Open Journal of Urology
Vol.3 No.2(2013), Article ID:31406,8 pages DOI:10.4236/oju.2013.32020
Distribution and Possible Function of Cannabinoid Receptor Subtype 1 in the Human Prostate*—An Inhibitory Role for Growth in the Human Prostate Cancer
1Department of Urology, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, Chuo City, Japan
2Department of Urology, Shiga University of Medical Science, Ohtsu City, Japan
Email: #matakeda@yamanashi.ac.jp
Copyright © 2013 Manabu Kamiyama et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 29, 2012; revised February 1, 2013; accepted February 9, 2013
Keywords: Prostate cancer; Prostate cell; Cannabinoid receptor; CB1
ABSTRACT
Background: Cannabinoid receptor subtype 1 (CB1) has a relationship to the proliferation of various cells including malignant tumoral cells. We investigated and compared the expression of CB1 in benign and malignant human prostate tissues and in benign and malignant human prostate cell lines, as well as its function for the proliferation of human prostate cancer cells. Methods: Real-time quantitative PCR was performed to compare its expressions in human prostate tissues (normal, benign hyperplasia, and cancer) and prostate cell lines (3 normal and 3 malignant). For localization of CB1, immunofluorescent staining with rabbit anti-CB1 polyclonal antibodies and tetramethyl isothiocyanate (TRITC)-labeled swine anti-rabbit immunoglobulin (DAKO) were used under fluorescence microscope. To further analyze whether cell death was induced by anandamide (non-selective agonist for CB1/CB2) via a receptor dependent mechanism, the viability of DU145 cells, which is known as androgen-insensitive prostate cancer cell, was measured using MTT assay. Results: CB1mRNA was found to be expressed in the all 3 human prostate tissues, however, CB1 protein was expressed in BPH and low grade malignant PC tissues, but not in high grade malignant PC tissues. CB1 as for cell lines, the expression of CB1 was low in malignant cell lines except for DU145. Anandamide elicited cell death, which was significantly inhibited by AM251 (selective antagonist for CB1), indicating that cell death induced by anandamide in DU145 cells was mediated by CB1. Anandamide time-dependently elicits up-regulation of CB1 in DU145 cells. Conclusions: CB1 may be an inhibitory regulator of androgen-insensitive human prostate cancer epithelial cell growth.
1. Introduction
In recent years, cannabinoids the active components of Cannabis sativa linnaeus (marijuana) and their derivatives are drawing renewed attention because of their diverse pharmacological activities such as cell growth inhibition, anti-inflammatory effects, and tumor regression [ 1 -6].
The cannabinoid receptor has two subtypes, CB1 and CB2, which are both guanine-nucleotide-binding protein (G-protein)-coupled receptors [7]. CB1 was initially characterized in rat brains [8], and later cloned from rat cerebral cortex [9] and human testis [10]. CB1 distributed mainly throughout the central nervous system, in the cerebellum, hippocampus, and cerebral cortex [ 11 ], and has been found present on peripheral neurons and in non-neuronal tissues including pituitary gland, adrenal gland, lung, testis, ovary, uterus, prostate, eye, and vascular tissue [ 11-15 ].
CB2 was cloned from rat spleen macrophages [16], and has also been found in the peripheral immune system tissues, such as spleen and tonsils, and particularly expressed on B-cells and natural killer cells [ ADDIN EN.CITE ADDIN EN.CITE.DATA 11 ].
As for ligands of these cannabinoid receptors, in addition to delta-9-tetrahydrocannabinol (THC), the major active component of marijuana (Cannabis sativa), arachidonoyl ethanolamide (anandamide) [ 17 ], 2-arachidonoyl glycerol [ 18,19 ], and 2-arachidonoyl glyceryl ether [ 20 ] were discovered to be endogenous ligands and later termed endocannabinoids. Anandamide is found in a wide range of brain tissues and non-neuronal tissues [ 21 ], and its localization overlaps with that of CB1 [22]. Cannabinoids are known to control the proliferation of various cells such as neural cells and malignant cells [23], and their effect is elicited mostly by CB1 [24].
Prostate cancer ranks as the most common non-cutaneous malignancy and the second leading cause of cancer-related deaths in American males, with similar trends in many Western countries. According to an estimate of the American Cancer Society, a total of 234,460 men will be diagnosed with prostate cancer in the United States in the year 2006 and 27,350 prostate cancer-related deaths are predicted [25]. The major cause of mortality from this disease is metastasis of hormone refractory cancer cells that fail to respond to hormone ablation therapy [26,27]. Because surgery and current treatment options have proven to be inadequate in treating and controlling prostatic cancer, the search for novel targets and mechanism-based agents for prevention and treatment of this disease has become a priority. Recent researches revealed the following new data regarding the role of cannabinoid receptors on the prostate cancer growth; Cellular 2-arachidonoyl glycerol, acting through the CB1 receptor, is an endogenous inhibitor of invasive prostate cancer cells [ 28 ]. Both CB1 and CB2 are present in several prostate cancer cell lines, and WIN 55,212-2, a mixed CB1/CB2 receptor agonist imparts cell growth inhibitory effects in LNCaP cells via an induction of apoptosis, not in the normal prostate epithelial cell at similar doses [ 29 ].
Treatment of with cannabinoid receptors (CB1/CB2) agonist WIN-55,212-2 elicited apoptosis in human prostate cancer LNCaP cells through a sustained activation of ERK1/2, induction of p27/KIP1, and inhibition of cyclin D1 [30].
