Agricultural Sciences
Vol.3 No.3(2012), Article ID:19041,12 pages DOI:10.4236/as.2012.33048
Isolation and pathogenicity of fungi associated to ambrosia borer (Euplatypus segnis) found injuring pecan (Carya illinoensis) wood
![]()
1Department of Agricultural Parasitology, Universidad Autónoma Agraria Antonio Narro, Saltillo, México; *Corresponding Author: fdanielhc@hotmail.com
2Department of Basic Sciences, Universidad Autónoma Agraria Antonio Narro, Saltillo, México
3Department of Food Research, School of Chemistry, Universidad Autónoma de Coahuila, Saltillo, México
Received 16 January 2012; revised 25 February 2012; accepted 7 March 2012
Keywords: Pathogenicity; Pecan Nut; Euplatypus segnis; Ambrosia Borer; Carya illinoensis
ABSTRACT
Euplatypus segnis is an insect pest of economic importance in pecan (Carya illinoensis) trees grown at Parras, General Cepeda and Torreón Coahuila, Mexico. The objectives in this studywere to identify the fungal strains associated to ambrosia borer body and diseased pecan wood and determine their pathogenicity. The results showed that the associated fungi to Euplatypus segnis and damaging the pecan wood were identified as: Helminthosporium sp., Aspergillus sp., Penicillium sp., Phoma sp., Ascochyta sp., Phaecylomices sp., Umbeliopsis sp., Torula sp., Fusarium solani, Alternaria alternata, Fusarum oxysporum, and Lasiodiplodia theobromae. The pathogenicity tests on healthy 3 year old pecan trees cv. western using Fusarium oxysporum, Fusarium solani, Alternaria alternata and Lasiodiplodia theobromae suspension conidia shown die back tree branches after 84 days inoculation. The insect in combination with the fungal invasion eventually cause the death of trees. Additionally, the insect contributes to the spread of fungi in pecan nut orchards.
1. INTRODUCTION
In Mexico, Euplatypus segnis also known as trunk and branch borer is distributed in the states of Coahuila, Durango, San Luis Potosi, Jalisco and Chihuahua [1-3]. In the Coahuila Southwest region there are approximately 525 thousand pecan (Carya illinoensis) trees on production, and annually are harvested about 19,345 tons of nuts. However, in more that 20 percent of the tree plantations the presence of ambrosia borer Euplatypus segnis has been reported causing yield losses of up to 4% (773 tons of nuts) [4]. In addition, E. segnis (Chapuis) (Coleoptera: Platypodinae), attacks wild trees such as: Bursera copallifera, Delonix regia, Ficus sp. Ficus lyrata, Ficus microcarpa, Ficus elastica and Ficus cotinifolia [5], fruit trees such as avocado, apple, peach, pomegranate, apricot, and mango, and some forestry trees like silver poplar, blackberry and tropical ash [6]. There are reports on symbiotic associations between ambrosia insects and fungus, this relation help both of them to survive, per example Xyleborus ferrugineus and Lasiodiplodia theobromae in avocado (Persea americana Mill) [7], Xyleborus ferrugineus and Fusarium solani, Cephalosporium sp. and Graphium sp. [8], Ips typographus and Ophiostoma polonicum on Picea abies [9], Hypotenemus hampei and Fusarium solani on Coffea genus trees [10]. Platypus quercivorus and Raffaelea quercivora on Quercus crispula and Q. serrata [11], Hypothenemus hampei and Penicillium brocae on Coffea arabiga [12], Platypus mutatus associated with a symbiotic fungus in commercial plantations of poplar [13]. In this study, fungi associated to ambrosia borer isolated from diseased pecan wood were morphologically and molecularly identified and incidence of diseases induced by these fungal species was determined using pathogenicity tests.
2. MATERIALS AND METHODS
2.1. Study Area
The sample collections of E. segnis specimens and pecan damaged tissues were obtained from Parras de la Fuente (25˚22'N 102˚11'W 1520 m), General Cepeda (25˚22'N 101˚28'W 1470 m) and Torreon (25˚42'N 103˚27'W 1120 m) all of these counties located in Coahuila, Mexico. Three orchards were sampled by locality and at each orchard were selected three 20-years old damaged trees cv. Western, the damaged trees were in the phase 3 (50% damaged leaf area, 25 to 50 holes entries and presence of sawdust at the stem base) [14]. Wood and insects samples were placed in plastic bags and were properly coded and carried to the entomology and phytopathology laboratories for examination.
2.2. Fungal Isolates
The fungi were obtained from xylem, phloem and wood contained entry points and borer insects. The borer insects were sectioning into head, thorax and abdomen. Each section was disinfected used sodium hypochlorite (1.5%) by immersion for 3 min, after those insect sections were washed with sterile distilled water for 1 min and dried on sterile paper towels. Wood and insect sections were placed on Petri dishes containing potato dextrose agar (PDA: 20 g potato, 20 g of dextrose, 18 g agar and 1000 mL distilled water) as a culture medium, placing 4 pieces per plate. The Petri dishes were incubated and a continuous black light lamp 40 W to 25˚C ± 1˚C was using during the day. The isolates were purified by monospore cultures on water-agar (AA: 18 g agar in 1000 mL distilled water), and increased on PDA. The strains were spread on liquid medium (Pontecorbo) in order to obtain spores and mycelium production for pathogenicity test, and freeze drying and preservation.
2.3. Identification of Pathogenic Fungi
2.3.1. Fungal Morphological Characterization
After 7 - 10 days, the isolated and purified fungi from wood and insects were observed under a composed microscope and identified by morphology using taxonomic keys for ascomycetes and imperfect fungi, in this case characterization was based on characteristics of isolate reproductive structures using Barnnet and Hunter [15], Hanlin [16], Boot [17], Rotem [18], Neergaard [19], Sutton [20] keys for genus and Wei et al. [21] key for species. To identify Lasiodiplodia theobromae were used Punithalingam [22], Sutton [20] and Burgess et al. [23] keys.
2.3.2. Molecular Identification of Isolated Fungi
To confirm the morphological identification of those fungi colonies that showed the highest pathogenicity on pecan trees under field conditions. DNA was isolated from Fusarium oxysporum, Fusarium solani, Alternaria alternata and Lasiodiplodia theobromae fungi at the Molecular Biology laboratory, CIIDIR-IPN Oaxaca. The DNA was isolated using the methodology proposed by Ahrens and Seemüller [24]. Amplification of internal transcribed spaces of ribosomal genes (rRNA) was performed by PCR using the primers ITS4 (TCC TCC GCT TAT TGA TAT GC) and ITS5 (GGA AGT AAA AGT CGT AAC AAG G) according to the methodology proposed by Ahrens and Seemüller [24] with minor modifications, PCR reaction was composed of sterile ultrapure water (13.22 μL), 10X TBE buffer (2.5 μL), MgCl2 at 2.5 mM (2.08 μL), dNTPs at 0.2 mM (2 μL), primers ITS4 and ITS5 to 20 ρmol (2 μL of each), DNA polymerase (biogenic®) 1 U (0.2 μL) and 80 ng of DNA sample (1 uL). The amplified product was purified with Wizard kit (Promega®) and sequenced with the Genetic Analyzer® Model 3100 (Applied Biosystems). The data base sequences with the highest value of similarity in were considered for comparison with the sequences obtained in this study. The ITS sequences obtained were analyzed using the software Lasergene® 2001, V5 (DNASTAR®, Inc.) and were aligned in the database of the National Gene Bank Center for Biotechnology Information (NCBI), by the BLAST program (Basic Local Alignment Search Tool)
(http://www.ncbi.nlm.nih.gov/BLAST/).
2.4. Pathogenicity Tests
Inoculum was incremented in PDA culture medium which was autoclaved three times continues with an interval of 24 hours among sterilizations. Then were inoculated each of the twelve fungi isolated from pecan damaged wood and borer insect body: Phoma, Fusarium oxysporum, Ascochyta, Phaecylomices sp Lasiodiplodia theobromae, Alternaria alternata, Umbeliopsis sp, Torula sp, Fusarium solani, Helminthosporium sp, Aspergillus sp and Penicillium sp. The isolates were multiplied in the culture media described above, then were kept in an incubator at 27˚C with a photoperiod of 12:12 and a relative humidity of 50% ± 10%. Once sporulation was observed, the conidia from each isolate were harvested adding to each Petri dish 50 mL of a solution of Tween 80 (0.05%) and sterile distilled water, and removing the mold with a spatula. Subsequently, the mold solution was liquefied for 60 sec and placed in a beaker of 250 mL (stock suspension). Then, were made three successsive dilutions of 100 uL of the stock suspension in 900 uL of sterile distilled water and were labeled 10–1, 10–2 and 10–3. Finally, spore concentration was determined using a Neubauer’s camera and microscope, the dilution samples were observed in 40× to calculate spore concentration of the stock solution which was adjusted to a final concentration of 109 conidia/mL.
