Journal of Biophysical Chemistry
Vol.2 No.4(2011), Article ID:8424,4 pages DOI:10.4236/jbpc.2011.24053
Variations induced in human erythrocytes by ultra-low X-ray doses
Department of Physics, Faculty of Science, Benha University, Benha, Egypt. drsmsallam@yahoo.com
Received 18 February 2011; revised 27 April 2011; accepted 17 May 2011.
Keywords: Erythrocytes; Ultra-Low X-Rays; Hemolysis; Ascorbic Acid; Electric Conductivity
ABSTRACT
In this work, the effect of different ultra-low doses of X-ray on human erythrocytes was investigated. Also, the effect of ascorbic acid added to erythrocyte suspension before X-rays was studied. The mean X-ray exposure level was about 10 µGy/h. Samples of erythrocytes suspension with and without ascorbic acid was exposed to X-ray doses in the range from 2.5 to 20 µGy. The obtained results showed pronounced radio-hemolysis of erythrocytes at doses starting from nearly 7.5 µGy. The effect was enhanced, for low doses, when ascorbic acid of relatively high concentration was added to erythrocyte samples. The changes may be attributed to a dose-dependent damage by oxidative stress at the level of the whole cell and to the production of reactive oxygen species, which can cause this damage. It may be concluded that X-rays, even at low levels of exposure, can induce oxidizing effect on erythrocytes. Accordingly, such results should be taken into account for workers operating on X-rays equipments.
1. INTRODUCTION
Radiological examinations are frequently requested for various medical reasons. In spite of recent technological advances and other laboratory investigations, radiology remains a corner stone, and has maintained a time-honored position in the detection of pathology of the skeletal system as well as for detecting some disease processes in soft tissue. Some notable examples are the very common chest X-ray, which can be used to identify lung diseases such as pneumonia, lung cancer or pulmonary edema, and the abdominal X-ray, which can detect intestinal obstruction, free air (from visceral perforations) and free fluid (in ascites). X-rays may also be used to detect pathology such as gallstones, which are rarely radiopaque, or kidney stones, which are often; but not always; visible. Traditional plain X-rays are less useful in the imaging of soft tissues such as the brain or muscle [1,2].
X-rays are part of the electromagnetic spectrum (ionizing radiation). The higher the voltage of the generator the more penetrating will be the radiation [2]. Penetration of ionizing radiation can destroy living cells or make them dysfunction. The mechanism behind cell futility due to ionizing radiation is the production of chemically active free radicals generated from the decomposition of cellular water [3], which damage the molecular structure, resulting in cell dysfunction (somatic effect) or mutations (genetic damage). Kayan et al. [1], have investigated the effects of vitamins C and E administration on induced oxidative toxicity for the blood of smoking and non-smoking X-ray technicians.
Ionizing radiation generates the production of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide and hydroxyl radical in a variety of cells. The hydroxyl radicals are the most reactive radiolytic species and are the predominant damaging species oxidatively modifying biological molecules [4]. ROS manifest high reactivity to cellular macromolecule components including DNA, lipids and proteins [5,6]. They are also implicated in cellular radiation responses associated with signal transduction. Radiation affects genetic instability that leads to delayed biological effect such as gene mutation, chromosome aberration and cell death [7-9].
In view of their clinical importance, ease of use and low cost, erythrocytes were used in this study to investigate membrane damage induced by ultra-low doses of X-rays, which may increase our knowledge about the possible mechanisms of radiohemolysis.
2. MATERIAL AND METHODS
2.1. Preparation of Erythrocyte Samples
Heparinized fresh blood samples (n = 20) were withdrawn from 10 healthy human volunteers, with an age range between 20 and 30 years without any known occupational exposure to chemical and/or ionizing radiation, and stored at 4˚C. Samples were exposed to X-rays and were used for experimentation in the same day. Erythrocytes were isolated by centrifugation for 15 min. at 1500 revolution/min, plasma and buffy coat were removed and erythrocytes were washed three times with isotonic saline (0.15 M NaCl) at room temperature. Washed erythrocytes were suspended in saline to obtain a hematocrit of about 1%.
2.2. Irradiation of Erythrocytes
Each erythrocyte suspension was placed in a pyrex glass cuvette at a distance of 2 cm from the window of the X-ray source to be irrdiated with a Teletron’s TELX-Ometer (TEL-Atomic Inc., Jackson, Michigan, USA). The device operates at 30 KV and 80 μA, and the mean exposure level was about 10 µGy/h. The erythrocyte suspensions were exposed to different doses by increasing exposure time to emitted X-rays.
2.3. Radiohemolysis
After X-rays exposure, erythrocyte suspensions were shacked gently and the absorbance was recorded using a JENWAY visible range spectrophotometer Model 6300 at 577 nm and 630 nm (Keison Products, Grants Pass, Oregon, USA).