Hence, we investigated and compared the distribution of CB1 in several different human prostate tissues, and several different prostate cell lines. Furthermore, we investigated the effect of CB agonist; anandamide, and CB1 selective antagonist; AM251, on the proliferation of DU145, which is known as androgen-insensitive prostate cancer cell line.
2. Materials and Methods@NolistTemp#2.1. Human Tissue Samples
Five normal human prostate specimens were obtained during cystoprostatectomy for bladder cancer, 5 benign prostatic hyperplasia (BPH) specimens came from transurethral prostatectomy (TUR-P) or suprapubic prostatectomy procedures, and 5 malignant prostatic tumor specimens were from radical prostatectomy or prostatic biopsy. The study protocol was approved by the Ethics committee of University of Yamanashi and all samples were used after receiving full informed consent from each patient.
2.2. Cell Culture
PC3 (ATCC CRL 1435), DU145 (ATCC HTB 81), and LNCaP (ATCC CRL 1740), human prostate carcinoma cell lines, were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). They were maintained in RPMI1640, Ham’s F12, and DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), respectively, along with antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin). The media and additives were purchased from Invitrogen (Carlsbad, CA, USA). PrEC (epithelial, CC-2555), PrSC (stromal, CC- 2508) and PrSMC (smooth muscle, CC-2587), which were derived in BioWhittaker (Walkersville, MD, USA), were normal human prostate cell lines. They were cultured in different growth medium (GM) according to the manufacturer’s instruction, PrEGM, SCGM, and SmGM- 2, respectively, which contained supplements and growth factors. These normal cell lines and media were obtained from SankoJunyaku (Tokyo, Japan). All cells were seeded on culture dishes and incubated in a 5% CO2 humidified atmosphere at 37˚C.
2.3. RT-PCR
Total RNA was isolated from cultured cells and prostate tissues using an RNeasy mini kit and RNase-Free DNase set (Qiagen, Hilden, Germany), according to the manufacturer’s protocols. Approximately 1 μg of extracted RNA was reverse transcribed into complementary DNA (cDNA) using avian myeloblastosis virus (AMV) reverse transcriptase (first strand cDNA synthesis kit; Roche, Basel, Switzerland). Reverse transcription was performed using a thermal program at 42˚C for 60 minutes and 99˚C for 5 minutes.
A qualitative PCR assay was performed with a thermal cycler system. Gene-specific primers were designed using the online program Primer 3 (Table 1). PCR amplification was performed at 94˚C for 5 minutes, followed by 30 cycles at 94˚C for 30 seconds, then 60˚C for 30 seconds, 72˚C for 1 minute, and 72˚C for 7 minutes, with a 50 μl mixture containing 0.2 μM of each pair of genespecific primers and a PCR core kit (Roche). PCR-amplified products were resolved on a 3.5% agarose gel and visualized with ethidium bromide. Some PCR products
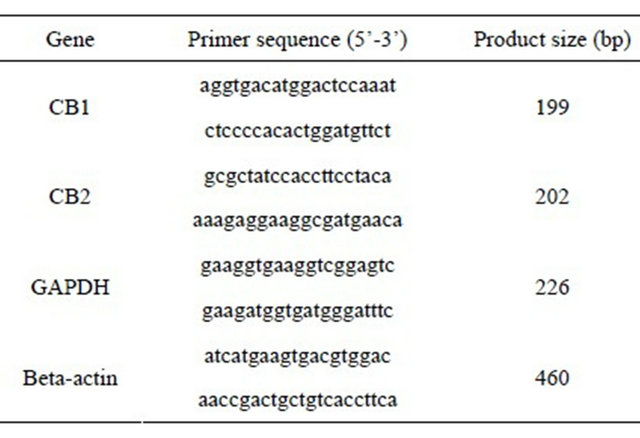
Table 1. Primers used for PCR.
were purified and sequenced using an automated sequencing machine to identify the target gene.
2.4. Real Time Quantitative RT-PCR
Quantitative PCR assay was performed with a Smart Cycler System (Cephied, Sunnyvale, CA, USA) using SYBR green I as the fluorogenic dye (Molecular Probes, Eugene, OR, USA) [31]. Each cDNA, the products of RT-PCR, was subjected to 40 PCR cycles of 94˚C for 10 seconds and 60˚C for 20 seconds, with a measuring point of 6 seconds, in a 25 μl mixture containing 0.2 μM of each pair of gene-specific primers and an Ex taq R-PCR kit for a hot start (Takara, Shiga, Japan). The measuring point was set about 3 degrees lower than the specific melting peak point of the target gene to reduce the amount of nonspecific DNA products. Gene expression in each cell line was quantified as the product of the target gene relative to that of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene.
2.5. Proliferation of DU145 Cells and Treatment of Cells
Anandamide (an endogenous cannabinoid receptor agonist) was purchased from Sigma-RBI (St. Louis, MO, USA). AM251 (a selective CB1 antagonist) was purchased from Tocris (Ellisville, MO, USA). Anandamide was dissolved and diluted by ethanol, while AM251 was by DMSO.
To evaluate anandamide-induced cell death in androgen-insensitive prostatic cancer cells, DU145 were cultured in serum-free medium and treated with 4 different concentrations (5, 10, 20, and 40 Um) anandamide solutions and control ethanol solution for the indicated times. To evaluate the effect of CB1 on the anandamide-induced cell death in DU145, 2 different concentrations (2, and 10 Um) of AM251 and control DMSA solution were used.
