relation between concentration of sodium hydroxide solution and lead ion concentration in treated effluent is apparently related to volumes of the alkali solution used in the treatment process. Figure 1 had earlier estimated that 0.405 L, 3.78 L and 8.2 L of 1 M, 0.1 M and 0.05 M NaOH solutions are required to modify the pH of the acidic wastewater from an initial value of 0.96 to desired value of 8.0. Smaller lead concentrations apparently occurs in the effluents treated with 0.1 M and 0.05 M NaOH solutions owing to 9 to 20 times greater dilution of the acidic wastewater in comparison to treatment with 1 M NaOH solution. Visual MINTEQ results however did not show differences in the soluble/precipitated lead concentrations of AWW treated to pH 8 with 1M, 0.1 M and 0.05 M NaOH solutions. The speciation results showed that identical levels (98.7 %) of lead precipitate (as Pb(OH)2, 2.02 × 10-5 M) on treating the AWW to pH 8 with the three NaOH solutions.
Figure 4 includes the Visual MINTEQ results for the soluble lead content (as a percentage) as function of pH on addition of 1 M NaOH solution. Treatment of AWW with NaOH solution renders significant fraction of lead insoluble (30 to 32 %) in the acidic pH range of 2.07 to 4.4 (Figure 4). Increasing the AWW pH to 8.0 (408 ml addition) rendered almost the entire lead fraction (98.7 %) insoluble. Figure 8 reveals that treating AWW with NaOH solution results in precipitation of PbSO4 species (anglesite) in the acidic pH range of 2.07 to 4.4. The much larger equilibrium constant (log Keq) for anglesite formation (log Keq = 7.79) in comparison to Pb(OH)2 formation (log Keq = -8) is apparently responsible for precipitation of lead in the acidic pH range.
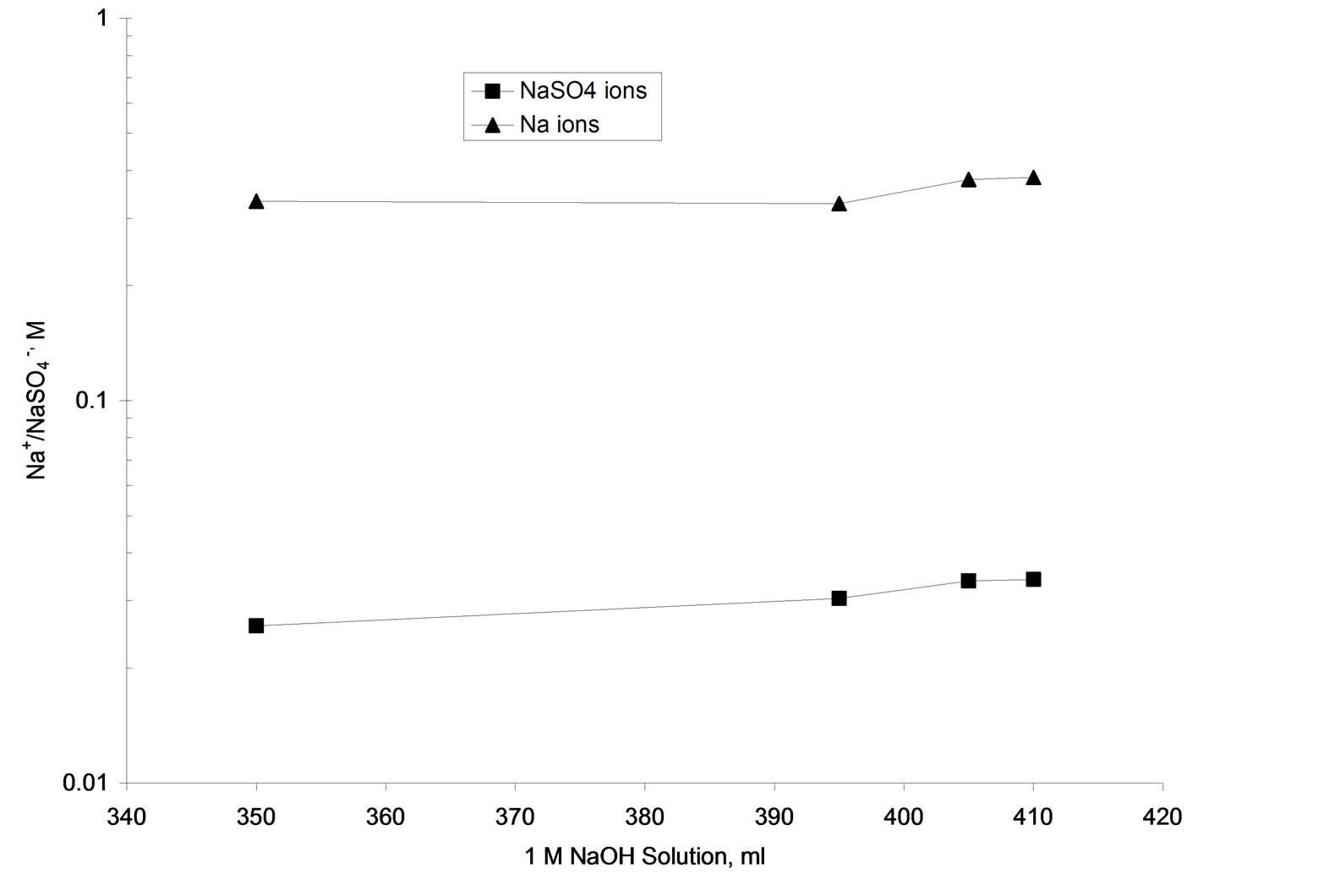
Figure 7. Variations in Na+ and NaSO4- ion concentrations upon AWW neutralization with 1 M NaOH solution-visual MINTEQ results.
