Food and Nutrition Sciences
Vol.4 No.2(2013), Article ID:27810,6 pages DOI:10.4236/fns.2013.42027
Influence of Synthetic Antioxidants on Extraction of All-trans-Lutein from Spinach under Air and Nitrogen Atmosphere
![]()
1Laboratory for Food Chemistry, National Institute of Chemistry, Ljubljana, Slovenia; 2Center for Validation Technologies and Analytics, National Institute of Chemistry, Ljubljana, Slovenia.
Email: katarinacernelic@yahoo.com
Received December 3rd, 2012; revised January 3rd, 2013; accepted January 11th, 2013
Keywords: Extraction; Lutein; Spinach; TBHQ; BHT; Nitrogen Atmosphere; HPLC
ABSTRACT
Extraction is most commonly used sample preparation technique in quantitative determination of all-trans-lutein in spinach. In our study RP-HPLC was applied to identify the constituents of spinach extract and to quantify all-translutein content with the mobile phase consisted of acetone and water. Due to sensitivity of all-trans-lutein, we compared effects of different extraction conditions; air and nitrogen atmosphere and ethanol, ethanol with added 0.1% TBHQ and ethanol with added 0.1% BHT as solvents. Results for the all-trans-lutein content in the spinach samples and spinach samples with standard addition obtained in air and in nitrogen confirmed the induced degradation of all-trans-lutein in oxygen from the air. Ethanol containing synthetic antioxidant TBHQ under nitrogen atmosphere gave the highest yield of extraction and recovery of all-trans-lutein from spinach.
1. Introduction
Carotenoids are tetraterpenes (composed from eight isoprene units) which are synthesized in bacteria, algae, fungi and green plants [1] where they form lipid-soluble yellow, orange, and red pigments [2]. Over 700 known carotenoids [3] are divided into oxygenated xanthophylls such as lutein, zeaxanthin, violaxanthin, neoxanthin and hydrocarbon carotenes such as β-carotene, α-carotene and lycopene. Because of their structure (several possible isomers), their instability, and so forth their extraction, isolation, identification and determination represent a considerable challenge. Fruits and vegetables are the primary dietary source of carotenoids for most mammalian species. From so many carotenoids identified in nature, more than 40 were, identified, in human body [4]. The most prevalent carotenoids in human serum are α-carotene, β-carotene, lycopene, lutein, zeaxanthin, and β-cryptoxanthin [2].
Being strong antioxidants they have a beneficial effect on human health, show antioxidant activity, immunity regulation, and inhibition of tumour cells proliferation [5-7].
Xanthophylls lutein, a non-provitamin A xanthophyll (Figure 1) and his structural isomer zeaxanthin selectively accumulate in the yellow spot of the eye retina with the highest concentration in macula lutea, where they acts as a blue light filter and serve as a protection against the development of age related macular degeneration (AMD) and cataract [8]. Beside the retina of the eye, dietary carotenoids accumulate in many tissues including the liver, adipose, serum, breast milk, adrenal, prostate, kidney, lung, brain, and skin [2].
Lutein is mostly found in dark green vegetables, such as spinach and kale [9-11]. Spinach (Spinacea olerace L.) is native to West Asia and it is widely cultivated in the world as one of the most popular vegetables. In an analytical chemistry, spinach is often used as a source of violaxanthin and neoxanthin standard since they are not commercially available [12,13].
There are some publications based on the effect of antioxidants during the preparation and analysis of lutein. The most extensively used antioxidant for keeping carotenoids stable is butylated hydroxytoluene (BHT) [14] (Figure 2).
In addition, the effect of tert-butlylhydroqinone (TBHQ) (Figure 2) was mentioned as a stabilizer during the spray-drying of β-carotene [15] and Nuutila noticed its positive effect on stability of flavonoids from onion during the extraction [16]. TBHQ is mostly thought as
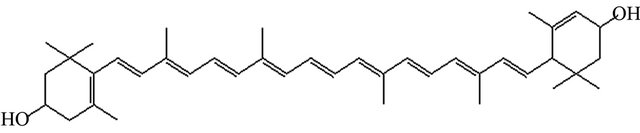
Figure 1. Structural formula of all-trans-lutein.