Cell viability was measured with MTT assay and expressed as the rate compared to non-treated controls in each period. 3-4,5-dimethylthiazole-2,5-diphenyltetrazolium bromide thiazole blue (MTT) assay kit was purchased from Chemicon. MTT assay was performed to measure cell proliferation. In this experiment, DU145 cells, from a hormone refractory cell line, were used for the cell proliferation assay. DU145 cells were suspended in serum-free medium and seeded at a density of approximately 2 × 104 cells/well into a 96-well culture plate. After 12 hours of seeding, cells were treated with these agonists and antagonists individually or in combination. After the appropriate times of treatment, MTT solution (0.5 mg/ml final concentration) was added to each well and incubation was continued for 4 hours. Since mitochondrial enzyme converts MTT to insoluble formazan crystals, 100 μl color development solution containing isopropanol and 0.04 N HCl were added to dissolve the crystals. Absorption values at 570 - 630 nm were determined with an automatic microplate reader (Bio-Rad, Hercules, CA, USA).
2.6. Immunofluorescent Staining
Frozen tissues embedded in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura, Tokyo, Japan) were cut serially into 7 μm thick sections, which were fixed in acetone (4˚C, 10 minutes), and non-specific reactions were blocked using blocking reagent (RT, 10 minutes; DakoCytomation, Glostrup, Denmark). Rabbit anti-CB1, and anti-CB2 polyclonal antibodies were purchased from Chemicon (Temecula, CA, USA), and used as primary antibodies at a dilution of 1:200 - 250 (RT, 1 hour). Controls were stained without primary antibodies. Antibody reactions were detected with tetramethyl isothiocyanate (TRITC)-labeled swine anti-rabbit immunoglobulin (DAKO) at a dilution of 1:100 (RT, 30 minutes), after which the labeled cells, colored red, were detected using a fluorescence microscope.
2.7. Statistical Analysis
Data are reported as means ± standard deviations of 3 independent experiments. Statistical analyses were performed by one-way analysis of variance or an unpaired t test (Microsoft Excel program). A p value of less than 0.05 was taken to indicate statistical significance.
3. Results@NolistTemp#3.1. Expressions of Cannabinoid Receptor mRNAs in Human Prostate Tissue
A qualitative PCR assay was performed to detect the gene expressions of cannabinoid receptors in human prostate tissues. CB1 and CB2 were detected to some degree in all normal, BPH, and prostatic cancer samples (Figure 1). In this experiment, the cancer samples might
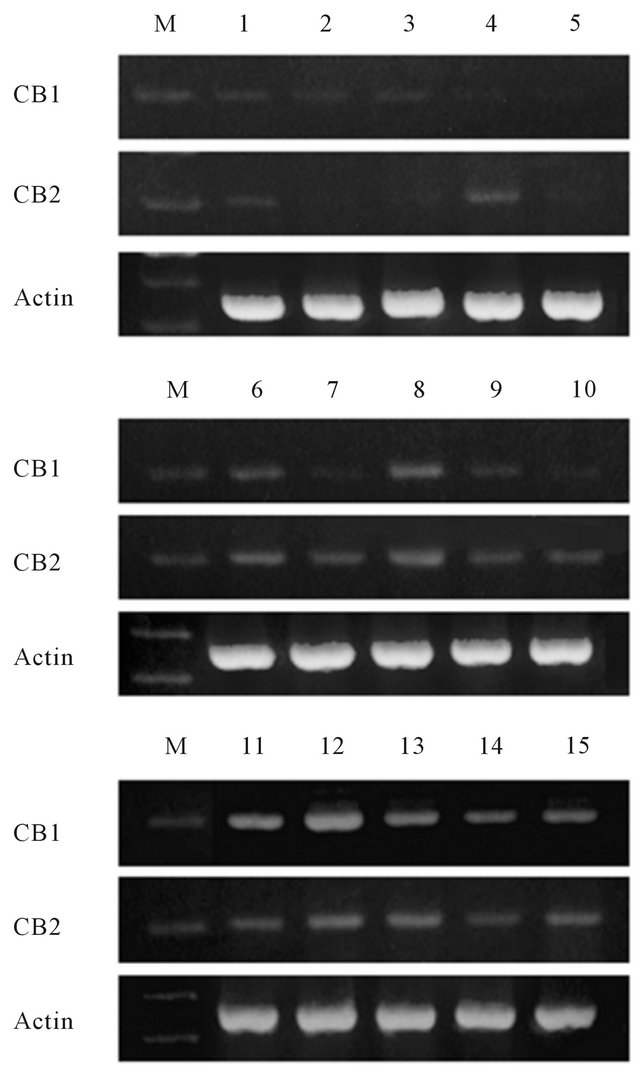
Figure 1. Qualitative PCR assay of the expression of cannabinoid receptors in human prostate tissues. The receptors were expressed in all of benign and malignant samples. Lanes 1 - 5, normal prostate; lanes 6 - 10, benign prostatic hyperplasia; lanes 11 - 15, prostate cancer. Cancer samples also contained non-cancerous regions. M: DNA molecular weight marker (100 bp).
also contain non-cancerous regions.