Pathogenicity tests were performed in vivo in twenty eight three years-old healthy pecan trees cv western (height (150 cm) and stem diameter (4 - 5 cm)) established in a high technology greenhouse at a temperature of 24˚C ± 2˚C, at the Universidad Autonoma Agraria Antonio Narro located in Saltillo, Coahuila. The inoculation technique used in this step was that proposed by Kuroda [11], briefly; trees bark at a height of 100 cm was sterilize with sodium hypochlorite (1.5%), then washed with sterile distilled water and dried on using sterile absorbent paper. To each tree, two holes were made on the stem until xylem with a sterile 3-mm drill diameter. Twenty four trees were inoculated with 1 mL of the conidial suspension at 1 × 109 conidia·ml–1 of each one of the twelve identified fungi and two trees were inoculated with a mixed suspension of all fungi spores and two trees with sterile distilled water (control). The inoculations were added using a manual sterile pipette, each wound was covered with parafilm to prevent rapid drying and possible contamination by other microorganisms. According to this technique was favored the low density against mass inoculations in the inoculated tree to prevent wilting and rapid reactions to characterize tissues in a cytological way in response to the inoculated fungi activities. The evaluations were done every 7 days, watching symptoms and disease progression for 84 days after inoculation. Later, from the infected tissues of inoculated stems was isolated the fungus in pure culture and compared morphologically with the fungus inoculated to confirm Koch’s postulates.
2.5. Statistical Analysis
The response variable was the presence of the fungal specie each case, which was evaluated in a quality way (presence and absence). For results analysis a categorical data analysis was performed. In this case Sx2 tables were used, where row and columns were nominally classified. Statistical analysis was performed using SAS (8.1 versions).
3. RESULTS
3.1. Disease Symptoms in Pecan Plantations under Field Conditions
In 20 years-old pecan trees were observed the characteristic symptoms of regressive dead and insect presence mainly in weakened and/or stressed (because age, water deficiency, injuries, fires, intense cold, snow or prolonged drought) trees. In trunk and branches were detected 2 mm-diameter entry holes and sawdust at the trees base, crystal reddish exudates that eventually became dark brown and pungent, after, taking off the bark, necrotic spots were observed with the typical form of diamond. On the other hand one cans heard a sound in trunks and branches; in addition one can see the sap out of the branches when phloem and xylem are piercing by the borer. Also the presence of adults, especially during morning and evening is detected, chlorosis is observed in the leaf area, wilting, premature abscission of stems and leaves, partial death of branches and downward total death of trees.
3.2. Morphological and Molecular Identification of Plant Pathogenic Fungi Associated with Plant Damage
The morphological characterization based on reproductive structures of the fungal isolates was performed using the taxonomic keys proposed by Barnnet and Hunter [15], Hanlin [16], Boot [17], Rotem [18], Neergaard [19], and Sutton [20] for genus and Wei et al. [21] keys were used for the specie identification. Lasiodiplodia theobromae was identified using the Punithalingam [22], Sutton [20] and Burgess et al. [23] keys. In Table 1 are shown the fungal species associated to die-back in pecan damaged wood.
The identification to genera and specie level by sequencing of the regions ITS1 and ITS2 of ribosomal genes (rRNA) was performed only to confirm identity of those fungal more common as pathogens. L. theobromae
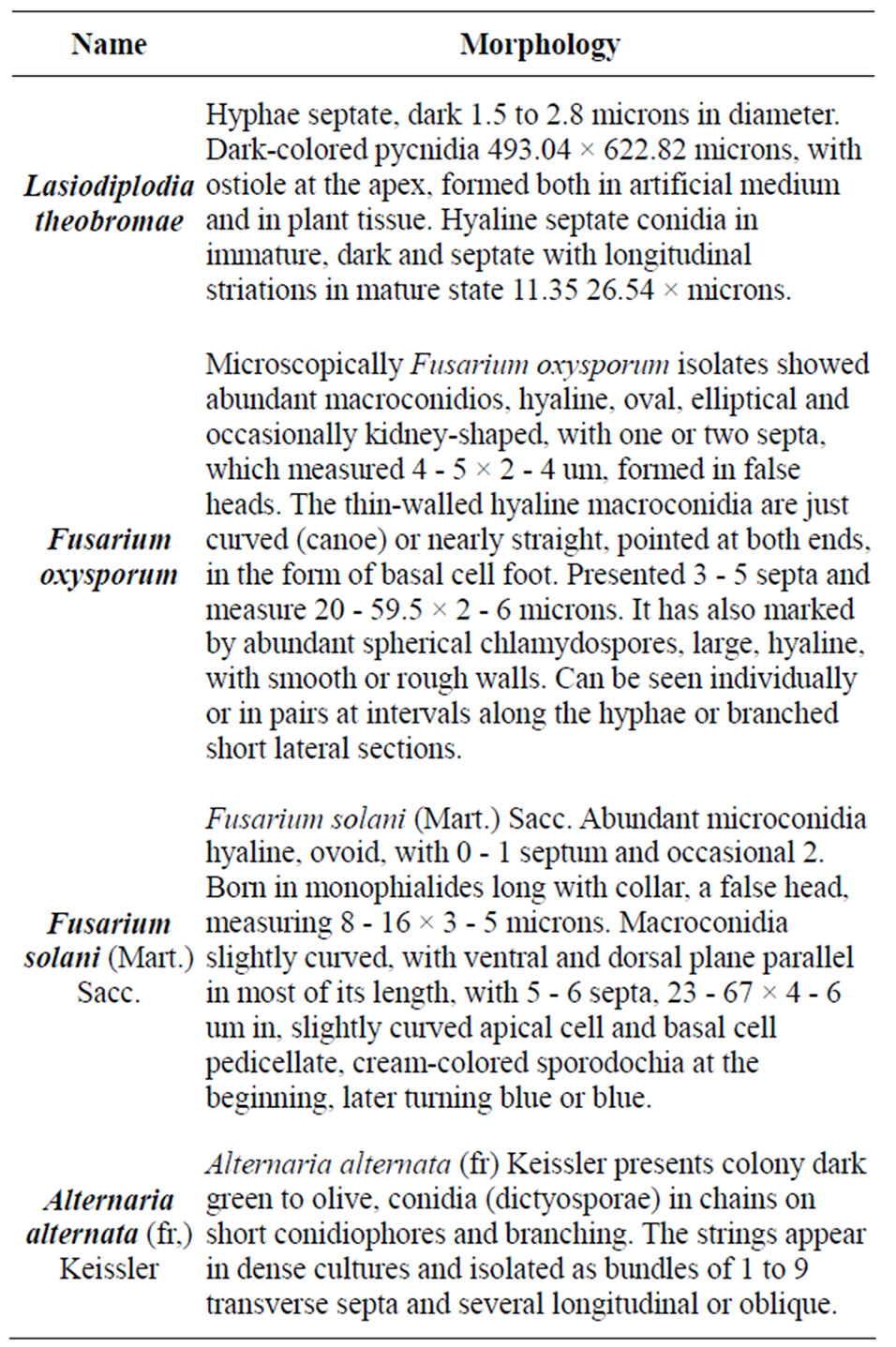
Table 1. Genus and species identified in samples of pecan with dieback symptoms and presence of ambrosia insects.
genome sequence was compared with the genetic sequences of L. theobromae deposited in the gene bank (NCBI) with accession numbers EF622073 (542 bp) and EU564805 (540 bp) in 98% and AY662402.1 in 99%; the Fusarium solani sequences was lineated with Fusarium solani sequences deposited in gene bank (NCBI) with accession numbers AF440567.1 (480 bp) in 94%, and AY310442.1 (554 bp) in 100%. The Fusarium oxysporum was aligned with F. oxysporum accession number EF495235.1 with a similarity index of 99%, Alternaria alternata gene sequence was compared and showed to be homologous to A. alternata with access numbers AF397236.1 with a similarity index of 100%, all these fungal species were confirmed as causal agents of dieback in pecan grown in Coahuila Southeast Region.
3.3. Fungi Associated to Insects and Damaged Pecan Trees in the Coahuila Southwest Region
There were isolated 459 fungal strains from insects and diseased pecan wood on artificial culture medium (PDA), distributed as follows: In Parras county were isolated 165 strains, in General Cepeda county 161 strains, and in Torreón county 131 strains. The genera isolated were described according to their morphological structures based on microscopic observations and literature guides.
In the Table 2 are displayed the statistics for fungi by incidence. The Mantel-Haenszel Chi-Square (Qcs), is valid for these data since fungi is ordinally scaled, and the response is dichotomous; it indicates that there is a strong association between fungi and incidence (Qcs = 73.53) which is highly significant. By looking at the percentages in the Figure 1, we can see that fungi F. solani and F. oxysporum had the highest frequency of incidence.