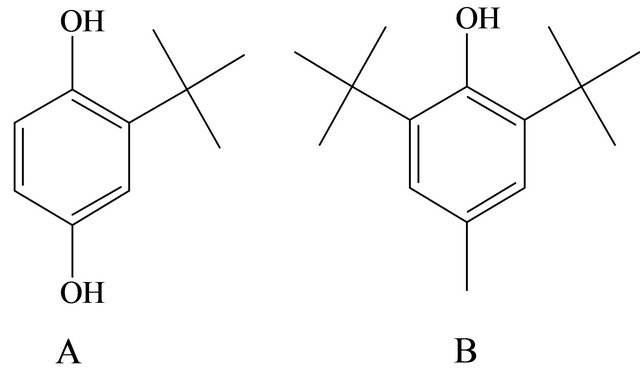
Figure 2. Structural formula of tert-butyl-hydroquinone (TBHQ) (A) and butylated hydroxytoluene (BHT) (B).
one of the most effective antioxidants for protection of fats and oils [17]. Firstly BHT and TBHQ were proposed to be used as an additive during extraction of carotenoids from spinach [18], but without comparing extraction in nitrogen atmosphere, which was suggested to avoid isomerization and degradation of carotenoids [12].
Since TBHQ and BHT are cheap and commercially available we studied their effect on extraction efficiency of all-trans-lutein from the spinach in air and under nitrogen atmosphere. However, the first and principal aim of present study was to develop a simple and the most efficient analytical procedure for extraction of all-translutein from spinach and separation method for carotenoids which could be applied also in other studies.
2. Materials and Methods
2.1. Chemicals
Lutein standard was from Extrasynthèse (Genay, France). Acetone, ethanol and (TBHQ, purity ≥97%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antioxidant 3,5-di-tert-butyl-4-hydroxytoluene (BHT, purity ≥99%) was purchased from Merck (Darmstadt, Germany). All solvents were of analytical grade. Bidistilled water was used.
2.2. Preparation of Standard Solution
The lutein stock solutions (65 µg/mL lutein in ethanol, 60 µg/mL lutein in ethanol with 0.1% TBHQ, and 57 µg/mL lutein in ethanol with 0.1% BHT) were sonicated in an ultrasonic bath (Sonis 3 GT, Iskra Pio d.o.o., Šentjernej, Slovenia) for 5 minutes. Additionally, standard solutions around 0.5 μg/mL were prepared with solvents (ethanol, ethanol with 0.1% TBHQ and ethanol with 0.1% BHT) separately. Concentration of standard solutions was checked spectrophotometrically (Lambda 45 UV/VIS, Perkin-Elmer, Massachusetts, USA) before the analysis, with their appropriate blank. Specific absorption coefficient of lutein at 445 nm in ethanol 2550 dm2/g was considered [19]. The solutions were stored at −80˚C prior to analysis.
2.3. Plant Material
Four different fresh spinach samples (Spinacia oleracea L.) were bought at the local market. Leaves of spinach without stems were lyophilized for 24 hours at −50˚C in lyophilirator (Micro Modulyo IMA Edwards, Bologna, Italy) equipped with a rotary vane vacuum pump (Pfeiffer, Asslar, Germany). Dry leaves were pulverized by Mikro-Dismembrator S (Sartorius, Göttingen, Germany) using liquid nitrogen. Obtained powder was weighted and taken as the dried material of fresh spinach leaves. Powdered and dried samples were kept well closed at −80˚C until analysis.
2.4. Extractions from Spinach
Several samples, 10 mg of lyophilized spinach were exactly weighed, and extracted for 1 hour at room temperature, protected from the sun light with 10 mL of solvents (ethanol, ethanol with 0.1% BHT, ethanol with 0.1% TBHQ). Extractions were performed in glass tubes in air and also under nitrogen atmosphere. The apparatus Carousel 12 Plus (Radleys, Saffron Walden, UK) with magnetic stirrer was used. After the extraction at room temperature extracts were centrifuged (Centric 322A, Tehtnica, Železniki, Slovenia) for 5 minutes at 4000 rpm and the concentrations of all-trans-lutein in supernatant was measured. All experiments were done in duplicate.