3.2. Quantification of Expression of Cannabinoid Receptor in Human Prostate Cell Lines
To measure the gene expressions of cannabinoid recaptors in human prostate cell lines, real time quantitative PCR was performed and the results were evaluated relatively (Figure 2). CB1 gene expression was found in DU145 cells to the same degree as in the normal epithelial cells, but more weakly in PC3 and LNCaP cells. The expression of CB2 was very low in all the prostate cell lines.
3.3. Cell Death Induced by Anandamide via a Receptor Dependent Mechanism
Cell viability and cytotoxicity were determined by the ability of the cells to yield dark blue formazan products

Figure 2. Relative expression to GAPDH of CB1 and CB2 genes in human prostate cell lines. Gene expressions of the receptors were measured using real time quantitative PCR. The expression of CB1 was nearly the same degree in DU145 and benign prostate cell lines. CB2 was also expressed in all cell lines, however, very weakly. MB231 cell: breast adenocarcinoma cell. Data are presented as means ± standard deviations of 3 independent experiments.
from pale yellow substrates (MTT) in proportion to the quantity of active mitochondria in viable cells. Examination of the action of cannabinoids in prostate cancer cells was initiated by analyzing the effect of anandamide on the viability of DU145 cells. DU145 cells were treated with different doses of anandamide for various durations. Figure 3 shows that cell viability became lower as the concentration of anandamide increased after 3, 6, 12, and 24 hours (p < 0.05). In addition, the cell viability curves clearly incline to the lower right, indicating that treatment with anandamide decreased the viability of DU145 cells in a doseand time-dependent manner. In order to analyze whether cell death induced by anandamide was mediated by a cannabinoid receptor, DU145 cells were incubated in the presence of 10 μM of anandamide with or without the selective antagonist of this receptor for 24 hours. The effect induced by 10 μM of anandamide was significantly prevented by 10 μM of AM251, but not by 2 μM AM251 (Figure 4).
To measure the gene expression of CB1 in DU145 cells treated with anandamide, real time quantitative PCR was performed and the result was evaluated relatively (Figure 5). The gene expression of CB1 increased until 24 hours after treatment with anandamide.
3.4. Expression of Cannabinoid Receptors (CB1 and CB2) in Human Prostate Tissue by Immunofluorescent Staining
Figure 6 shows characteristic patterns of CB1 and CB2 in prostatic tissues. In BPH tissues, CB1 and CB2 were localized in the epithelial regions. In the prostatic cancer
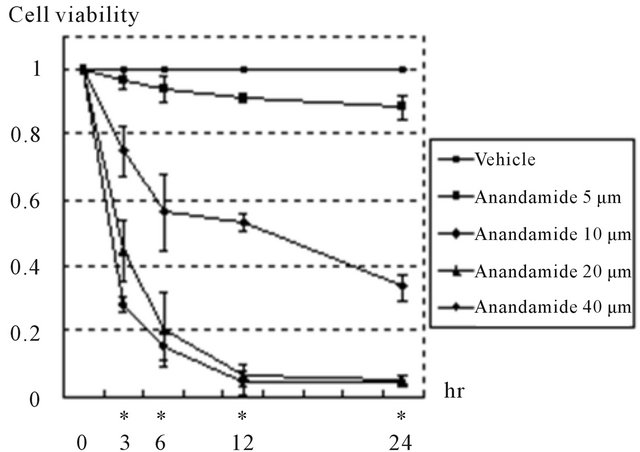
Figure 3. Anandamide induced cell death in DU145 cells. Cells were cultured in serum-free medium and treated with anandamide for the indicated times. Cell viability was measured with MTT assay and expressed as the rate compared to non-treated controls in each period. Treatment with anandamide reduced cell viability in a doseand timedependent manner. Data are presented as means ± standard deviations of 3 independent experiments. *Significant differences (p < 0.05), shown in each period of treatment by one-way analysis of variance.

Figure 4. Anandamide induced DU145 cell death via CB1. Cells cultured in serum-free medium in the presence of 10 μM of anandamide with or without AM251 for 24 hours. Cell death induced by 10 μM of anandamide was significantly prevented by 10 μM of AM251. A significant effect on cell viability was not seen in the vehicles alone (data not shown). Cell viability was expressed as the rate compared to non-treated controls. Data are presented as means ± standard deviations of 3 independent experiments. Significant differences (p < 0.05): *versus no-treatment, #versus treatment with 10 μM of anandamide.
tissues, with Gleason score 4 + 5 = 9 and moderatelydifferentiated adenocarcinoma, the expressions of either CB1 or CB2 in epithelial cells were almost negative (figure 6).
Figure 7 revealed the comparison between low grade malignant and high grade malignant PC. In PC with Gleason score 3 + 3 = 6, well differentiated adenocarci-

Figure 5. Anandamide elicited the up-regulation of target receptor genes in DU145 cells. Gene expressions of receptors in treated cells were measured by real time quantitative PCR. A, anandamide elicited the up-regulation of CB1. Gene expressions were expressed as the rate compared to that at 0 hour. Data are presented as the means ± standard deviations of 3 independent experiments. *Significant differences (p < 0.05) versus 0 hour.