In the Table 3 are displays the Mantel-Haenszel results for the stratified analysis where the strata are all combinations of county and tissue used (insect o wood) is QcsMH = 73.4498, which is strongly significant, in this case some fungal species where observed only in some counties and a specific tissue (wood or insect) but no in others (Figures 2 and 3). The association of fungi and incidence controlling for counties is QcsMH = 73.4878, which is highly significant. In this case some fungal species appeared only in samples taken in specific counties (Figures 3 and 4). On the other hand, the Mantel-Haenszel results for the stratified analysis where the strata are all combinations of tissue used (insect o wood) is QcsMH = 73.5103, respectively, which is highly significant. In
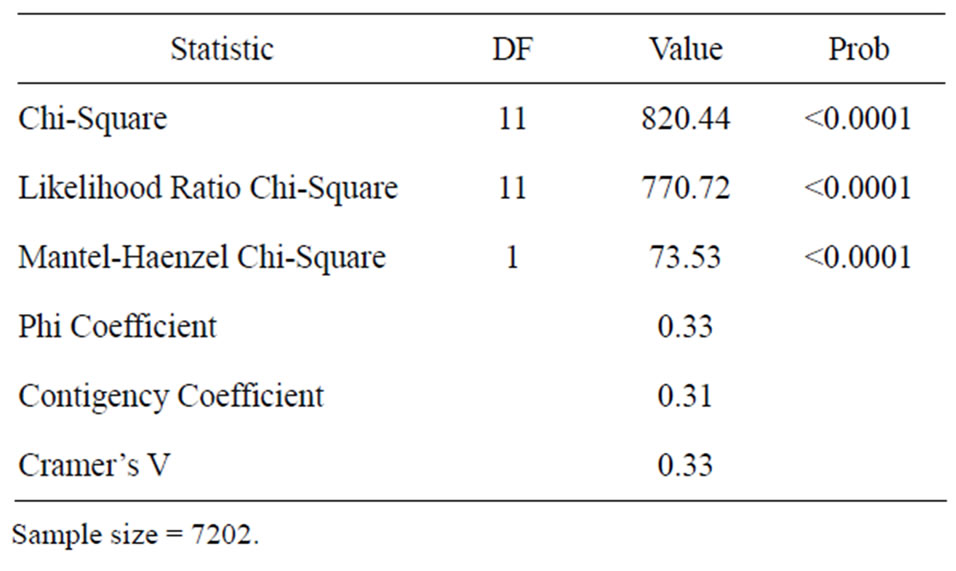
Table 2. Statistics for fungi by incidence.
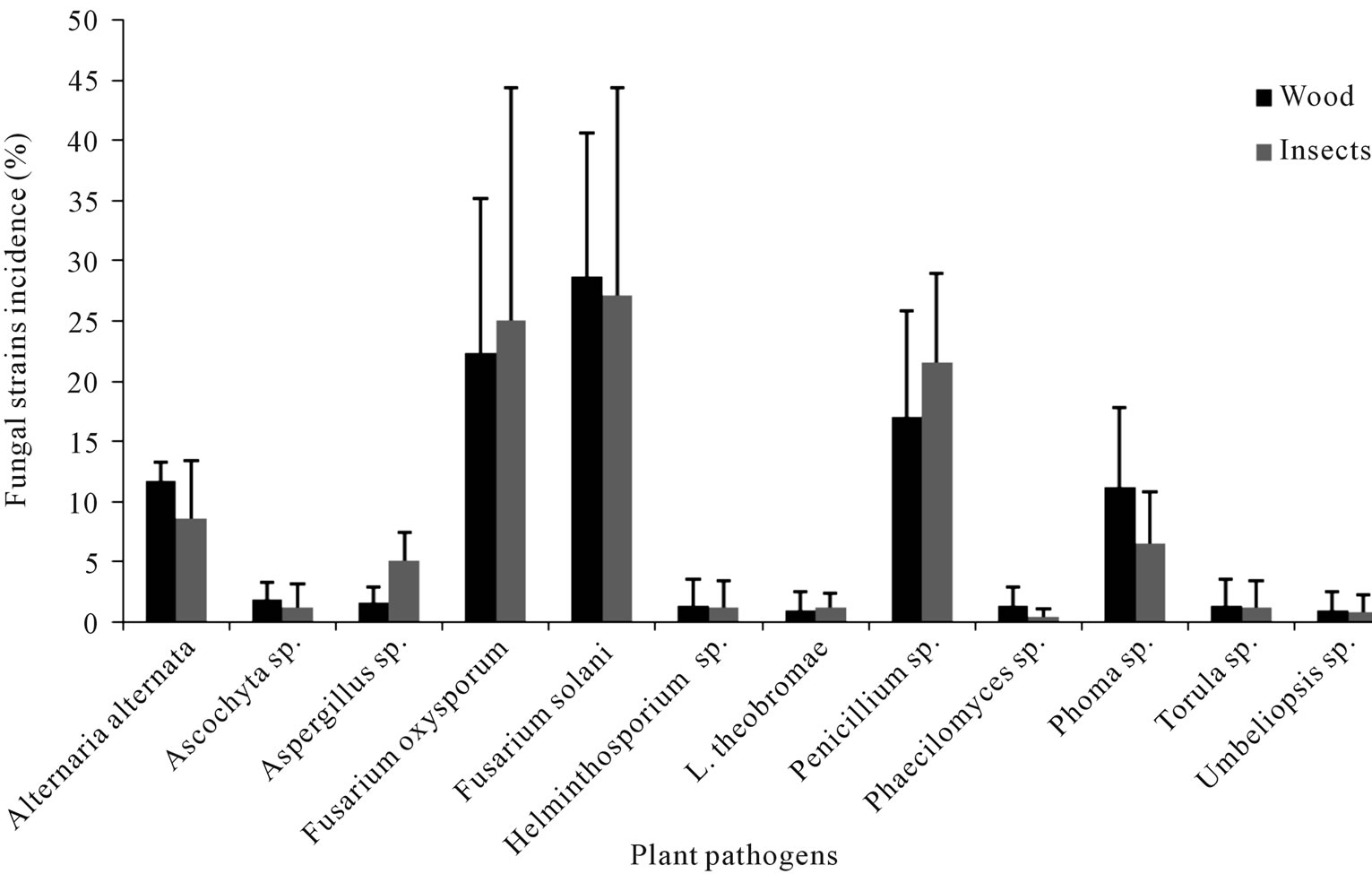
Figure 1. Fungi species isolated from the Euplatypus segnis collected attacking pecan branches and trunks at three Coahuila counties.
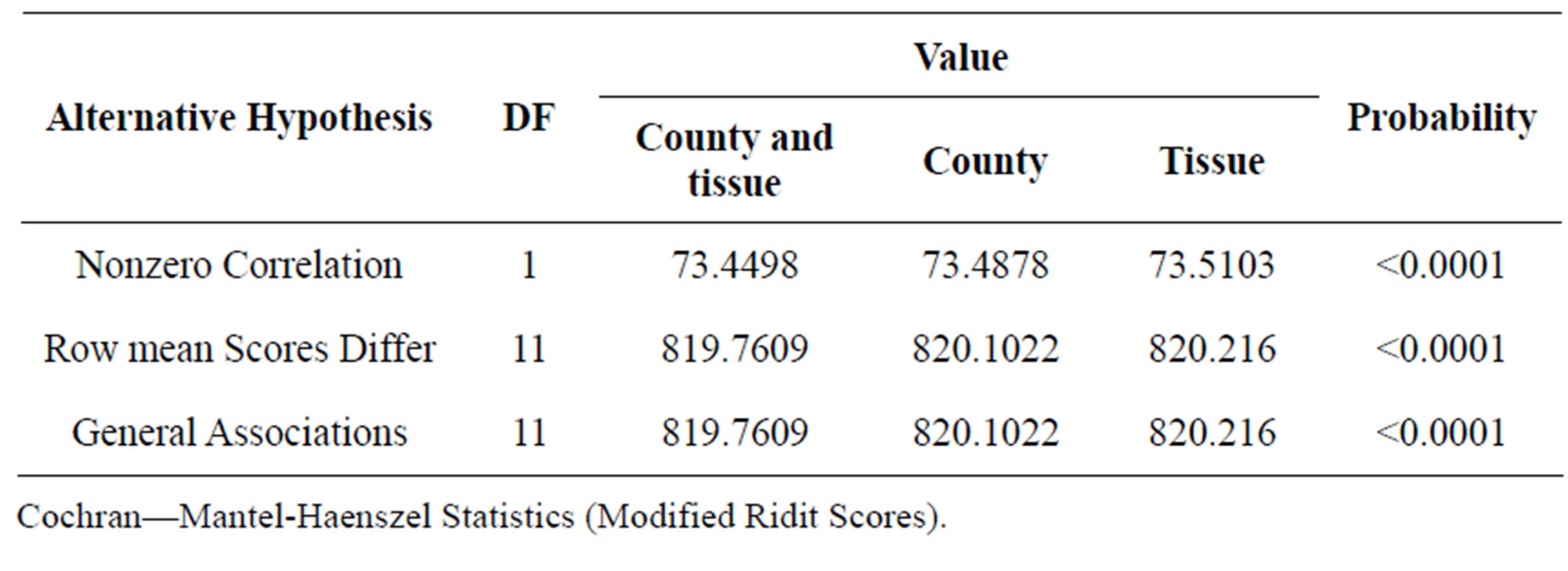
Table 3. Statistics for stratified by county and tissue used, stratified by county and stratified by tissue used.