2.4. Conductivity Measurements
We measured the conductivity of erythrocyte suspendsions using a conductance bridge (Griffen and George Ltd., London, UK), where a glass tube that contains two platinum disc electrodes was filled with the erythrocyte suspension. Conductivity of erythrocyte suspensions without irradiation (σ˚) were carried out first, then it was measured for each irradiated sample of different doses (σ). The relative conductivity (σ/σ˚) for irradiated erythrocyte suspensions was then calculated.
2.5. Effect of Ascorbic Acid
Hemolysis of erythrocytes treated with ascorbic acid at a concentration of 5 mM were recorded using a JENWAY visible range spectrophotometer Model 6300 at 577 nm, where the decrease in intensity of the peak at 577 nm represents the degree of hemoglobin breakdown or the degree of hemolysis.
2.6. Effect of Ascorbic Acid on Pre-Irradiated Erythrocytes
Ascorbic acid at a concentration of 5 mM was added to pre-irradiated erythrocyte suspensions by a specific dose of X-rays, and the absorbance was recorded using a JENWAY visible range spectrophotometer Model 6300 at 577 nm.
2.7. Statistical Analysis
Results were represented as the mean ± SD of 8 - 10 experiments and appropriate statistical analysis were carried out for different comparisons.
3. RESULTS AND DISCUSSION
Ionizing radiation induces oxidation process of biomolecules. The effects are dependent to a large degree on the dose of the oxidizing agent [10]. In the present work, exposure of human erythrocytes to increasing doses of X-rays gives the curve reported in Figure 1. In this curve the process of hemoglobin oxidation begins with a nearly lag period of about 45 min. irradiation, which corresponds to an X-ray dose of approximately 7.5 µGy, then a dramatic dose-dependent decrease in absorbance appears reflecting a strong hemoglobin break down and consequently hemolytic effect. The last effect may be due to the production of ROS, which can cause this damage and also may be involved in peroxidation of membrane lipids [11].
It is known that in normal states, erythrocytes spectrum doesn’t show a peak at 630 nm and its appearance in spectrum can be considered as an indication of oxidative hemolysis of erythrocytes and the production of methemoglobin. According to the obtained results shown in Figure 2, a dose dependent increase in absorbance at 630 nm was observed, indicating a conversion of hemoglobin into methemoglobin and that the conversion is dose-dependent relationship. The electric conductivity
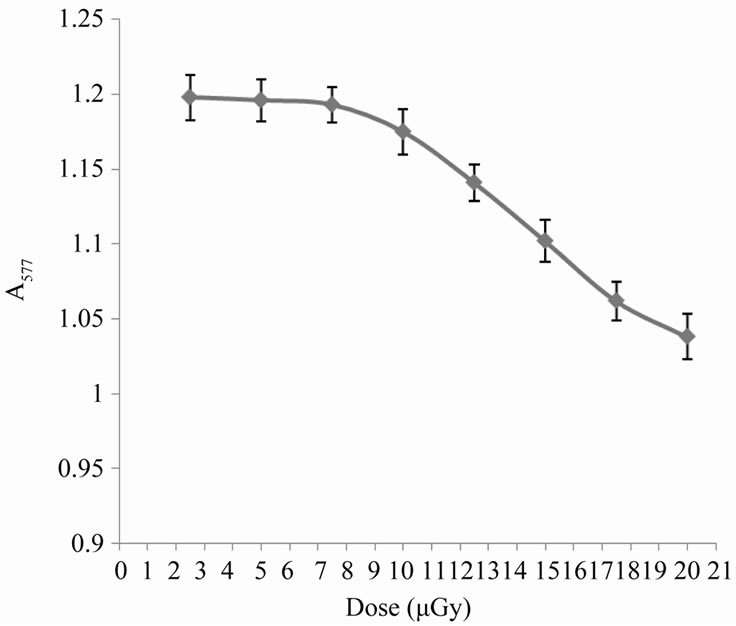
Figure 1. Variation in erythrocyte absorbance at 577 nm with the applied dose of X-rays.