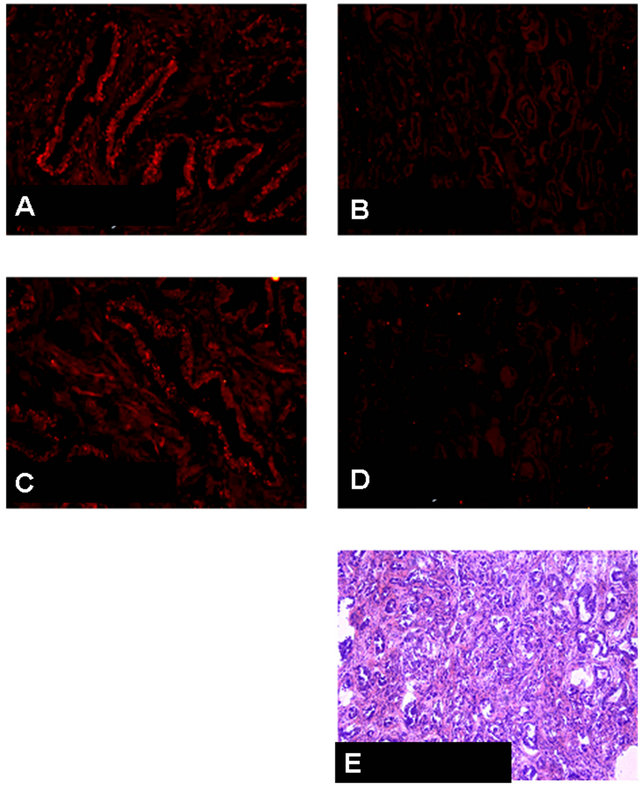
Figure 6. CB1 and CB2 immunohistochemical findings, and H&E staining in benign prostatic hyperplasia (BPH) and protstatic cancer (PC) with Gleason score 4 + 5 = 9, moderately differentiated adenocarcinoma. (A) CB1 immunostaining for BPH tissue. Positive staining in the epithelial cells is shown. ×400; (B) CB2 immunostaining for (BPH) tissue. Positive staining in the epithelial cells is shown. ×400; (C) CB1 immunostaining for PC tissue with Gleason score 4 + 5 = 9. Negative staining in the epithelial cells. ×200; (D) CB2 immunostaining for PC tissue same as C. Negative staining in the epithelial cells. ×200; (E) H & E staining for PC tissue same as C and D.
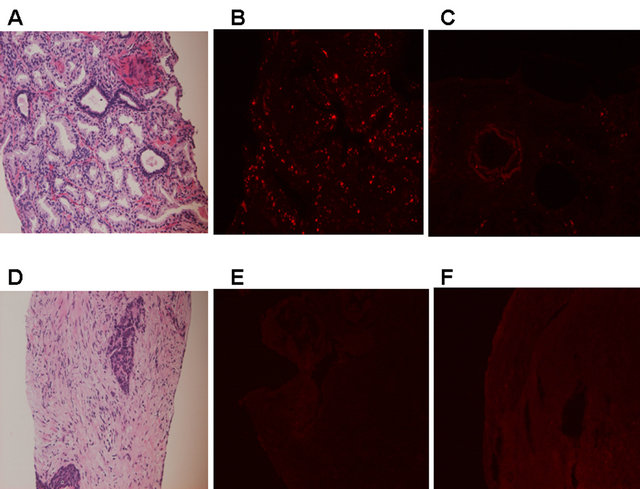
Figure 7. CB1 immunohistochemical findings in protstatic cancer (PC) in 2 patients with different grades. (A)-(C): PC with Gleason score 3 + 3 = 6, well differentiated adenocarcinoma; (D)-(F): PC with undifferentiated, neuroeodocrine carcinoma. (A): H & E staining. ×200; (B): CB1 immunostaining. Positive staining in the epithelial cells is shown. ×200; (C): Negative control of CB1 immunostaining. ×200; (D): H & E staining. ×200; (E): CB1 immunostaining. Negative staining throughout the tissue. ×200; (F): Negative control of CB1 immunostaining. ×200.
noma, CB1-positive staining in the epithelial cells is shown (figures 7(A)-(C)). However, negative CB1 staining in the epithelial cells is shown in PC with undifferentiated, neuroeodocrine carcinoma (figures 7(D)- (F)).
4. Discussion
It is known that CB1 is distributed mainly in the nerve system, however, this receptor has also been detected in various non-neuronal tissues including prostate [ ADDIN EN.CITE ADDIN EN.CITE.DATA 11 ]. We found CB1 gene expression in both several human prostate tissues, and cell lines, regardless of whether the samples were benign or malignant. According to our results, gene expression of CB1 in LNCaP and PC3, but not DU-145, are lower than benign prostate cells (epithelial cell, stromal cell, and muscle cell).
According to these gene expression studies, we assumed that DU145 is much more suitable for growth inhibition experiment with CB agonist than LNCaP and PC3. As expected, DU145 revealed that concentration-dependent inhibition of cell growth by anandamide, a non-selective agonist for CB1/CB2. Furthermore, addition of 10 uM of AM251, a selective antagonist for CB1, significantly reversed the growth inhibition by anandamide. These results indicate that cannabinoid-induced cell death is mediated by CB1 in DU145. Similar results have been reported in thyroid carcinoma , and other cancer cells [ ADDIN EN.CITE ADDIN EN.CITE.DATA 1 , 2 , 4 ,32, 33 ].
We might consider that CB2 positivity in prostate cancer indicated possible existence of inflammation and immunocyte infiltration, since CB2 was more weakly detected than CB1 in all cell lines by RT-PCR. However, other report showed that both CB1 and CB2 activation might inhibit cell growth [ ADDIN EN.CITE ADDIN EN.CITE.DATA 29 ].