Spinach samples with addition of lutein standard were performed as described above. The difference was that the spinach sample was spiked with 0.5 μg/mL of lutein standard solution. The recovery experiments were done in duplicates in glass tubes on the apparatus Carousel 12 Plus in air and under nitrogen, for all spinach samples with three different solvents (ethanol, ethanol with 0.1% TBHQ and ethanol with 0.1% BHT).
2.5. HPLC Chromatographic Analysis
RP-HPLC was applied to identify the constituents of spinach extract and to quantify all-trans-lutein content. HPLC analysis was carried out using Surveyor Plus HPLC system (Thermo Finnigan, San Jose, CA, USA), equipped with a thermostated autosampler Surveyor Autosampler Plus, a quaternary pump Surveyor LC Pump Plus and a diode-array detector Surveyor PDA Plus. ChromQuest 4.2 software was used for the data evaluation. For the separation ProntoSIL C30 column, 250 × 4.6 mm, 5 μm particle size (Bischoff Chromatography, Leonberg, Germany), equipped with a Phenomenex C18 guard column (Torrance, California, USA) was used. Mobile phase consisted of acetone (solvent A) and water (solvent B). The following gradient was applied: 80% A to 95% A from 0 to 25 min, 95% A from 25 to 30 min, 95% to 100% A from 30 to 32 min, 100% A from 32 to 37 min, 100% A to 80% A from 37 to 40 min, and 80% A from 40 to 42 min The acquisition wavelength was set at 450 nm and the flow rate was 1 mL/min and injection volume was 20 μL. Quantitative determination of lutein was carried out using external standard method with average response factor, which was injected after every four samples.
2.6. Statistical Analysis
All sample test solutions were prepared in duplicate and the mean values were calculated. Statistical analyses were conducted using SPSS software, with Duncan’s multiple range tests. The difference between the means was considered significant at a level of P < 0.05.
3. Resultes and Discussion
Considering the complexity of carotenoid profile in spinach a gradient solvent system of acetone and water was used. Typical chromatogram of carotenoids from spinach is presented in Figure 3, where all principal carotenoids: all-trans-lutein (β,ε-carotene-3,3’-diol), β- carotene (β,β-carotene), α-carotene, violaxanthin (5,6,5’, 6’-diepoxy-5,6,5’,6’-tetrahydro-β,β-carotene-3,3’-diol) and neoxanthin (5’,6’-epoxy-6,7-didehydro-5,6,5’,6’-tetrahydro-β,β-carotene 3,5,3’-triol) are separated within 34 min. Moreover, we managed to achieve baseline separation with all-trans-lutein and all-trans-zeaxanthin, both dihydroxycarotenoids differing only in the position of one of their double bonds.
3.1. Influence of Antioxidants Addition and Atmospheric Conditions on Extraction Efficiency
Sample preparation is the vital step in reliable analysis of lutein. The extraction was made from the lyophilized spinach to avoid possible enzymatic changes, which may occur during the storage of the spinach samples. Powdering the plant material in liquid nitrogen with Dismembrator enabled to get a representative sample despite low weight (10 mg). Enhanced extraction efficiency from the fine particles was achieved. The saponification was not necessary, due to the fact that all-trans-lutein in spinach is in free form and not esterified with fatty acids and that our developed method had separated carotenoids with chlorophylls, which according to Dachtler and coworkers may hinder HPLC analysis [20]. The results were calculated as mg of all-trans-lutein per 100 g of fresh spinach.
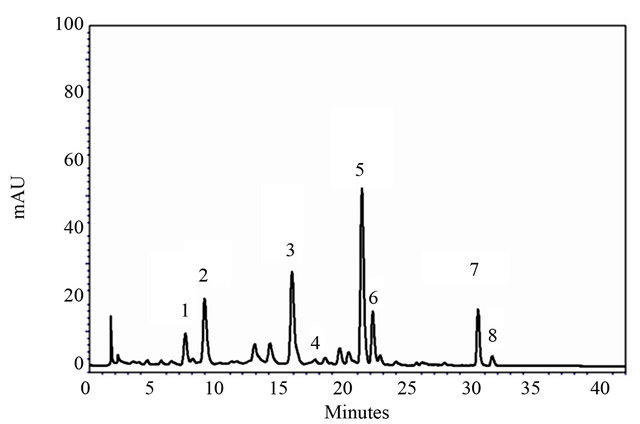
Figure 3. Chromatogram of carotenoids from spinach extract separated with gradienton Prontosil C30 column and recorded at 450 nm. Designated peaks represent: neoxantin (1), violaxanthin (2), lutein (3), zeaxanthin (4), chlorophyll b (5), chlorophyll a (6), β-carotene (7), α-carotene (8).