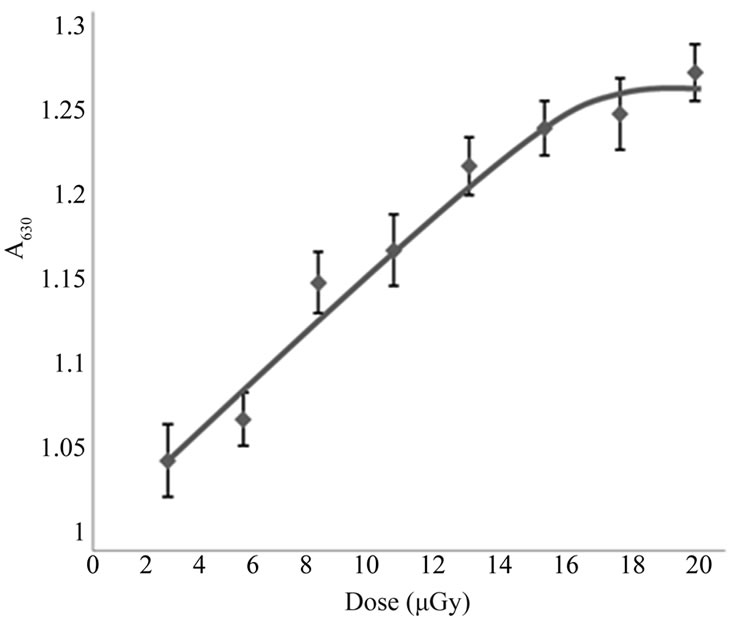
Figure 2. Variation in erythrocyte absorbance at 630 nm with the applied dose of X-rays.
measurements shown in Figure 3 confirm these the last observations, which showed a dose-dependent decrease in relative conductivity that may be due to the transformation of hem Fe2+ to Fe3+ [12].
For exposure doses lower than 7.5 µGy, X-ray irradiation effects could be confirmed using another oxidative agent like ascorbic acid. Figure 4 shows the changes in absorbance for the erythrocyte suspensions with time at wavelength 577 nm for the sample treated by 5 mM ascorbic acid only and for samples pre-irradiated by Xrays of low doses in the range form 1.7 µGy to 6.7 µGy, before affecting by 5 mM ascorbic acid. Ascorbic acid is considered to be an oxidative agent at high concentrations, which cause oxidative hemolysis due to its interaction with erythrocytes causing lipid peroxidation of their membranes, the formation of spectrin-hemoglobin complex in the inner surface of their membranes and the oxidation of hemoglobin to methemoglobin. This process leads to hemolysis of erythrocytes [13]. It is clear from Figure 4 that the exposure of erythrocyte suspensions to X-rays accelerated the process of oxidation produced by ascorbic acid when used alone, also that the process is dose dependent.
From the last Figure 4, there is a time interval during which the variations in absorbance with time is nearly linear, the slope of this interval gives the hemolysis rate (H.R); the rate at which the number of cells in the suspension decreases. The intersection of the slope line with time axis gives the hemolysis time (H.T); the time at which there is no cells in the suspension [14]. Figure 5 shows the variation of HR with different doses of X-rays. From this figure it is clear that the hemolysis rate increases by increasing the dose. Figure 6 shows that the HT is a dose-dependent at which the hemolysis time decreases by increasing dose. That is, results of HR and
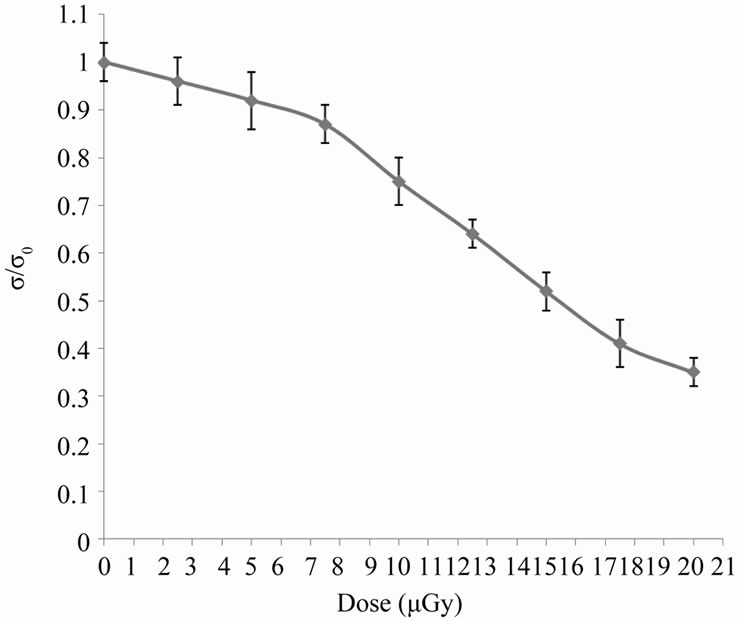
Figure 3. The change in relative conductivity due to different doses of X-rays.

Figure 4. Kinetics of hemoglobin breakdown in erythrocytes due to the effect of different X-rays doses.

Figure 5. Kinetics of hemoglobin breakdown in erythrocytes due to the effect of different X-rays doses.
