Advances in Chemical Engineering and Science
Vol.3 No.1(2013), Article ID:27042,7 pages DOI:10.4236/aces.2013.31012
Kinetics and Mechanism of Interaction between Chromium(III) and Ethylenediaminetetra-3-Propinate in Aqueous Acidic Media
Chemistry Department, Faculty of Science, Ain Shams University, Cairo, Egypt
Email: michel_lhay86@yahoo.com
Received August 20, 2012; revised September 22, 2012; accepted October 2, 2012
Keywords: Kinetic; Mechanism; Substitution; Ethylenediaminetetra-3-Propinate; Chromium(III)
ABSTRACT
The kinetics of the formation of 1:1 complex of chromium(III) with ethylenediaminetetra-3-propinate (EDTP) was followed spectrophotometrically at lmax = 557 nm. The reaction was found to be first order in chromium(III) and was accelerated by EDTP. Increasing the pH from 3.3 to 4.7 accelerated the reaction rate, the reaction rate was retarded by increasing ionic strength and dielectric constant of the reaction medium. A mechanism was suggested to account for the results obtained which involves ion pair formation between the various species of the reactants. Values of the activation parameters obtained indicate an associative mechanism.
1. Introduction
Synthetic chelating agents are used in many industrial applications because of their capability to bind and mask metal ions (such as Cr(III), Co(III), Fe(III)). Amongst these, ethylenediaminetetra-3-propinate is a synthetic organic metal chelating agent whose metal binding properties are exploited in a wide range of applications. These include detergent, food, pharmaceutical, cosmetic, metal finishing, photographic, textile and paper industries [1-3]. It is also used as a component in decontamination formulation of nuclear reactors and in nuclear waste processing [4,5].
Although the experimental system and reaction studied here is very simple in nature, elucidation of the mechanism in this model system has implications for a variety of more complex homogeneous and heterogeneous phenomena involving metal-organic complexes (e.g., metal ion transport, bioavailability, and toxicity).
In this study, the reaction of chromium(III) and ethylenediaminetetra-3-propinate in weak acid solution is investigated. Factors affecting the rate of reaction were the goal of this study.
2. Experimental
2.1. Reagents and Solutions
All chemicals were of pure grade and were used without further purification. All solutions were prepared using bidistilled water. The ethylenediaminetetra-3-propinate was prepared using a previously described procedure [6]. Stock solution of (0.1 mol∙dm−3) of hexaaquachromium (III) was prepared by dissolving CrCl3 in bidistilled water and leaving the solution for 48 hours at 45˚C, where upon green color of CrCl3 changed to blue color of aquachromium(III) [7].
2.2. Instrumentals
The absorbance measurements were performed using thermostatted 292 Cecil spectrophotometer and pH measurements were conducted with Griffin pH meter fitted with glass-calomel electrode standardized by potassium hydrogen phthalate.
2.3. Kinetic Measurements
Kinetic experiments were conducted by mixing thermostatted solutions of chromium(III) and the ethylenediaminetetra-3-propinate and adjusting hydrogen ion concentration to the required value with potassium hydroxide or perchloric acid. Ionic strength was adjusted by sodium perchlorate solution. The solution was then introduced into the reaction vessel, which was previously thermostatted to the desired temperature and the reaction was followed spectrophotometrically at lmax = 557 nm for the complex formed. The reaction rate was followed under pseudo first order conditions where at least ten fold excess of the ligand concentration over the reactant chromium(III) concentration was always ensured. Values of the observed first order rate constant, kobs, were determined graphically for each run by plotting log (A¥-At) versus time, t, where A denotes the measured absorbance and the subscripts refer to time of reaction. The absorbance (A¥) was obtained directly after ensuring completion of the reaction. First order plots were linear for more than 85% of the reaction progress.
3. Results and Discussion
3.1. Kinetics
3.1.1. Dependence on [Cr(III)]T
The reaction was found to be first order in chromium(III), the observed first order rate constants, kobs, did not vary with chromium(III) concentration, (Table 1) ensuring first order kinetics in chromium(III).