Furthermore, the effect of cannabinoids on cancer cell growth, especially breast cancer cells, has been studied. Anandamide was found to elicit a remarkable anti-proliferative effect on the human breast cancer cell lines MCF-7, EFM-19, and T-47D via CB1 [ 32-34 ]. Anandamide inhibits the proliferation of human breast cancer cells which are stimulated by prolactin and nerve growth factor (NGF), and down-regulates both the long form (100 kDa) of the prolactin receptor (PRLr) and the high affinity NGF receptor (Trk) via CB1 activation, which leads the inhibition of the adenylyl cyclase (AC)-cAMP/ protein kinase A (PKA) pathway and the activation of the Raf-1/extracellular signal-regulated kinase (ERK) cascade [ 33 ].
NGF receptors are related to the neoplastic progression of human prostate. During malignant transformation, expression of the low affinity NGF receptor (p75LNGFR) is reduced [35], however, that of Trk is retained at the same level. While p75LNGFR is expressed in normal prostate, the expression is partially lost in BPH and malignant prostate tissues, and completely lost in the metastatic cell lines PC3, DU145, LNCaP, and TSU-prl [36]. Furthermore, expression of p75LNGFR in transfected TSU-prl cell reduces NGF-induced cell growth by activation of program cell death. Therefore, p75LNGFR is a negative regulator of human prostate epithelial cell growth, whereas Trk A is a positive regulator [ 37,38 ]. The same as breast cancer cells, anandamide also may elicit a down-regulation on PRLr/Trk levels and inhibits the proliferation of DU145 cells, which are stimulated by prolactin and NGF, via a CB1-dependent mechanism [34]. Cannabinoid receptor can be deeply concerned with NGF receptors in the proliferation of prostate cancer cell induced by cannabinoids.
In addition to our gene expression study, either CB1 or CB2 protein expression in high grade malignant PC tissues was almost negative. On the other hand, BPH and low grade malignant PC tissues showed higher degree of expression of CB1 protein than in high grade malignant PC tissues. CB receptor agonists can inhibit cancer cell invasion via increased expression of tissue inhibitor metalloproteinase-1 in lung cancer cells, and also in other cancer cells [5]. In a recent report, tetrahydrocannabinoid (THC), agonist for CB1 and CB1, has inhibited in vitro motility, and in vivo growth and metastasis [ 39 ]. In another very recent report, CB1 expression is not correlated with the grade of malignancy in PC [ 40 ]. Our present gene expression experiments are that PC3, highest malignant grade PC cells revealed lowest degree of CB1 gene expression, and that DU145, lower or moderate malignant grade PC cells revealed highest degree of CB1 gene expression. According to these gene expression data with cultured PC cells and protein expression with human PC tissues, we may hypothesized that benign tissue and low malignant PC tissues express higher degree of CB1 protein expression, and that growth of these tissues can be inhibited by CB1 agonists. On the other hand, higher grade malignant PC tissues do not express CB1 protein, hence, growth of these tissues can not be inhibited by CB1 agonists. To overcome the protective effect in higher grade malignant PC tissues, induction of CB1 into these tisseus or cells may be effective.
In summary, anandamide inhibits the proliferation of human prostate cancer epithelial cells, DU145, via CB1 receptor. It suggests that cannabinoids have a potential tumor suppression effect, though further study of this receptor as new targets in prostate cancer therapy is required.
5. Acknowledgements
We are grateful to Kazuhiro Mitsui, Kiyomi Tanabe and Yukiko Tsuchiya for their technical assistances.”
REFERENCES
- I. Galve-Roperh, C. Sanchez, M. L. Cortes, T. G. del Pulgar, M. Izquierdo and M. Guzman, “Anti-Tumoral Action of Cannabinoids: Involvement of Sustained Ceramide Accumulation and Extracellular Signal-Regulated Kinase Activation,” Nature Medicine, Vol. 6, No. 3, 2000, pp. 313-319. doi:10.1038/73171
- M. Bifulco, C. Laezza, G. Portella, et al., “Control by the Endogenous Cannabinoid System of Ras Oncogene-Dependent Tumor Growth,” Faseb Journal, Vol. 15, No. 14, 2001, pp. 2745-2747.
- C. Sanchez, M. L. de Ceballos, T. G. del Pulgar, et al., “Inhibition of Glioma Growth in Vivo by Selective Activation of the CB(2) Cannabinoid Receptor,” Cancer Research, Vol. 61, No. 15, 2001, pp. 5784-5789.
- M. L. Casanova, C. Blazquez, J. Martinez-Palacio, et al., “Inhibition of Skin Tumor Growth and Angiogenesis in Vivo by Activation of Cannabinoid Receptors,” Journal of Clinical Investigation, Vol. 111, No. 1, 2003, pp. 43-50.
- M. Guzman, “Cannabinoids: Potential Anticancer Agents,” Nature Reviews Cancer, Vol. 3, No. 10, 2003, pp. 745- 755. doi:10.1038/nrc1188
- T. W. Klein, “Cannabinoid-Based Drugs as Anti-Inflammatory Therapeutics,” Nature Reviews Immunology, Vol. 5, No. 5, 2005, pp. 400-411. doi:10.1038/nri1602
- A. C. Howlett, J. M. Qualy and L. L. Khachatrian, “Involvement of Gi in the Inhibition of Adenylate Cyclase by Cannabimimetic Drugs,” Molecular Pharmacology, Vol. 29, No. 3, 1986, pp. 307-313.