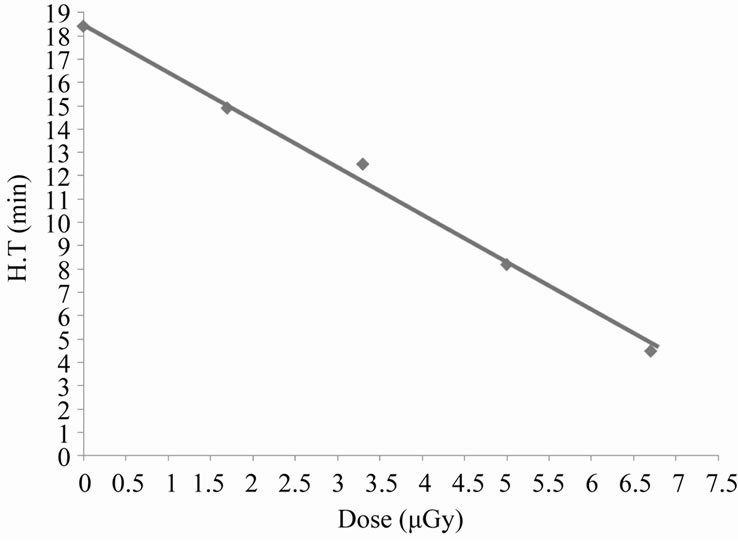
Figure 6. Kinetics of hemoglobin breakdown in erythrocytes due to the effect of different X-rays doses.
HT may lend support to the fact that the number of erythrocytes decreases rapidly at higher doses (hemolysis).
4. CONCLUSIONS
X-rays even at low levels of exposure may have oxidizing effect on erythrocytes, which must be taken into account for workers operating on X-rays equipments.
REFERENCES
- Kayan, M., Naziroglu, M., Celik, O., Yalman, K. and Koylu, H. (2009) Vitamin C and E combination modulates oxidative stress induced by X-ray in blood of smoker and non smoker radiology technicians. Cell Biochemistry and Functions, 27, 424-429. doi:10.1002/cbf.1589
- Huda, W. (2009) Review of radiologic physics. 3rd Edition, Lippincott Williams & Wilkins, Philadelphia.
- Kocer, I., Taysi, S., Ertekin, M.V., Karslioglu, I., Gepdiremen, A., Sezen, O. and Serifoglu, K. (2007) The effect of L-carnitine in the prevention of ionizing radiation-induced cataracts: A rat model. Graefe’s Archive for Clinical and Experimental Ophthalmology, 65, 27-33.
- Riley, P.A. (1994) Free radicals in biology: Oxidative stress and the effects of ionizing radiation. International Journal of Radiation Biology, 65, 27-33. doi:10.1080/09553009414550041
- Agrawal, A. and Kale, P.K. (2001) Radiation induced peroxidative damage: Mechanism and significance. Indian Journal of Experimental Biology, 39, 291-309.
- Prithivirajsingh, S., Story, M.D., Bergh, S.A., Geara, F.B., Ang, K.K., Ismail, S.M., Stevens, C.W., Buchholz, T.A. and Brock, W.A. (2004) Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Letters, 30, 227-232. doi:10.1016/j.febslet.2004.06.078
- Tominaga, H., Kodama, S., Matsuda, N., Suzuki, K. and Watanabe, M. (2004) Involvement of reactive oxygen species (ROS) in the induction of genetic instability by radiation. Journal of Radiation Research, 45, 181-188. doi:10.1269/jrr.45.181
- Maffei, F., Angelini, S., Forti, G.C., Vidante, F.S., Lodi, V., Mattioli, S. and Hrelia, P. (2004) Spectrum of chromosomal aberrations in peripheral lymphocytes of hospital workers occupationally exposed to low doses of ionizing radiation. Mutation Research, 547, 91-99.
- Hagelstrom, A.H., Gorla, N.B. and Larripa, I.B. (1995) Chromosomal damage in workers occupationally exposed to chronic low level ionizing radiation. Toxicology Letters, 76, 113-117. doi:10.1016/0378-4274(94)03204-K
- Krokosz, A. (2003) The effect of hypochlorite on human erythrocytes pretreated with x-radiation. Cellular & Molecular Biology Letters, 8, 215-219.
- Klucinski, P., Wojcik, A., Bochenek, R.G., Gminski, J., Mazur, B., Hrycek, A., Cieslik, P. and Martirosian, G. (2008) Erythrocyte antioxidant parameters in workers occupationally exposed to low levels of ionizing radiation. Annals of Agricultural and Environmental Medicine, 15, 9- 12.
- Ibrahim, I. H. (1997) Variation in electrical conductivity of human erythrocytes caused by ascorbic acid (a new method for following hemolysis). The 3rd International Conference on Engineering Mathematics and Physics, Cairo, 2, 653-656.
- Ibrahim, I. H. (2006) Oxidative hemolysis of erythrocytes induced by various vitamins. International Journal of Biochemistry Science, 2, 100-103.
- Ibrahim, I. H. (1988) Akislitelnei gemoliz erythrothitov. Ph.D. Thesis, Belarussia University, Minsk.