3.1.2. Dependence on [EDTP]T
The effect of varying ethylenediaminetetra-3-propinate concentration, on the rate of reaction was also studied at different pH values (Table 1) and a plot of the first order rate constant, kobs, against EDTP concentration was nonlinear, (Figure 1), indicating formation of ion pair [8,9].
3.1.3. Dependence on Ionic Strength
Increasing the ionic strength, I, of the reaction medium from 0.6 to 1.5 mol∙dm−3 (adjusted by sodium perchlorate) the reaction rate (Table 1). Applying Bronsted Bjerrum equation [10,11], a linear relationship was obtained by plotting log kobs versus √I, (Figure 2) indicating that reaction involves ion pairing formation.
3.1.4. Dependence on Dielectric Constant
The effect of the dielectric constant on the rate of reaction was studied using different ratios of ethanol-water mixtures. The values of the observed first order rate con-
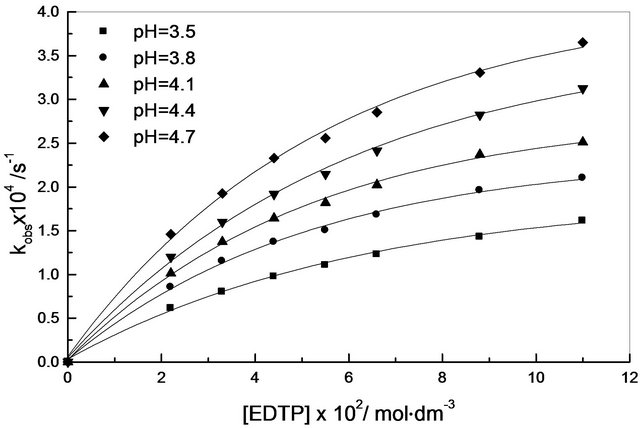
Figure 1. Variation of kobs with [EDTP] at various pH; I = 0.6 mol∙dm−3, T = 35˚C, [Cr(III)] = 8.8 × 10−3 mol∙dm−3.
stant, kobs increased with decreasing the dielectric constant of the reaction medium, ε, (Table 1). Applying Bjerrum’s equation [10], a plot of log kobs versus 1/ε was linear with positive slopes, (Figure 3) indicating that the reaction is an ion pair type [12].
3.1.5. Dependence on pH
The effect of pH on the rate of reaction was studied in the range from 3.0 to 4.7 at various temperatures, (Table 2). The results obtained show that the reaction is accelerated by lowering hydrogen ion concentration.
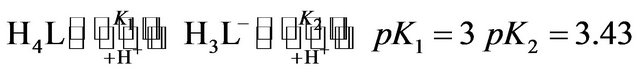
The dependence of kobs on hydrogen ion concentration can be explained by in following equilibriums between the various species of each reactant which are present in the reaction medium [13,14].
 (1)
(1)
 (2)
(2)
3.2. Mechanism of Reaction
The pentaaquahydroxochromium(III) species is more

Figure 2. Variation of log kobs with /I; [EDTP] = 11 × 10−2 mol∙dm−3, pH = 4.1, [Cr(III)] = 8.8 × 10−3 mol∙dm−3, T = 35˚C.
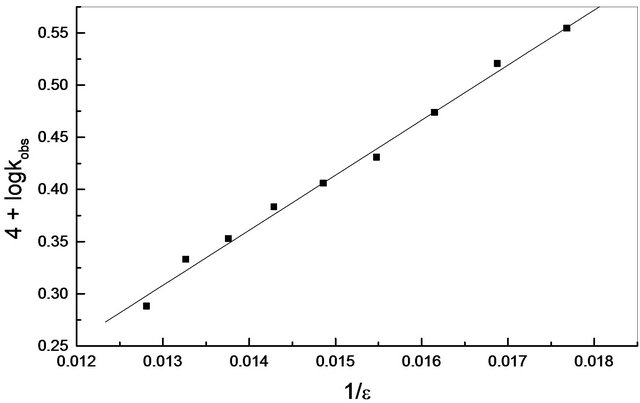
Figure 3. Variation of log kobs with 1/ε in ethanol-water mixture; T = 25˚C, pH = 4.1, [Cr(III)] = 8.8 × 10−3 mol∙dm−3, I = 0.6 mol∙dm−3, [EDTP] = 11 × 10−2 mol∙dm−3.