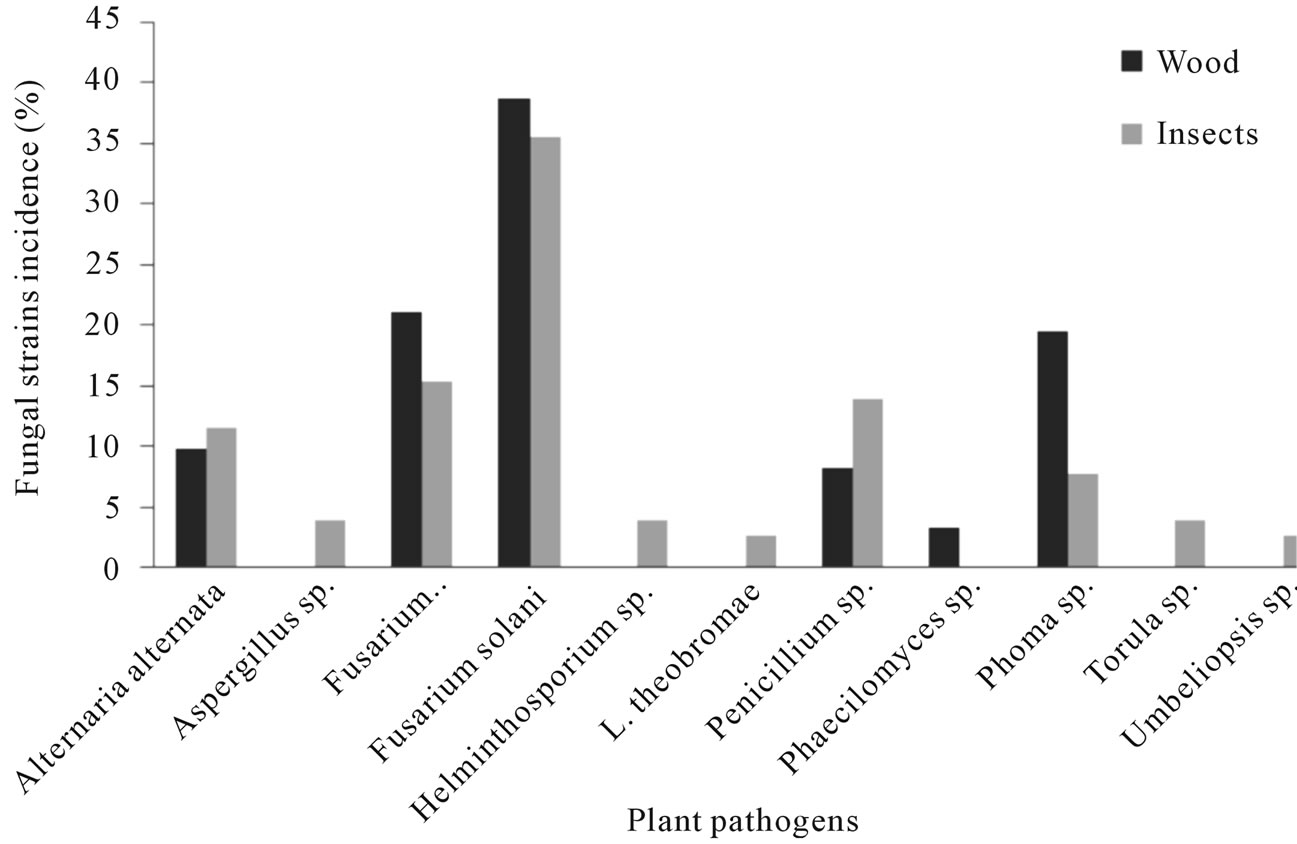
Figure 2. Fungal species found associated with Euplatypus segnis and diseased pecan (Carya illinoensis) wood collected at Parras Coahuila, Mexico.
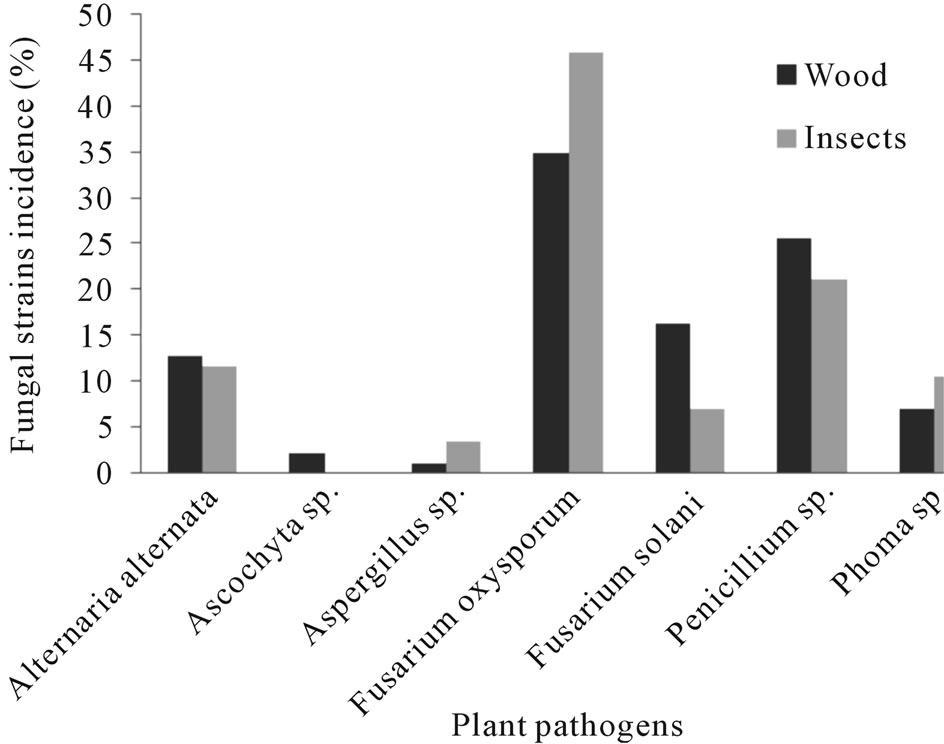
Figure 3. Fungal species found associated with Euplatypus segnis and diseased pecan (Carya illinoensis) wood collected at General Cepeda, Coahuila, Mexico.
this case some fungal species appeared only in samples taken in a specific tissue but not in the others (Figure 2). The significant association between incidence and fungal specie remains evident. QGMH and QSH are significant too in all the analyses.
Eighty nine fungal strains were isolated from borers insect body (Euplatypus segnis) collected in pecan branches and trunks at Parras county, 35% of the isolates belongs to Fusarium solani, 15.2% to Fusarium oxysporum, 13.9% to Penicillium, 11.4% to Alternaria alternata, 7.6% to Phoma sp., 3.8% to Helminthosporium sp., Torula sp., Aspergillus sp., and 2.5% to Lasiodiplodia theobromae and Umbeliopsis sp., (Figure 2), also 76 fungal strains were isolated from diseased pecan wood, 38.7% belongs to Fusarium solani, 21% to Fusarium oxysporum, 19.4% to Phoma sp., 9.7% to Alternaria alternata, 8.1% to Penicillium sp., and 3.2% to Phaecylomices sp. (Figure 2).
Eighty five fungal strains were isolated from borer insect (E. segnis) body collected in pecan branches and trunks at General Cepeda county, these strains were distributed as follow: 45.9% belonging to Fusarium oxysporum, 21.2% to Penicillium, 11.4% to Alternaria alternata, 10.6% to Phoma sp., 7.1% to Fusarium solani and 3.5% to Aspergillus sp. (Figure 3). On the other hand 76 fungal strains were isolated from pecan (C. illinoensis) diseased wood, distributed as follow 38.7% belongs to Fusarium oxysporum, 21.2% to Penicillium sp., 16.3% to Fusarium solani, 11.8% to Alternaria alternata, 7% to Phoma sp., 2.3% to Ascochyta sp. and 1.2% to Aspergillus sp. (Figure 3).
From the samples collected in the Torreon county, sixty nine fungal stains were isolated from borer insect (E. segnis) body collected attacking pecan branches and trunks. These strain were distributed as follow: 38.5% belongs to Fusarium solani, 28.6% to Penicillium sp., 10.3% toFusarium oxysporum, 7.7% to Aspergillus sp., 4.0% to Helminthosporium sp. And Alternaria alternata, 3.3% to Ascochyta sp., 2.2% to Phoma sp. and 1.1% toPhaecylomices sp. and Lasiodiplodia theobromae (Figure 4), also were isolated 62 fungal strains from diseased pecan (C. illinoensis) wood distributed as follow 34.7% belongs to Fusarium solani, 14.7% to Penicillium sp., 9.3% to Fusarium oxysporum, 7.7% to Aspergillus sp., 12.0% to Alternaria, 9.3% to Phoma sp., 4.0% to Torula and Helminthosporium sp., and 2.7% to Lasiodiplodia theobromae, Umbelliopsis sp., Ascochyta sp. and 1.3% to Aspergillus sp. and Phaecylomices sp. (Figure 4).
3.4. Pathogenicity of Fungi Strains Isolated from Pecan Trees with Dieback
At 14 days after inoculation were observed the first signs of disease in some trees (Table 4). In trees inoculated with Fusarium solani had discharge in the drilling at 21 and 28 days, chlorosis at 42 and 49 days, wilting at 63 and 70 days and dieback after 77 days after inoculation, the inoculated trees with Fusarium oxysporumshown exudates at the inoculation point in the two treatments at 14 and 21 days, and brown leaves at 35 and 42 days, chlorosis at 45 and 56 days, at 63 and 70 days caused wilting and dieback at 77 and 84 days after inoculation. The inoculated trees by Alternaria alternata showed chlorosis at 63 days, wilting to 70 days and dieback to 84 days after inoculation. The trees infected with Lasiodiplodia theobromae presented leaves chlorosis at 56 days, premature abscission at 70 days, defoliation at 77 days and dieback at 84 days, the other tree shoots inoculated only presented reddish tip at 77 and chlorosis at 84 days; Torula sp. Showed brown leaves at 77 days and defoliation at 84 days, the second treatment presented only brown leaves at 84 days, the two treatments behaved similarly. Phoma sp., caused wilting only 70 days to complete the evaluation at 84 days, the trees inoculated with Phaecylomices sp. presented chlorotic symptoms at the end of the evaluation, Ascochyta sp., caused brown leaves at 63 days, wilting from 77 to 84 days, in the second treatment was observed brown leaves at 70 days and defoliation at 84 days; Helminthosporium sp., caused chlorosis at 77 days and wilting at 84 days, the other treatment only showed a slight chlorosis; The treatments that received a mixture of spores showed the most damaged where trees were killed at 77 and 84 days in both treatments, Penicillium sp. and Aspergillus sp. Umbeliopsis sp. and the control treatment did not cause any outward symptoms.