3.2. Quantification of All-trans-Lutein in Spinach Samples of Antioxidants Addition and Atmospheric Conditions on Extraction Efficiency
According to the literature data, the content of all-translutein was about 3 - 13 mg per 100 g of fresh spinach [21-24].
Due to the sensitivity of carotenoids the stabilization in food and foodstuffs samples against oxidation and degradation during the sample preparation and analysis of carotenoids has always been of most importance. Because of proand antioxidant behavior of carotenoids during sample preparation effects of supplemented antioxidants on the extracting procedure were determined.
The content of all-trans-lutein in each of our four spinach samples was extracted with all three solvents. Obtained results are presented in Table 1. The average content of all-trans-lutein in spinach extracted under air atmosphere was 7.16, 7.61 and 7.36 mg of all-trans-lutein per 100 g of spinach when ethanol, ethanol with added TBHQ and ethanol with added BHT was used, respectively. Under nitrogen atmosphere contents of alltrans-lutein were higher, 7.74, 8.16 and 7.91 mg of alltrans-lutein per 100 g of spinach when ethanol, ethanol with added TBHQ and ethanol with added BHT was used, respectively. These results show that the extraction of all-trans-lutein under nitrogen atmosphere is much more efficient. Comparison between extractions under nitrogen and extractions in air atmosphere shows that high concentration of oxygen in the air promotes the instability of carotenoids, so it has a significant effect on the yield of all-trans-lutein from the spinach extracts, despite the presence of antioxidants. Those results may be explained so, that in the extraction solution added antioxidant protected lutein from existing free radicals, see Figure 4, but

Figure 4. Schematic presentation of some possible path of lutein degradation during the extraction with ethanol. Elimination of Radicals (Reaction 1), restoration of lutein (Reaction 2), quenching (Reaction 3), peroxyl aducts (Reaction 4).
Table 1. Yields of all-trans-lutein from the lyophilized spinach samples (calculated in mg of all-trans-lutein/100g of fresh spinach).
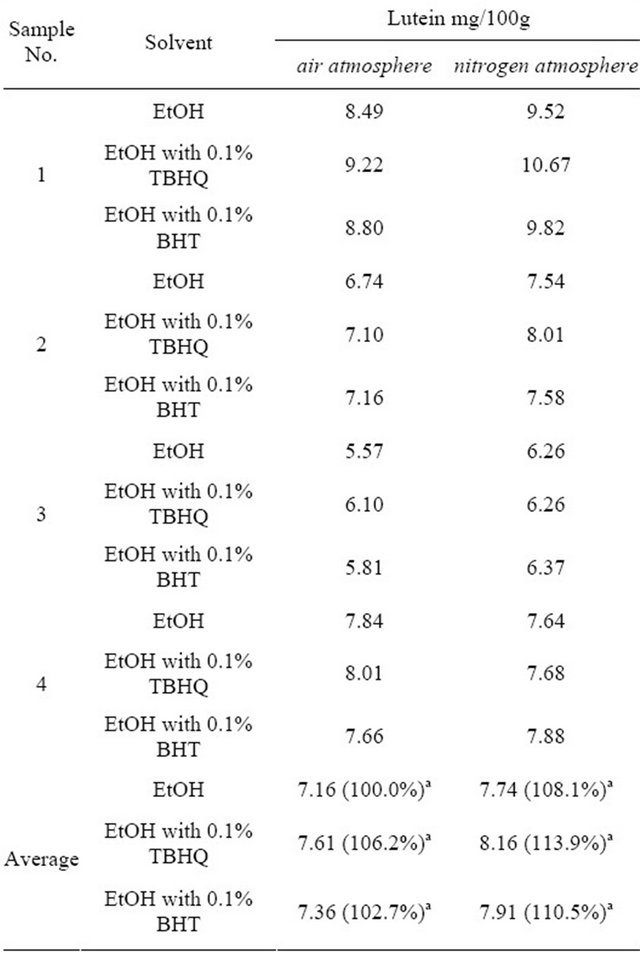
aContent of lutein in the lyophilized spinach samples normalised on content of lutein obtained by extraction with ethanol in air atmosphere (%).
at the same time nitrogen atmosphere protects lutein from formation of hydroperoxide and further formation of peroxyl radical adducts which could lead to autoxidation.