Table 1. Values of kobs under various conditions.

reactive than the hexaaquachromium(III) due to the presence of OH− which causes an increase of water labilities due to its π-bonding ability [15-22].
The results obtained can be explained by the following mechanism for the interaction between the predominant species of chromium(III) with the predominant species of EDTP in the pH range under investigation
 (1)
(1)
Table 2. Kinetic data for the interaction of Cr(III) with EDTP at various temperature and pH; [Cr(III)] = 8.8 × 10−3 mol∙dm−3, [EDTP] = 11 × 10−2 mol∙dm−3, I = 0.6 mol∙dm−3.

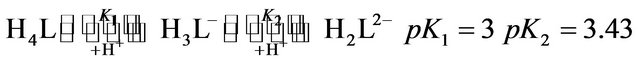 (2)
(2)
 (3)
(3)
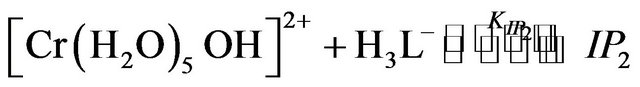 (4)
(4)
 (5)
(5)
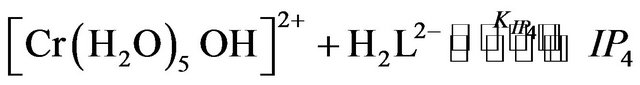 (6)
(6)
 (7)
(7)
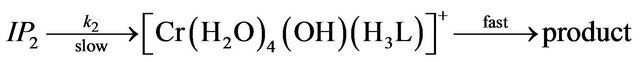 (8)
(8)
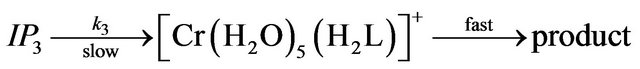 (9)
(9)
 (10)
(10)
where IP1-IP4 are the hexaaquo and pentaaquohydroxy ion pair complexes of chromium(III) and EDTP.
The rate of exchange of the first ligand molecule, in the inner coordination sphere of the metal center is slow and therefore the rate determining Equations (7)-(10) [19-21]. As soon as one carboxyl group of the ligand enters into the inner sphere, the electron density on the chromium center increases owing to the inductive effect and as results the remaining ligands are labilized easily and its substitution is rapid. From the previous mechanism, the first order rate constant is derived as
 (11)
(11)
by inversing Equation (11) we get equation
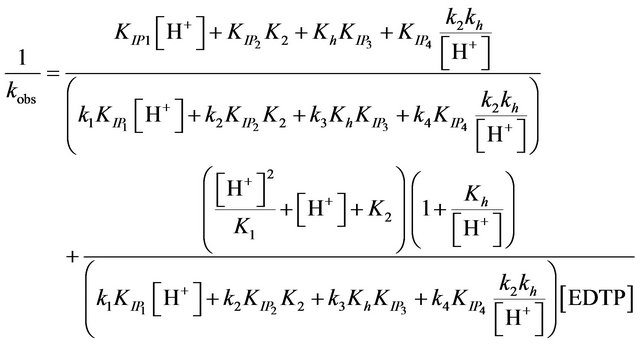 (12)
(12)
and a plot of 1/kobs versus 1/[EDTP] gave s straight line with slopes
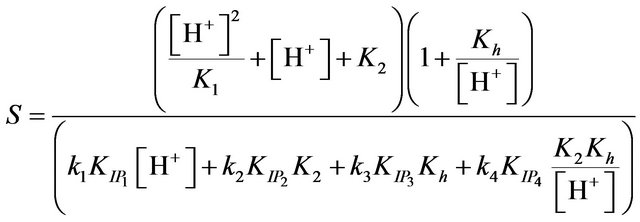 (13)
(13)
and intercepts, I
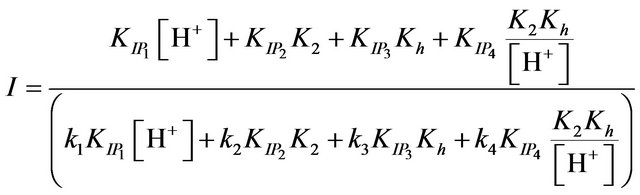 (14)
(14)
and
 (15)
(15)
The values of the ion pair formation constants, KIP and the rate constants of the rate determining steps, k, were calculating by plotting 1/kobs versus 1/[EDTP] at different pH, (Figure 4). Values 7.11, 14.78, 10.15 and 16.81 mol−1∙dm3 for the ion pair formation constants, KIP and 1.83, 4.81, 2.97 and 5.23 × 10−3 s−1 for the rate determining steps, k, respectively were calculating by applying Equations (13)-(15) at different hydrogen ion concentrations and taking the values of K1, K2 and Kh from Equations (1) and (2). The unexpected values of ion pair formation constants, KIP, (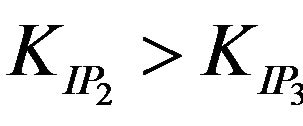 ) indicate the mechanism is not only via electrostatic attraction but also include hydrogen bonding between the acetate groups and the first coordination sphere H2O.