4. DISCUSSION
4.1. Insect-Fungi Association
Euplatypus segnis is is an ambrosia specie with monogenic reproductive behavior [25], polyphagous in its degree of specificity with respect to the host and xylomicetophagus by feeding habit, consuming the conidia producing by
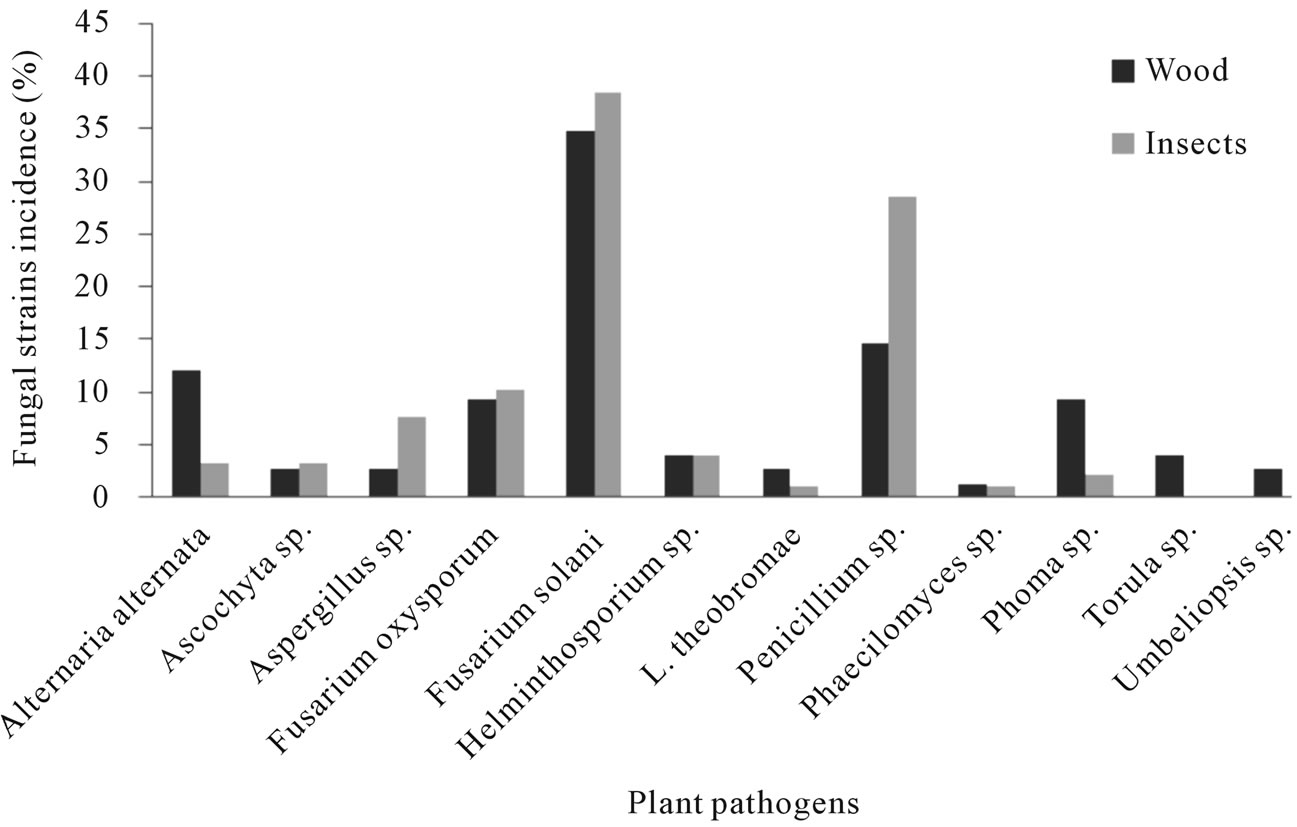
Figure 4. Fungi species found associated with Euplatypus segnis and diseased pecan (Carya illinoensis) wood collected at Torreon Coahuila, Mexico.
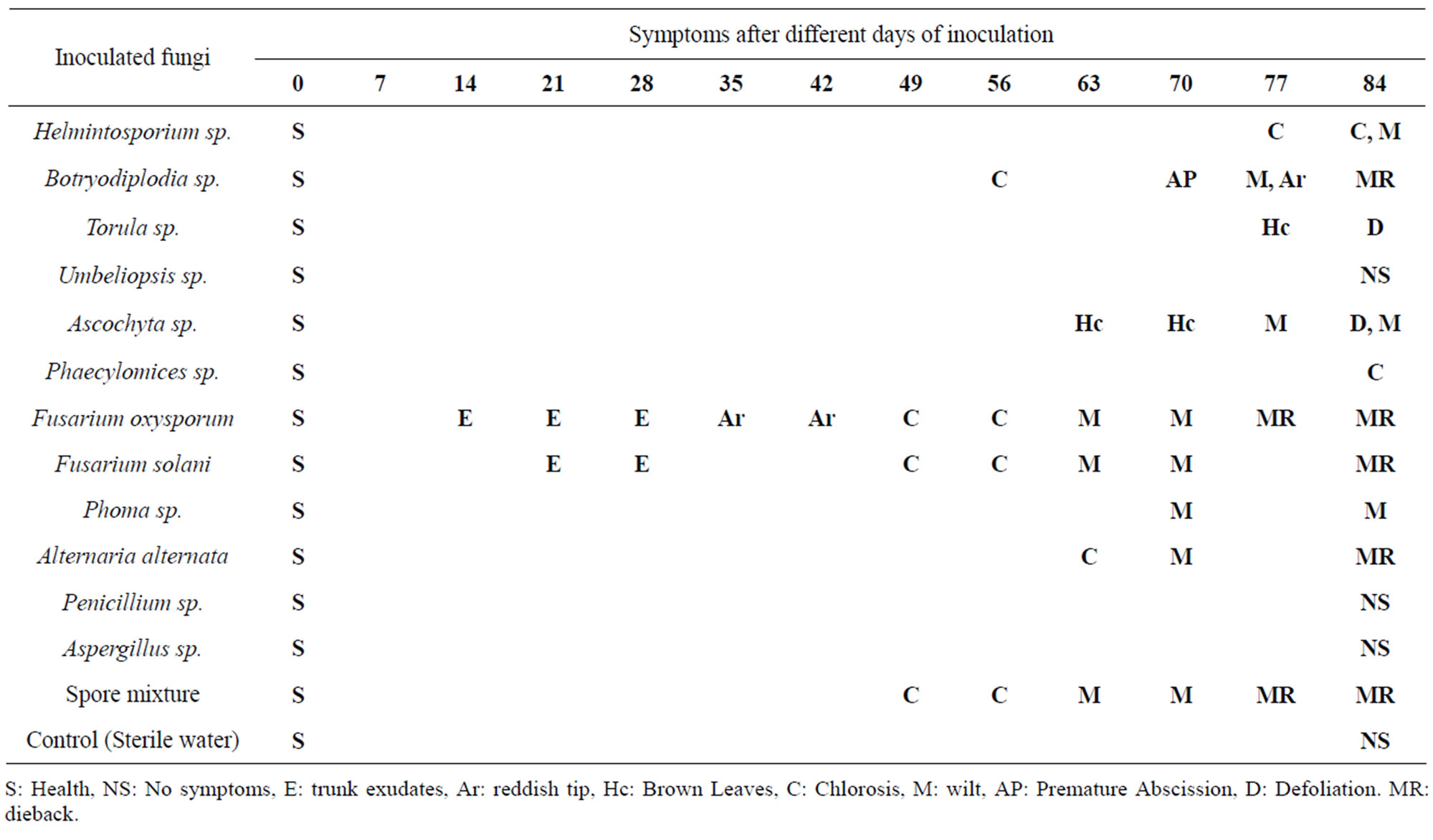
Table 4. Development of symptoms and signs in pecan trees inoculated with fungal strains isolated from E. segnis and damaged pecan wood.