- W. A. Devane, F. A. Dysarz, 3rd, M. R. Johnson, L. S. Melvin and A. C. Howlett, “Determination and Characterization of a Cannabinoid Receptor in Rat Brain,” Molecular Pharmacology, Vol. 34, No. 5, 1988, pp. 605-613.
- L. A. Matsuda, S. J. Lolait, M. J. Brownstein, A. C. Young and T. I. Bonner, “Structure of a Cannabinoid Receptor and Functional Expression of the Cloned cDNA,” Nature, Vol. 346, No. 6284, 1990, pp. 561-564. doi:10.1038/346561a0
- C. M. Gerard, C. Mollereau, G. Vassart and M. Parmentier, “Molecular Cloning of a Human Cannabinoid Receptor Which Is Also Expressed in Testis,” Biochemical Journal, Vol. 279, Pt. 1, 1991, pp. 129-134.
- S. Galiegue, S. Mary, J. Marchand, et al., “Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations,” European Journal of Biochemistry, Vol. 232, No. 1, 1995, pp. 54-61. doi:10.1111/j.1432-1033.1995.tb20780.x
- A. J. Straiker, G. Maguire, K. Mackie and J. Lindsey, “Localization of Cannabinoid CB1 Receptors in the Human Anterior Eye and Retina,” Investigative Ophthalmology & Visual Science, Vol. 40, No. 10, 1999, pp. 2442- 2448.
- J. Liu, B. Gao, F. Mirshahi, et al., “Functional CB1 Cannabinoid Receptors in Human Vascular Endothelial Cells,” Biochemical Journal, Vol. 346, Pt. 3, 2000, pp. 835-840. doi:10.1042/0264-6021:3460835
- U. Pagotto, G. Marsicano, F. Fezza, et al., “Normal Human Pituitary Gland and Pituitary Adenomas Express Cannabinoid Receptor Type 1 and Synthesize Endogenous Cannabinoids: First Evidence for a Direct Role of Cannabinoids on Hormone Modulation at the Human Pituitary Level,” Journal of Clinical Endocrinology & Metabolism, Vol. 86, No. 6, 2001, pp. 2687-2696. doi:10.1210/jc.86.6.2687
- R. G. Pertwee, “Evidence for the Presence of CB1 Cannabinoid Receptors on Peripheral Neurones and for the Existence of Neuronal Non-CB1 Cannabinoid Receptors,” Life Science, Vol. 65, No. 6-7, 1999, pp. 597-605. doi:10.1016/S0024-3205(99)00282-9
- S. Munro, K. L. Thomas and M. Abu-Shaar, “Molecular Characterization of a Peripheral Receptor for Cannabinoids,” Nature, Vol. 365, No. 6441, 1993, pp. 61-65. doi:10.1038/365061a0
- W. A. Devane, L. Hanus, A. Breuer, et al., “Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor,” Science, Vol. 258, No. 5090, 1992, pp. 1946-1949. doi:10.1126/science.1470919
- T. Sugiura, S. Kondo, A. Sukagawa, et al., “2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain,” Biochemical and Biophysical Research Communications, Vol. 215, No. 1, 1995, pp. 89-97. doi:10.1006/bbrc.1995.2437
- R. Mechoulam, S. Ben-Shabat, L. Hanus, et al., “Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors,” Biochemical Pharmacology, Vol. 50, No. 1, 1995, pp. 83-90. doi:10.1016/0006-2952(95)00109-D
- L. Hanus, S. Abu-Lafi, E. Fride, et al., “2-Arachidonyl Glyceryl Ether, an Endogenous Agonist of the Cannabinoid CB1 Receptor,” Proceedings of the National Academy of Sciences, Vol. 98, No. 7, 2001, pp. 3662-3665. doi:10.1073/pnas.061029898
- C. C. Felder, A. Nielsen, E. M. Briley, et al., “Isolation and Measurement of the Endogenous Cannabinoid Receptor Agonist, Anandamide, in Brain and Peripheral Tissues of Human and Rat,” FEBS Letters, Vol. 393, No. 2-3, 1996, pp. 231-235. doi:10.1016/0014-5793(96)00891-5
- A. Szallasi and V. Di Marzo, “New Perspectives on Enigmatic Vanilloid Receptors,” Trends in Neurosciences, Vol. 23, No. 10, 2000, pp. 491-497. doi:10.1016/S0166-2236(00)01630-1
- M. Guzman, C. Sanchez and I. Galve-Roperh, “Control of the Cell Survival/Death Decision by Cannabinoids,” Journal of Molecular Medicine, Vol. 78, No. 11, 2001, pp. 613-625. doi:10.1007/s001090000177
- V. Di Marzo, T. Bisogno and L. De Petrocellis, “Anandamide: Some Like It Hot,” Trends in Pharmacological Sciences, Vol. 22, No. 7, 2001, pp. 346-349. doi:10.1016/S0165-6147(00)01712-0
- A. Jemal, R. Siegel, E. Ward, et al., “Cancer Statistics, 2006,” CA: A Cancer Journal for Clinicians, Vol. 56, No. 2, 2006, pp. 106-130. doi:10.3322/canjclin.56.2.106