) indicate the mechanism is not only via electrostatic attraction but also include hydrogen bonding between the acetate groups and the first coordination sphere H2O.
The effect of temperature on the rate reaction was also studied at different hydrogen ion concentrations (Table 2). The activation parameters were calculating using Arrhenius plots values and the Eyring equation and were found to be 57.2 ± 3 kJ∙mol−1 for the energy of activation and −128 ± 8 J∙K−1∙mol−1 for the entropy of activation.
It is well known that substitution reactions of hexaaquachromium(III) with a variety of ligands proceed by associative [12,20-22] and dissociative [23,24] mechanisms. Swaddle [25,26] and Lincoln [27] have reviewed that the activation parameters and mechanism of octahedral substitution and concluded that an associative mechanism is operative for octahedral cationic complexes of trivalent metal ions except for Co(III) with ionic radii greater than 60 pm, which demand associative character for substitution reaction of [Cr(H2O)6]3+ The associative mechanism is further supported by 1) lowering of enthalpy and large negative entropy for substitution of water by ligand compare to water exchange (for water exchange ∆H* = 109.6 kJ∙mol−1 and ∆S* = +12 J∙K−1∙mol−1 [28]); 2) the straight line obtained from plotting of log k1 with log k2 [29], (Figure 5) (Where k1 and k2 are the first order rate constants at different temperature) for the substitution of water in [Cr(H2O)6]3+ by valine [16], glycine [17], serine [18], Aspartic acid [19], L-glutamic acid [21], DL-lysine [21], DL-leucine [22]
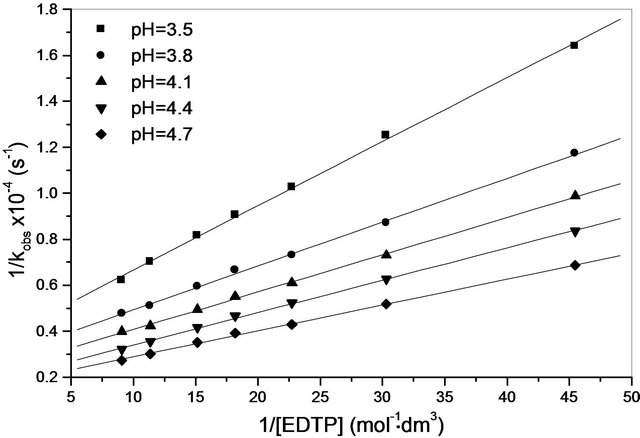
Figure 4. Variation of 1/kobs with 1/[EDTP]; [Cr(III)] = 8.8 × 10−3 mol∙dm−3, T = 35˚C, I = 0.6 mol∙dm−3.

Figure 5. Plot of log k1 with long k2 for the substitution of water in [Cr(H2O)6]3+ by different ligands.
and EDTP (this work).