the fungi inoculated to the trees [26]. Ulloa [27] mentions that borers have the habit of feeding on fungi introduced by them in the gallery system built into the wood. These fungi appear to be relatively non-specific and need basically moisted wood for their development. Insect species that grow fungus in galleries are called beetles or ambrosia borers and they are dominant in tropical areas [28]. Most of the buildings in the tunnel systems of ragweed borer (galleries) are in the vigorous host tissues, weakened or recently dead, though some species are specialize in colonize the bone marrow, large seeds, fruits and petiole leaves [26,29]. The term ambrosia refers to the fungi that are cultivated by beetles in the gallery walls, which are feed exclusively for insects. Beetles are necessarily dependent on fungi, from which acquired vitamins, amino acids, and essential sterols [30, 31]. The fungi are the main source of food for larvae and adults and are essential to complete insect life cycle [10]. In some insect tribes, only females perform tasks inside the culture, while males are short lived and little flight [32]. After mating, females disperse to new host substrate, carrying fungi in specialized pockets called mycangia. Once inside, the founder females planted the fungi on the walls of tunnels, lay eggs, and serve the growing brood [32]. In a way not understood, the female beetles can control fungal growth and degree of multispecies composition [30,33-35]. If the female dies, the culture is quickly contaminated by other fungi and bacteria, which ultimately leads to the brood death [32,36]. The ambrosia fungi cultured by borers are not pure monocultures, also which consist of a mixture of fungi mycelium, yeasts, and bacteria [37,38]. Norris [39] calls these complex multi-species mixtures. However, recent work has shown that a fungus always dominates in primary cultures of the insect [37,40-42]. In addition, beetles carry only the primary fungus in the mycangia (although sometimes secondary fungi are isolated from the mycangia), females tend to favor the primary fungal culture, which gives them a nutritional benefit [32,41,43, 44], some auxiliary fungi also support beetle development [32]. These observations imply the primary fungus as the primary crop, while fungi, yeasts and bacteria may be secondary “weeds” or with additional auxiliary roles to play in the cultures. The fungus growing transmission among generations of farmer’s beetles is transmitted vertically from parent to offspring generations [38,43,45]. Reproductive females acquire original inoculum from their crops, they take with them in specialized pockets during dispersal flights and use this as seed inoculum for their new crops. Ambrosia borers are only associated with a particular kind of primary cultures within a specific geographic region [37,41]. However, although most of the beetles are associated with a specific species of fungus in their primary area, ambrosia beetle species distantly related are sometimes associated with cultivating the same fungus, involving the exchange, direct or indirectly. The fungal exchange between and within species of beetles can occur when several females colonize the same tree and fungal associations contaminate adjacent galleries [41]). The primary fungi are primarily asexual [46,47], while less specific auxiliary fungi are often sexual [43].
4.2. Fungi Associated with Pecan in the Coahuila Southwestern Region
The identification of different fungal isolates from both wood and insect body, one may infer that diebackmay be associated with Fusarium since it is the fungal specie most frequently isolated from pecan tissue and insect body (Figure 1).
This association of Fusarium solani and Fusarium oxysporum as a primary agent on pecan tree is due to exudates or gummosis presented at 14 days, later inoculated trees showed marked chlorosis and red apices, finally trees declined to dead. Inside of stalks were initially observed brown areas and when these areas were cut transversely, staining was observed in the vascular system in ring forms, this situation was observed in leaf petiole. When the fungal pathogen completely invaded the conducting vessels caused widespread of wilt and tree death. From all the isolated fungal species, Fusarium solani and Fusarium oxysporum are highlighted in this study because they have a recognized importance as ambrosia fungus [30]. Fusarium solani had the highest percentage of occurrence at Parras county, 35% in insects and 38.7% in diseased wood and in Torreon county, 38.5% in insects and 34.9% in diseased wood, by this reason it is considered as the primary ambrosia fungus, in contrast Fusarium oxysporum was the most frequent fungal specie at General Cepeda county, 45.9% in insects and 38.7% in diseased wood. As genera Fusarium are a ubiquitous necrotic parasite [48], and may tolerates pH changes [49], and host susceptibility to this fungus is directly influenced by temperature and osmotic pressure [50], Fusarium is widely distributed in soils and organic substrates, and it has been isolated from permafrost in the Arctic to Sahara sands, it presence is abundant in cultivated soils from temperate and tropical areas and it is the most frequently isolated fungi from plants by pathologists [17]. Fusarium species are commonly associated with some group of insects like Scolytidae and Platypodidae families habit in wood [51]. Regarding the association of Fusarium species and order Coleoptera insects in woods, Norris [32] mention Fusarium solani as a primary ambrosia fungus associated with species from Xyleborus genus, this report is consistent with the results obtained in this study, since 27.1% of our samples from the three different locations showed damage associated with insects of the Coleoptera order. Gil et al., [52] identified Corthylus sp. (Coleoptera: Scolytidae), causing damage to alder (Alnus acuminata) trees, which showed wood rot and death, in this case the inset was associated to some ambrosia fungi like Fusarium solani, Fusarium sp. Ceratocystis sp., Verticillium sp. and a yeast identified as Pichia sp. Bonello et al. [53] found that the ambrosia beetle Xylosandrus germanus associated with Fusarium lateritium and F. oxysporum, is related with vascular stained, cancers, wilting and dieback in black walnut (Juglans nigra). Tisserat et al. [54] found that the mortality of black walnut (Juglans nigra) in Colorado, USA, is the result of association of Pityophthorus juglandis (Coleoptera: Scolytidae) with Fusarium solani and Geosmithia sp. this fungus was latter isolated from trunk marginal cancer in the final stages of decline. F. solani has been mentioned as a primary symbiotic with the coffee berry borer (Hypothenemus hampei) [55,56]. The immature stages of X. ferrugineus are unable to complete the development in absence of F. solani [34,57-59]. Ergosterol is essential for development and reproduction of X. ferrugineus [60,61]. This sterol is absent in wood, but F. solani offer it in sufficient quantities [31]. This fungus also provides to X. ferrugineus essential fatty acids and phospholipids [62]. Norris [32] found that X. ferrugineus offspring was highly associated to the primary ambrosia fungus Fusarium solani, but with Cephalosporium sp. association was reduced by 50% and with Graphium sp. up to 70%. Pityophthorus juglandis is associated with F. solani in black walnut (Juglans nigra) trees in North America and South Africa; it is believed that trees are predisposed to cancer formation by stress factors such as optimum site conditions, improper pruning and adverse weather conditions [54], such as abandoned orchards and poor management. In our study, F. solani and F. oxysporum were consistently isolated from bone, xylem, phloem, cambium and galleries, as well as all Euplatypus segnis body parts. This observation and pathogenicity studies suggest an important role of F. oxysporum and F. solani in the pecan tree mortality of all ages in our region.
In the pathogenicity tests, Lasiodiplodia theobromae induced necrotic lesions approximately 10 cm long and 1.0 cm wide, that caused necrosis in the cortical tissue and deepened to the bone in the site of inoculation, at 56 days showed a slight chlorosis followed by early abscission, leaves with red tips, wilting and dieback tree at 84 days, the slow progression of the disease coincides with Milholl and [62] who mentioned that this type of fungus grow slowly and progress in living tissue host due to its saprophytic condition. Optionally, L. theobromae is a parasite that usually infects its host plants by penetrating through wounds and decaying tissue. Occurrence of this fungus is common in tropical and subtropical regions and in different ecological areas, where it has been identified as the cause of disease in approximately 280 species of vascular plants, including avocado, apple, mango, grapes, pine, rose, rubber, cotton, cocoa, coffee, sugar cane, peanuts, tobacco, etc. [63]. Umezurike [64] mentions that Botryodiplodia theobromae has cellulolytic activity in Bombax buonopozense wood. The fungus attacks plant cells in a similar manner to soft rot fungi, use starch and other saccharides present in the initial wood substrates before degradation of cellulose and hemicellulose, but not degrade lignin. Rajput et al. [65] mention that decline and death of mango (Mangifera indica L.) trees in India, are related to the presence of Botryodiplodia theobromae, Alternaria alternata, Fusarium moniliforme, Cephalosporium sp., Chaetomium sp. Aspregillus ellipticus, A. niger, Penicillium sp., Curvularia lunata, Gloeosporium mangiferae, C. globosum, Daldinia sp associated to the attack of ambrosia beetles, termites, mechanical damage to trunk, branches and pruning. Rondon [7] established the relationship between the wood borer Xyleborus ferrugineus F. (Coleoptera: Scolytidae) and Botryodiplodia theobromae Pat attacking avocado trees in Venezuela, this author found that B. theobromae is the most frequently isolated fungi from the body of this wood borer insect. Flores et al. [66] detected the association of Scolytus sp. and Botryodiplodia theobromae fungus, which causes circular lesions to elongated oval up to 15 cm long, dark color that can be covered by large masses of spores in dry periods, causing the death of tips, and branches of Tectona grandis trees in Ecuador. Masood et al. [67] identified the association of Hypocryphalus mangiferae (Coleoptera: Scolytidae) with Lasiodiplodia theobromae, Phomopsis fimbriata and Ceratocystis sp. in the sudden death syndrome in Pakistan associated with symptoms of gummosis, dry rot and vascular discoloration. Tress inoculated with Alternaria alternata showed at 63 days marked chlorosis, wilting leaf area at 70 days and dieback at 84 days as external symptoms and necrotic areas, browning of tissues and a deep dehydration of inoculated area. The genus Alternaria contains cosmopolitan species found in a wide range of materials and products, can damage food and feed, producing biologically active compounds such as mycotoxins. As pathogens reduce crop yields or affect stored plants therefore requires a precise identification of the species because each has specific characteristics (preferences for growth, pathogenicity, and production of secondary metabolites) that predict the behavior of this fungus [68]. Armengol et al. [69] mentioned that A. alternata is normally a saprophytic fungus, but in Spain some strains have been described as pathogenic on different citrus cultivars like Fortune, Nova, and Mineola. Negron et al. [70] described Alternaria as parasites of annual and bi-annual plants or saprophytes on organic substrates, however found that Alternaria alternata associated with Scolytus schevyrewi (Coleoptera: Scolytidae) and other fungi affect some species of elms (Ulmus spp) in Colorado and Utah, which showed necrotic areas, browning of xylem tissue, wilting of foliage and general death of elms, symptoms consistent with the disease progression in the pecan trees of this study inoculated with A. alternata in our pathogenicity tests. Reyes [71] found that 8% of fungal strains isolated from wood samples and insect remains of adult weevils (Coleoptera: Platypodidae) belong to Alternaria alternata and 7% to Phoma sp.