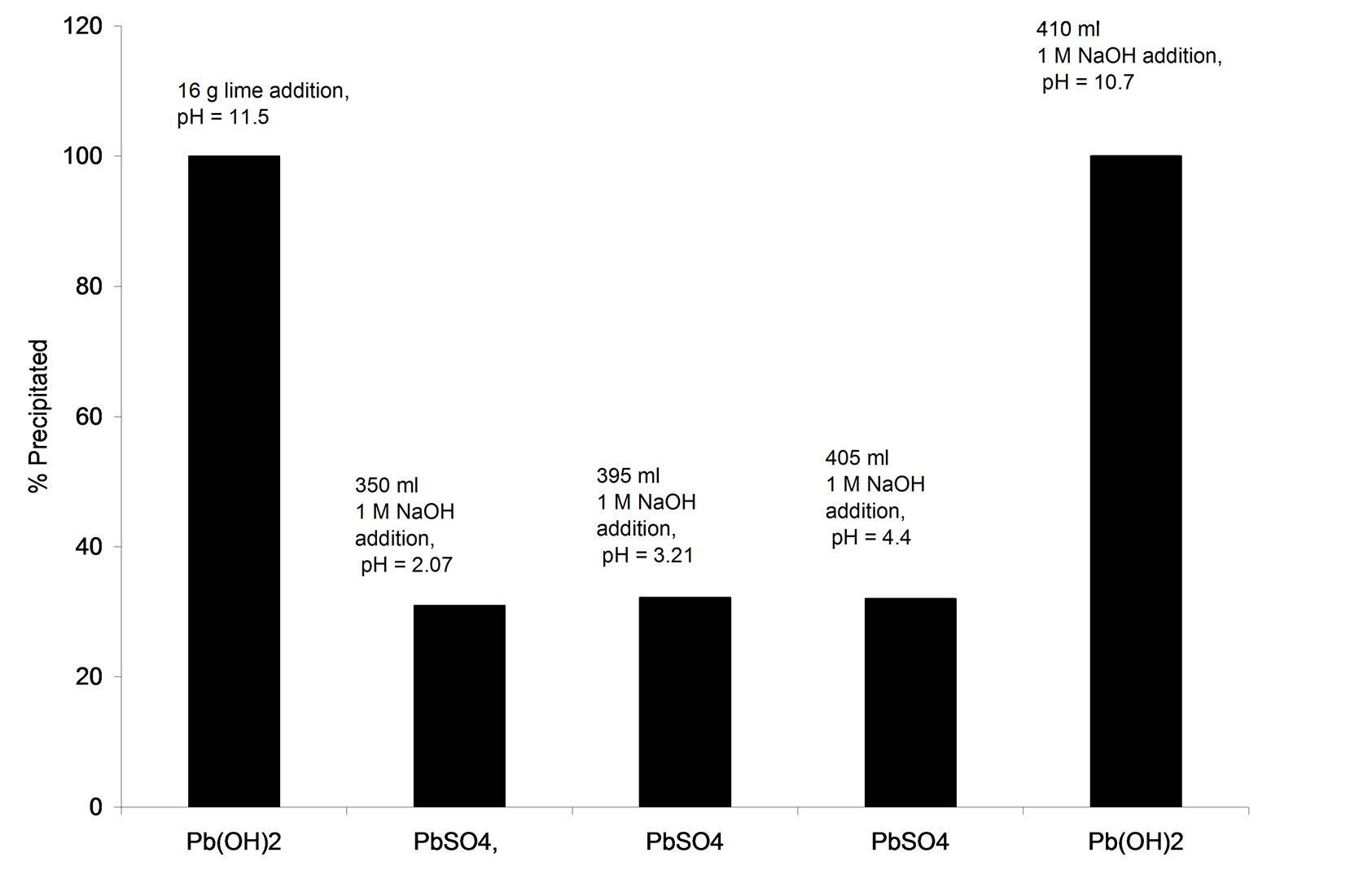
Figure 8. Relative distribution of precipitated lead as a function of pH upon AWW neutralization with lime and 1 M NaOH solution.