- S. R. Denmeade, X. S. Lin and J. T. Isaacs, “Role of Programmed (Apoptotic) Cell Death during the Progression and Therapy for Prostate Cancer,” Prostate, Vol. 28, No. 4, 1996, pp. 251-265. doi:10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G
- D. G. Tang and A. T. Porter, “Target to Apoptosis: A Hopeful Weapon for Prostate Cancer,” Prostate, Vol. 32, No. 4, 1997, pp. 284-293. doi:10.1002/(SICI)1097-0045(19970901)32:4<284::AID-PROS9>3.0.CO;2-J
- K. Nithipatikom, M. P. Endsley, M. A. Isbell, et al., “2-Arachidonoylglycerol: A Novel Inhibitor of Androgen-Independent Prostate Cancer Cell Invasion,” Cancer Research, Vol. 64, No. 24, 2004, pp. 8826-8830. doi:10.1158/0008-5472.CAN-04-3136
- S. Sarfaraz, F. Afaq, V. M. Adhami and H. Mukhtar, “Cannabinoid Receptor as a Novel Target for the Treatment of Prostate Cancer,” Cancer Research, Vol. 65, No. 5, 2005, pp. 1635-1641. doi:10.1158/0008-5472.CAN-04-3410
- S. Sarfaraz, F. Afaq, V. M. Adhami, A. Malik and H. Mukhtar, “Cannabinoid Receptor Agonist-Induced Apoptosis of Human Prostate Cancer Cells LNCaP Proceeds through Sustained Activation of ERK1/2 Leading to G1 Cell Cycle Arrest,” Journal of Biological Chemistry, Vol. 281, No. 51, 2006, pp. 39480-39491. doi:10.1074/jbc.M603495200
- L. De Petrocellis, D. Melck, A. Palmisano, et al., “The Endogenous Cannabinoid Anandamide Inhibits Human Breast Cancer Cell Proliferation,” Proceedings of the National Academy of Sciences, Vol. 95, No. 14, 1998, pp. 8375-8380. doi:10.1073/pnas.95.14.8375
- D. Melck, D. Rueda, I. Galve-Roperh, L. De Petrocellis, M. Guzman and V. Di Marzo, “Involvement of the cAMP/ Protein Kinase A Pathway and of Mitogen-Activated Protein Kinase in the Anti-Proliferative Effects of Anandamide in Human Breast Cancer Cells,” FEBS Letters, Vol. 463, No. 3, 1999, pp. 235-240. doi:10.1016/S0014-5793(99)01639-7
- D. Melck, L. De Petrocellis, P. Orlando, et al., “Suppression of Nerve Growth Factor Trk Receptors and Prolactin Receptors by Endocannabinoids Leads to Inhibition of Human Breast and Prostate Cancer Cell Proliferation,” Endocrinology, Vol. 141, No. 1, 2000, pp. 118-126. doi:10.1210/en.141.1.118
- M. Perez, T. Regan, B. Pflug, J. Lynch and D. Djakiew, “Loss of Low-Affinity Nerve Growth Factor Receptor during Malignant Transformation of the Human Prostate,” Prostate, Vol. 30, No. 4, 1997, pp. 274-279. doi:10.1002/(SICI)1097-0045(19970301)30:4<274::AID-PROS8>3.0.CO;2-L
- B. R. Pflug, M. Onoda, J. H. Lynch and D. Djakiew, “Reduced Expression of the Low Affinity Nerve Growth Factor Receptor in Benign and Malignant Human Prostate Tissue and Loss of Expression in Four Human Metastatic Prostate Tumor Cell Lines,” Cancer Research, Vol. 52, No. 19, 1992, pp. 5403-5406.
- B. Pflug and D. Djakiew, “Expression of p75NTR in a Human Prostate Epithelial Tumor Cell Line Reduces Nerve Growth Factor-Induced Cell Growth by Activation of Programmed Cell Death,” Molecular Carcinogenesis, Vol. 23, No. 2, 1998, pp. 106-114.
- S. Krygier and D. Djakiew, “Neurotrophin Receptor p75(NTR) Suppresses Growth and Nerve Growth FactorMediated Metastasis of Human Prostate Cancer Cells,” International Journal of Cancer, Vol. 98, No. 1, 2002, pp. 1-7.
- R. Ramer and B. Hinz, “Inhibition of Cancer Cell Invasion by Cannabinoids via Increased Expression of Tissue Inhibitor of Matrix Metalloproteinases-1,” Journal of the National Cancer Institute, Vol. 100, No. 1, 2008, pp. 59- 69.
- A. Preet, R. K. Ganju and J. E. Groopman, “Delta9-Tetrahydrocannabinol Inhibits Epithelial Growth Factor-Induced Lung Cancer Cell Migration in Vitro as Well as Its Growth and Metastasis in Vivo,” Oncogene, Vol. 27, No. 3, 2008, pp. 339-346.
- G. Czifra, A. Varga, K. Nyeste, et al., “Increased Expressions of Cannabinoid Receptor-1 and Transient Receptor Potential Vanilloid-1 in Human Prostate Carcinoma,” Journal of Cancer Research and Clinical Oncology, Vol. 135, No. 4, 2009, pp. 507-514.
NOTES
*This work is supported by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology to I. Araki (No. 21592068), T. Nomura (No. 21659372), and M. Yoshiyama (No. 22591787), S. Kudo (No. 21791497) and M. Takeda (No. 20390423).
#Corresponding author.