The fungus Paecilomyces sp. is a pathogen of wide host range and wide geographical distribution, which has been isolated from soil and insects of various orders such as Coleoptera, Homoptera and Collembola, Lepidoptera, Diptera, Homoptera, Hymenoptera and spiders [72]. Paecilomyces farinosus may inhibit some strains of pathogenic fungi [73]. Finally, Aspergillus, Paecilomyces, and Penicillium, have been isolated from insect gut Triatoma sp. [74], which has been found sporadically in pecan orchards at the Coahuila Southeast region, Mexico.
5. CONCLUSIONS
Twelve genera of fungi were isolated from the body of E. segnis and diseased pecan wood. These fungi were identified as Phoma sp., Fusarium sp., Ascochyta sp., Phaecylomices sp., Lasiodiplodia sp., Alternaria sp., Umbeliopsis sp., Torula sp., Helminthosporium sp., Aspergillus sp., and Penicillium sp., and inoculated into healthy pecan trees, only Fusarium oxysporum, Fusarium solani, Alternaria alternata and Lasiodiplodia theobromae were highly pathogenic causing dieback at 87 days of inoculation, they were characterized morphologically and molecularly to the specie level.
In Mexico, this is the first study that determines to Fusarium solani, Fusarium oxysporum, Alternaria alternata and Lasiodiplodia theobromae, as the causal agent of pecan (Carya illinoensis) dieback in association with Euplatypus segnis ambrosia borer.
Fusarium solani and F. oxysporum were the most prevalent fungi and isolated from insects and diseased wood at all studied locations. It has been suggested that some species of fungi associated with ambrosia borer are in a symbiotic relationship with the insect. Although, there may be a symbiotic relationship between fungi and insects, there is no conclusive evidence to show that this relationship is sufficient, due to the great diversity of fungi found in association with E. segnis.
REFERENCES
- Equihua-Martínez, A. and Burgos-Solorio, A. (2007) Platypodidae y Scolytidae (Coleoptera) de Jalisco, México. Dugesiana, 14, 59-82.
- Galván, L.O.A. (2000) Euplatypus segnis (Chapuis): Fluctuación poblacional y magnitud de daño a nogales en Parras, Coahuila. In: Vázquez, N.J.M., Ed., Memoria del II Curso de Actualización Fitosanitaria en Nogal. 10 y 11 de Marzo. Instituto Tecnológico de Estudios Superiores de Monterrey (ITESM), Torreón, 45-47.
- García, M.O. (1999) El barrenador ambrosia Euplatypus segnis (Chapuis) del tronco y ramas del nogal (Carya illinoensis). Memorias del Séptimo Simposium Internacional Nogalero NOGATEC. 23, 24 y 25 de septiembre. Instituto Tecnológico de Estudios Superiores de Monterrey, Torreón, 39-42.
- Cesaveco (2010) Comité estatal de sanidad vegetal del estado de Coahuila. Boletin, 86, 20.
- Atkinson, T.H., Fernández, E.M., Céspedes E.S. and Burgos, A.S. (1986) Scolytidae y platypodidae (coleoptera) asociados a selva baja y comunidades derivadas en el estado de Morelos, México. Folia Entomologica Mexicana, 69, 41-82.
- Samaniego-Gaxiola, J.A., Ramírez-Delgado, M., Pedroza-Sandoval, A. and Nava-Camberos, U. (2008) Association between cotton root rot (Phymatotrichopsis omnivore) and borer insects of pecan tree (Carya illinoensis). Agricultura Técnica en México, 34, 21-32 (in Spanish).
- Rondon, A. and Guevara, Y. (1984) Algunos aspectos relacionados con la muerte regresiva del aguacate (Persea Americana Mill). Agronomía Tropical, 34, 119-129.
- Kok, L.T. and Norris, D.M.J. (1972) Symbiotic interrelationships between microbes and ambrosia beetles. VI Amino-acid composition of ectosymbiotic fungi of Xyleborus ferrugineus. Annals of the Entomological Society of America, 65, 598-602.
- Brignola, C., Lacroix B., Lieutier F., Sauvard D., Drouet A., Claudot, C., Yart, A., Berryman, A. and Christiansen, E. (1995) Induced Responses in phenolic metabolism in two norway spruce lnoculations with Ophiostoma polonicumand bark beetle-associated fungus. Plant Physiology, 109, 821-827.
- Morales, R.J.A., Rojas, M.G., Bhatkar, H.S. and Saldaña, G. (2000) Symbiotic relationships between Hypothenemus hampei (coleoptera: scolytidae) and Fusarium solani (moniliales: tuberculariaseae). Annals of the Entomological Society of America, 93, 541-547. doi:10.1603/0013-8746(2000)093[0541:SRBHHC]2.0.CO;2
- Kuroda, K. (2001) Responses of Quercus sapwood to infection with the pathogenic fungus of a new wilt disease vectored by the ambrosia beetle Platypus quercivorus. Japan Wood Society, 47, 425-429.
- Peterson, W.S., Pérez, E.J., Vega, F. and Infante, F. (2003) Brocae Penicillium, a new species associated coffee berry borer with the in Chiapas, Mexico. Mycologia, 95, 141- 147. doi:10.2307/3761973
- Alfaro, R. (2003) El taladrillo grande de los forestales, Platypus mutatus (=sulcatus): Importante plaga de la populicultura Argentina. Forestal, 28, 17.
- Rosas, R.E., Avila, G.M.R. and Cano, R.P. (2004) El metodo Laguna tecnica para conbatir el barrenador del tronco del nogal (Euplatypus segnis Chapuis). Memorias de la XVI Semana Internacional de Agronomia FAZUJED. Gomez Palacios, del 6-10 de Septiembre 2004.
- Barnett, H.L. and Hunter, B.B. (2006) Illustrated genera of imperfect fungi. 4th Edition, The American Phytopatological Society, St. Paul Minnesota.
- Hanlin, R.T. (1990) Illustrated genera of ascomycetes I. APS Press, Saint Paul.
- Booth, C. (1971) The genus Fusarium. Commonwealth Mycological, Kew.
- Rotem, J. (1988) The genus Alternaria, biology, epidemiology and pathogenicity. APS Press, St. Paul.
- Neergaard, P. (1977) Seed pathology. The McMillan Press Ltd., Surrey.
- Sutton, B.C. (1980) The fungi imperfecti with pycnidia coellomycetes, acervuli and stromata. Commonwealth Mycological Institute, Surrey.
- Wei, R.R., Sorger, P.K. and Harrison, S.C. (2005) Molecular organization of the Ndc80 complex, an essential kinetochore component. Proceedings of the National Academy of Sciences, 102, 5363-5367. doi:10.1073/pnas.0501168102
- Punithalingam, E. (1976) Botryodiplodia theobromae. CMI description of pathogenic fungi and bacteria No. 519. Commonwealth Mycological Institute, Surrey.
- Burgess, T.I., Barber, P.A., Mohali, S., Pegg, G., De Beer W. and Wingfield, M.J. (2006) Three new Lasiodiplodia spp. Fromthe tropics, based on DNA sequence recognized comparisons and morphology. Mycologia, 98, 423-435. doi:10.3852/mycologia.98.3.423
- Ahrens, U. and Seemüller, E. (1992) Detection of DNA of plant pathogenic mycoplasmalike organisms by polymerase chain reaction amplifying a sequence of 16S rRNA gene. Phytopathology, 82, 828-832. doi:10.1094/Phyto-82-828
- Kirkendall, L.R. (1983) The evolution of mating system in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zoological Journal of the Linnean Society, 77, 293-352. doi:10.1111/j.1096-3642.1983.tb00858.x
- Wood, S.L. (1982) The bark and ambrosia beetles of north and central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Naturalist Memoirs, 6, 1359.
- Ulloa, M. (1991) Illustrated dictionary of mycology. UNAM, Mexico.
- Flechtmann, C.A.H. (1995) Scolytidae em reflorestamentos com pinheiros tropicais. IPEF, Piracicaba.
- Harrington, C.T. (2005) Ecology and evolution of mycophagous bark beetles and their fungal partners. In: Vega, F.E. and Blackwell, M., Eds., Ecological and Evolutionary Advances in Insect-Fungal Associations, Oxford University Press, Oxford, 257-291.
- Beaver, R.A. (1989) Insect-fungus in the bark relationships and ambrosia beetles. Academic Blackwell, London.
- Kok, L.T, Norris, D.M and Chu, H.M. (1970) Sterol metabolism as a basis for a mutualistic symbiosis. Nature, 225, 661-662. doi:10.1038/225661b0
- Norris, D.M. (1979) The mutualistic fungi of xyleborine beetles. Halsted Press, Chichester. http://www.ncbi.nlm.nih.gov
- French, J.R.J. and Roeper, R.A. (1972) Interactions of the ambrosia beetle Xyleborus dispar (Coleoptera: Scolytidae) with STI symbiotic fungus, Ambrosiella hartigii (Fungi Imperfecti). Canadian Entomologist, 104, 1635-1641. doi:10.4039/Ent1041635-10
- Kingsolver, J.G. and Norris, D.M. (1977) External morphology of Xyleborus ferrugineus (Fabr.) (Coleoptera: Scolytidae) I. Head and prothorax of adult male and female. Journal of Morphology, 154, 147-156. doi:10.1002/jmor.1051540110
- Roeper, R.A, Treeful, L.M., O’Brien, K.M., Foote, R.A. and Bunce, M.A. (1980) Life history of the ambrosia beetle Xyleborus affinis (Coleoptera: Scolytidae) from in vitro culture. Great Lakes Entomologist, 13, 141-144.