Figure 5 reveals that alkaline treatment of AWW (pH 8) with 0.05 M, 0.1 M and 1 M NaOH solutions produce 0.0021 kg, 0.0045 kg and 0.0048 kg of moist sludge respectively. Figure 6 compares the total dissolved solid concentration (TDS) of AWW treated to pH 8 with 1 M, 0.1 M, 0.05 M NaOH solutions respectively. Expectedly, the acidic wastewater treated with 1 M NaOH solution exhibits maximum TDS level of 13,310 mg/L; the acidic wastewater treated with 0.05 M NaOH solution exhibits the minimum TDS level (2800 mg/L) which is higher than TDS limit recommended [4] for disposal of treated leachate to land, inland surface water or public sewers (2100 mg/L).
3.4. Comparison of Lime and Sodium Hydroxide Treatments
Figure 1 reveals that NaOH solutions are more effective than lime for alkaline treatment of AWW as the pH of the acidic wastewater is modified to 8.0 at lower OH concentrations (377 to 410 meq/L) on addition of NaOH solutions than on lime addition (OH concentration = 422 meq/L at pH=8). Experimental results in Figure 5 show that treatment with NaOH solutions produce much lesser (45 to 103 folds) masses of moist sludge than lime. Alkaline treatment (pH 8) with 0.1 M and 0.05 M NaOH solutions generate acceptable levels of residual lead concentrations (< 0.1 mg/L) in the treated AWW, while lime treatment results in residual lead concentration (0.11 mg/L) which is slightly in excess of the permissible limit. The disadvantage of using NaOH solutions is that the treated wastewater is characterized by higher total dissolved salt concentrations (2860 to 13310 mg/L, Figure 6). For example, acidic wastewater treated with 0.05 M NaOH solution has 33 % higher TDS (TDS=2860 mg/L) than the sample treated with lime (TDS = 2100 mg/L).
4. Conclusions
Speciation results showed that AWW is characterized by the presence of 0.196 molar H+ and 0.08 molar HSO4- ion concentrations. Upon lime/NaOH treatment, hydroxyl ions released by alkali dissociation, reduce hydrogen ions to form H2O molecules. Decrease in HSO4- concentration is attributed to the reduction of bisulfate to sulfate ions; the sulfate ions combine with calcium/sodium ions to form CaSO4(aq)/NaSO4- species. AWW is characterized by lead concentration of 4.24 mg/L. Speciation results showed that almost the entire lead fraction is precipitated (98.7 to 99.48 %) upon increasing the pH of AWW to 8.0 with lime or 1 M NaOH solution. Treating AWW with 0.1 M and 0.05 M NaOH solutions generated acceptable residual lead concentrations (lower than the permissible limit of 0.1 mg/L) in the treated wastewater owing to greater dilution of AWW by the weaker alkali solutions.
Comparison of NaOH and lime treatments reveal that lower OH concentrations (in meq/L) are required to modify the pH of AWW from 0.96 to 8.0. Further, smaller mass of moist sludge and environmentally acceptable levels of residual lead concentrations (for additions of 0.1 M and 0.05 M solutions) are generated upon NaOH treatment in comparison to lime treatment of AWW. Based on the minimal mass of moist sludge produced, acceptable residual lead concentration and lowest (among NaOH solutions) TDS of treated AWW, 0.05 M NaOH solution is suggested as an alternative agent to remediate the acidic wastewater from lead-acid battery manufacturing industry. The treated AWW will however have to be subjected to some form of desalination before it is discharged on land or to inland water bodies to meet environmental norms for discharge of treated effluent into the environment.
REFERENCES
- G. Macchi, M. Pagang, M. Santori, and G. Tiravanti, “Battery industrial wastewater: Pb removal and produced sludge,” Water Research, Vol. 27, pp. 1511–1518, 1993.
- J. W. Patterson, “Lead in wastewater treatment technology,” Ann Arbor Science, Ann Arbor, Michigan, pp. 129–138, 1975.
- R. M. Harrisom and D. P. H. Laxen, “Lead pollution causes and control,” Chapman and Hall, London, 1981.
- Ministry of Environment and Forests. Municipal Solid Wastes (Management and Handling) Rules 2000. MOEF, New Delhi, 2000.
- “Liquid effluent discharge standards for lead acid battery manufacturing,” Central Pollution Control Board. http:// cpcb.nic.in.
- CPCB. “Guidelines for proper functioning and upkeep of disposal sites,” Hazardous Waste Management Series: HAZWAMS/32/2005–2006, Central Pollution Control Board (CPCB), New Delhi, 2005.