4. Conclusion
In the Present study, the kinetics of the reaction between chromium(III) and EDTP in weak acid aqueous solutions was investigated. The reaction was found to be first order in chromium(III). The reaction rate accelerated with increasing the EDTP concentration, pH, temperature, decreasing ionic strength and dielectric constant of the reaction medium. An associative mechanism was suggested to account for the results obtained
REFERENCES
- G. McLendon, H. Hull, K. Larkin and W. Chang, “Metal Binding to the HIV Nucleocapsid Peptide,” Journal of Biological Inorganic Chemistry, Vol. 4, No. 2, 1999, pp. 171-174. doi:10.1007/s007750050301
- S. Tandy, K. Bossart, R. Mueller, J. Ritschel, L. Hauser, R. Schulin and B. Nowak, “Extraction of Heavy Metals from Soils Using Biodegradable Chelating Agents,” Environmental Science and Technology, Vol. 38, No. 3, 2004, pp. 937-944. doi:10.1021/es0348750
- C. A. Tolman, “Steric Effects of Phosphorus Ligands in Organometallic Chemistry and Homogeneous Catalysis,” Chemical Reviews, Vol. 77, No. 3, 1977, pp. 313-348. doi:10.1021/cr60307a002
- Y. V. Nancharaiah, N. Schwarzenbeck, T. V. K. Mohan, S. V. Narasimhan, P. A. Wilderer and V. P. Venugopalan, “Biodegradation of Nitrilotriacetic Acid (NTA) and Ferric—NTA Complex by Aerobic Microbial Granules,” Water Research, Vol. 40, 2006, pp. 1539-1546. doi:10.1016/j.watres.2006.02.006
- H. Bolton Jr., D. C. Girvin, A. E. Plymale, S. D. Harvey and D. J. Workman, “Degradation of Metal—Nitrilotriacetate Complexes by Chelatobacter heintzii,” Environmental Science and Technology, Vol. 30, No. 3, 1996, pp. 931-938. doi:10.1021/es950397k
- D. J. Radanovic´, S. R. Trifunovic´, M. S. Cvijovic´, C. Maricondi and B. E. Douglas, “Synthesis and Characterization of Hexadentate Cobalt(III) Complexes with Novel Edta-Type Ligands Part 2. Circular Dichroism of the Trans (O5O6) and Trans (O6) Isomers of a Cobalt(III) Complex of 1,3-propanediamine-N, N’-diacetic-N, N’-Di- 3-Propionic Acid,” Inorganica Chimica Acta, Vol. 196, No. 2, 1992, pp. 161-169. doi:10.1016/S0020-1693(00)86119-8
- K. Emorson and W. M. Graven, “Equilibria in Acidic Solutions of Chromium(III) Perchlorate,” 1959, Journal of Inorganic and Nuclear Chemistry, Vol. 11, No. 4, pp. 309-313. doi:10.1016/0022-1902(59)80045-2
- T. W. Swaddle, “Substitution Reactions of Divalent and Trivalent Metal Ions,” Advanced Inorganic and Bioinorganic Mechanism, Vol. 2, 1983, p. 95.
- T. W. Swaddle, “Operationism, Mechanism, and High Pressure,” Review of Physical Chemistry of Japan, Vol. 50, 1980, pp. 230-242.
- F. Basolo and R. G. Pearson, “Mechanism of Inorganic Reaction,” 2nd Edition, Wiley, New York, 1958.
- A. A. Forst and R. G. Pearson, “Kinetic and Mechanism,” John Wiley and Sons, New York, 1961.
- M. Shahid, I. A. Khan and Kabir-ud-Din, “Kinetic and Mechanistic Studies on the Complexation of Aquachromium(III) with DL-Tryptophan in Aqueous Acidic Media,” Journal of the Chemical Society, Dalton Transactions, 1990, pp. 3007-3012.
- K. Emorson and W. M. Graven, “Equilibria in Acidic Solutions of Chromium(III) Perchlorate,” Journal of Inorganic and Nuclear Chemistry, Vol. 11, No. 4, 1959, pp. 309-313. doi:10.1016/0022-1902(59)80045-2
- P. Djudjević, D. J. Radanović, M. S. Cvijović and D. Veselinović, “Potentiometric Determination of the Protonation Constants of 1,3-Propanediamine-N,N’-DiacetateN,N’-di-3-Propionate,” Talanta, Vol. 38, No. 4, 1991, pp. 455-460.