- Borden, J.H. (1988) The striped ambrosia beetles. In: Berryman, A.A., Ed., Dynamics of Forest Insect Populations, Plenum, New York.
- Batra, L.R. (1966) Ambrosia fungi: Extent of specificity to ambrosia beetles. Science, 153, 193-195. doi:10.1126/science.153.3732.193
- Haanstad, J.O. and Norris, D.M. (1985) Microbial symbionts of the ambrosia beetle Xyloterinus politus. Microbial Ecology, 11, 267-276. doi:10.1007/BF02010605
- Norris, D.M. (1965) The complex of fungi essential to growth and development of Xyleborus sharpie in wood. Material und Organismen Beiheft, 1, 523-529.
- Baker, J.M. (1963) Ambrosia beetles and their fungi, with particular reference to Platypus cylindricus Fab. Symposium of the Society for General Microbiology, 13, 232- 265.
- Gebhardt, M., Bergerow, D. and Oberwinkler, F. (2004) Identification of the ambrosia fungus of Xyleborus monographus and X. dryographus (Curculionidae, Scolytinae). Mycologia, 3, 95-102.
- Kinuura H. (1995) Symbiotic fungi associated with ambrosia beetles. Japan Agricultural Research Quarterly, 29, 57-63.
- Francke-Grosmann, H. (1967) Wood-inhabiting ectosymbiosis in insects. Academic, New York.
- Morelet, M. (1998) Une nouvelle raffaelea spec, isolee cylindrus platypus, coleoptera des chenes xylomycetophage. Extr. Annales de la Societe des Sciences Naturelles, 50, 185-193.
- Fernandez-Marin, H., Zimmerman, J.K. and Wcislo, W.T. (2004) Ecological traits and evolutionary sequences of nest establishment in fungus growing ants (Hymenoptera, Formicidae, Attini). Biological Journal of the Linnean Society, 81, 39-48.
- Jones, K.G. and Blackwell, M. (1998) Phylogenetic analysis of ambrosia species in the genus Raffaelea based on 18S rDNA sequences. Mycological Research, 102, 661-665. doi:10.1017/S0953756296003437
- Rollins, F., Jones, K.G., Krokene, P., Solheim, H. and Blackwell, M. (2001) Phylogeny of asexual fungi associated with bark beetles and ambrosia. Mycologia, 93, 991- 996. doi:10.2307/3761761
- Nicholson, P. (2001) Molecular Assays as aids in the detection, diagnosis and Quantification of Fusarium species in plants. APS Press, St. Paul, 176-192.
- Carrillo, L. (1990) Micotoxinas de Fusarium spp en frutos deteriorados de Cucurbita ficifolia. Revista Argentina de Microbiología, 22, 212-215.
- Doohan, F.M., Brennan, J. and Cooke, B.M. (2003) Influence of climatic factors on Fusarium species pathogenic to cereals. European Journal of Plant Pathology, 109, 755-768.
- Cooke, R. (1977) Mutualistic symbioses with insects. John Wiley & Sons, London.
- Gil, P.Z.N., Bustillo, P.A.E., Gómez, D.D.S. and Marín, M.P. (2004) Corthylus novo sp. (Coleoptera: Scolytidae), plaga del aliso en la cuenca del rio Blanco en Colombia. Revista Colombiana de Entomología, 30, 171-178.
- Bonello, R., McNee, W.R., Storer, A.J., Wood, D.L. and Gordon, T.L. (2001) The role of olfactory stimuli in the location of weakened hosts by twig-infesting Pityophthorus sp. Ecological Entomology, 26, 8-15. doi:10.1046/j.1365-2311.2001.00288.x
- Tisserat, N., Cranshaw, W., Leatherman, D., Utley, C. and Alexander, K. (2009) Mortality in colorado black walnut caused by the walnut twig beetle and thousand cankers disease. Plant Health Progress. doi:10.1094/PHP-2009-0811-01-RS
- Morales-Ramos, J.A., Rojas, M.G. and Harrington, T. (1999) Association between the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae) and Fusarium solani (Moniliales: Tuberculariaceae). Annals of the Entomological Society of America, 92, 98-100.
- Morales-Ramos, J.A., Rojas, M.G., Sittertz-Bhatkar, H. and Saldaña, G., (2000) Symbiotic relationship between Hypothenemus hampei (Coleoptera: Scolytidae) and Fusarium solani (Moniliales: Tuberculariaceae). Annals of the Entomological Society of America, 93, 541-547. doi:10.1603/0013-8746(2000)093[0541:SRBHHC]2.0.CO;2
- Norris, D.M. and Baker, J.K. (1967) Symbiosis effects of a mutualistic fungus upon the growth and reproduction of Xyleborus ferrugineus. Science, 156, 1120-1122. doi:10.1126/science.156.3778.1120
- Norris, D.M. and Chu, H.M. (1971) Maternal Xyleborus ferrugineus transmission of sterol or sterol-dependent metabolites necessary for progeny pupation. Journal of Insect Physiology, 17, 1741-1745. doi:10.1016/0022-1910(71)90071-0
- Norris, D.M., Baker, J.K. and Chu, H.M. (1969) Symbiotic interrelationships between microbes and ambrosia beetles. III. Ergosterol as the source of sterol to the insect. Annals of the Entomological Society of America, 62, 413- 414.
- Chu, H.M., Norris, D.M. and Kok, L.T. (1970) Pupation requirement of the beetle, Xyleborus ferrugineus: Sterols other than cholesterol. Journal of Insect Physiology, 16, 1379-1387. doi:10.1016/0022-1910(70)90137-X
- Kok, L.T. (1979) Lipids of ambrosia fungi and the life of mutualistic beetles. In: Batra, L.R., Ed., Insect-Fungus Symbiosis, Halsted Press, Sussex.
- Milholland, R.D. (1970) Histology of Botryosphaeria canker of highbush blueberries susceptible and resistant. Phytopathology, 60, 70-74. doi:10.1094/Phyto-60-70
- Riva, R. (1996) Tecnología del cultivo de camu camu en la Amazonía Peruana. INIA, Pucallpa-Perú.
- Umezurike, G.M. (1969) Cellulolytic activities of Botryodiplodia theobromae pat. Annals of Botany, 33, 451- 462.
- Rajput, K.S. and Rao, K.S. (2007) Death and decay in the trees of Mango (Mangifera indica L.). Microbiological Research, 162, 229-237. doi:10.1016/j.micres.2004.07.003
- Flores, T.V., Crespo, R.G. and Cabezas, G.F. (2010) Plagas y enfermedades en plantaciones de Teca (Tectona grandis L.F) en la zona de Balzar, Provincia del Guayas. Ciencia y Tecnología, 3, 15-22.
- Masood, A., Saeed, S., Silveira, S.F., Akem, C.N., Hussain, N. and Farooq, A.M. (2011) Of mango quick decline in Pakistan: Survey and pathogenicity of fungi isolated from bark beetle and mango tree. Pakistan Journal of Botany, 43, 1793-1798.
- Andersen, B., Krøger, E. and Rodney G.R. (2001) Chemical and morphological segregation of Alternaria alternata, A. gaisen and A. longipes. Mycological Research, 105, 291-299. doi:10.1017/S0953756201003446
- Armengol, J., Sales, R., Garcia-Jimenez, J. and AlfaroLassala, F. (2000) First report of Alternariabrown spot of citrus in Spain. Plant Disease, 84, 1044. doi:10.1094/PDIS.2000.84.9.1044B
- Negron, J.F., Witcosky, J.J., Cain, R.J., LaBonte, J.R., Duerr, D.A., McElwey, S.J., Lee, J.C. and Seybold, S.J. (2005) The banded elm bark beetle: A new threat to elms in North America. American Entomologist, 51, 84-94.
- Capurro, M. and Reyes, S. (2007) Fungi, insect borers Association present in samples of wood entered into the regional laboratory of the agricultural and livestock service. Bachelor Thesis, Universidad Austral de Chile.
- Chan-Cupul, W., Ruiz-Sánchez, E., Cristóbal-Alejo, J., Pérez-Gutiérrez, A., Munguía-Rosales, R. and LaraReyna, J. (2010) In vitro development of four Paecilomyces Fumosoroseus isolates and their pathogenicity on immature whitefly. Agrociencia, 44, 587-597.
- Gemma, J.N., Hartmann, G.C. and Wasti, S.S. (1984) Interaction between inhibitory Ceratocystis ulmi and several species of entomogenous fungi. Mycologia, 76, 256-260. doi:10.2307/3793101
- Moraes, A.M.L. Junqueira, A.C.V., Costa, G.L., Celano, V., Oliveira, P.C. and Coura, J.R. (2000) Fungal flora of the digestive tract of triatomines of 5 species vectors of Trypanosoma cruzi, Chagas 1909. Mycopathologia, 151, 41-48. doi:10.1023/A:1010905420001