- M. E. Mendola, T. Paul, T. J. Strathmann and R. F. Carbonaro, “Investigation of the Kinetics of Aquation of the 1:2 Complex between CrIII and Nitrilotriacetic Acid,” Polyhedron, Vol. 28, No. 2, 2009, pp. 269-278. doi:10.1016/j.poly.2008.11.018
- I. A. Khan and Kabir-ud-Din, “Kinetics of Anation of Hexaaquochromium(III) Ion by Valine in Aqueous Acidic Medium,” Indian Journal of Chemistry, Vol. 23A, 1984, p. 98.
- I. A. Khan and Kabir-ud-Din, “Anation of Hexaaquochromium(III) by Glycine,” Journal of Inorganic and Nuclear Chemistry, Vol. 43, No. 5, 1981, pp. 1082-1085. doi:10.1016/0022-1902(81)80188-1
- I. A. Khan, M. Shahid and Kabir-ud-Din, “Kinetics of Aanation of Hexaaquochromium(III) Ion by Serine in Aqueous Acidic Medium,” Indian Journal of Chemistry, Vol. 22A, 1983, pp. 382-385.
- I. A. Khan and Kabir-ud-Din, “Kinetics of Anation of Hexaaquachromium(III) Ion by Aspartic Acid: Mechanism and Activation Parameters,” Transition Metal Chemistry, Vol. 11, No. 10, 1986, pp. 391-395. doi:10.1007/BF01225990
- M. F. Abdel-Messih and Z. M. Abou-Gamra, “Kinetics and Mechanism of the Reaction between Chromium(III) and Picolinic Acid in Weak Acidic Aqueous Solution,” Monatshefte für Chemie, Vol. 143, No. 2, 2012, pp. 211- 216. doi:10.1007/s00706-011-0598-z
- N. M. Guindy, Z. M Abou-Gamra. and M. F. AbdelMessih, “Kinetic Studies on the Complexation of Chromium(III) with Some Amino Acids in Aqueous Acidic Medium,” Monatshefte Fur Chemie, Vol. 131, No. 8, 2000, pp. 857-866. doi:10.1007/s007060070063
- N. M. Guindy, Z. M. Abou-Gamra and M. F. AbdelMessih, “Kinetic Studies on the Complexation of Aqua Chromium(III) with DL-Leucine in Aqueous Acidic Media,” Journal of Chemical Physics, Vol. 96, No. 5, 1999, pp. 851-864. doi:10.1051/jcp:1999175
- J. H. Espenson, “Formation Rates of Monosubstituted Chromium(III) Complexes in Aqueous Solution,” Inorganic Chemistry, Vol. 8, No. 7, 1971, pp. 1554-1556. doi:10.1021/ic50077a047
- S. C. Tyagi and A. A. Khan, “The Studies on the Composition and Kinetics of the Complex Formed by the Interaction of Hexaaquochromium(III) with Salicylic Acid,” Journal of Inorganic and Nuclear Chemistry, Vol. 40, No. 11, 1978, pp. 1899-1901. doi:10.1016/0022-1902(78)80251-6
- T. W. Swaddle, “Activation Parameters and Reaction Mechanism in Octahedral Substitution,” Coordination Chemistry Reviews, Vol. 14, No. 3, 1974, pp. 217-268. doi:10.1016/S0010-8545(00)80306-9
- F. C. Xu, H. R. Krourse and T. W. Swaddle, “Conjugate Base Pathway for Water Exchange on Aqueous Chromium(III)—Variable-Pressure and Variable-Temperature Kinetic Study,” Inorganic Chemistry, Vol. 24, No. 3, 1985, pp. 267-270.
- S. F. Lincoln and A. E. Merbach, “Substitution Reactions of Solvated Metal Ions,” Advances in Inorganic Chemistry, Vol. 42, 1995, pp. 1-88. doi:10.1016/S0898-8838(08)60051-3
- R. A. Plane and H. Taube, “Observations of the Kinetics of the Exchange of Water between Cr(H2O)6+++ and Solvent,” Journal of Physical Chemistry, Vol. 56, No. 1, 1952, pp. 33-38. doi:10.1021/j150493a008
- G. C. McBane, “Chemistry from Telephone Numbers: The False Isokinetic Relationship,” Journal of Chemical Education, Vol. 75, No. 7, 1998, p. 919. doi:10.1021/ed075p919