In our experiment lutein is protected against free radicals (Reaction 1) and also against lutein radical (Reaction 2). This antioxidant protection could not stop the formation of hydroperoxides and autoxidation of lutein when certain higher concentration of oxygen was present (Reactions 4). For this reasons the addition of antioxidants is more preferred when extraction is performed in air atmosphere. When lutein physical quenches singlet oxygen (1O2), the excited lutein molecule is not necessary destroyed. The addition of antioxidants may enable excited triplet state of lutein to safely return to lower level, from where it can undergo into further cycles of singlet oxygen quenching (Reaction 3) [25]. It is also possible that the addition of antioxidants prevent pro-oxidation of alltrans-lutein which may occur during extraction. The addition of TBHQ to solvent shows statistically better antioxidant protection according to pure solvent, when extracting all-trans-lutein regardless to chosen atmospheric condition (air or nitrogen), while in nitrogen atmosphere there were significant differences only when TBHQ was added, but not in the case of addition of BHT (P < 0.05). The higher efficiency of synthetic antioxidant TBHQ could be related with his additional hydroxyl group or some steric effects. Addition of TBHQ into extraction solvent gave approximately 6% higher yield than experiments where only pure solvent was used.
From the results it is evident that atmosphere with a low concentration of oxygen is the most relevant factor, during the extraction procedure. Antioxidants could reduce degradations of carotenoids during the extraction procedure to certain level, meanwhile the shortage of oxygen prevents formation of hydroperoxides and diminishes the autoxidation and therefore successfully minimizes degradation of all-trans-lutein.
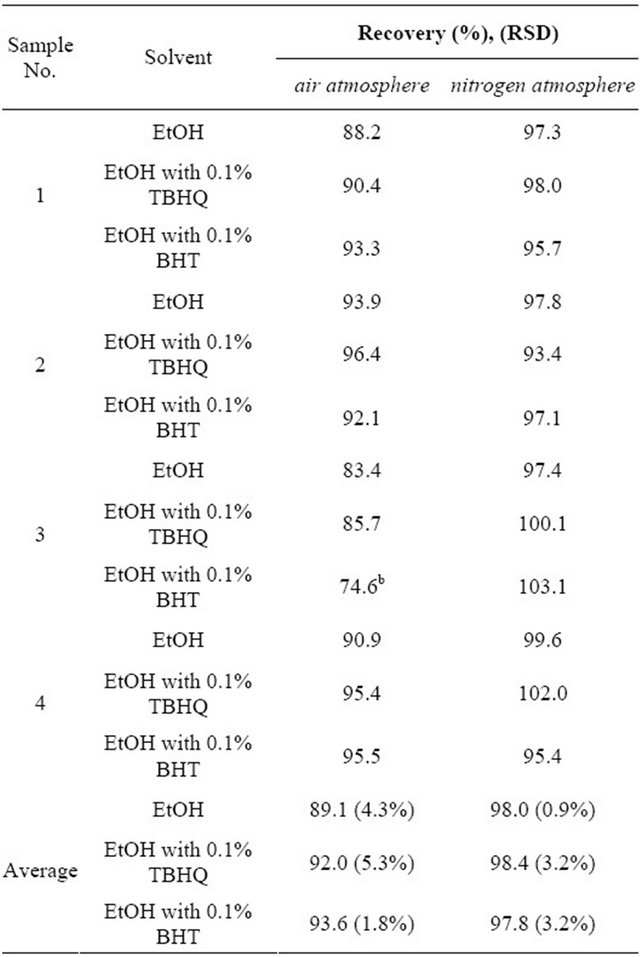
In all four spinach samples, with three different solvents the recovery was also studied (Table 2). The average recoveries are between 89.1% and 93.6% in air condition, and approximately 98% when extraction was performed under nitrogen atmosphere. The highest recovery was obtained by extraction all-trans-lutein under nitrogen atmosphere and added TBHQ. This result shows how sensitive antioxidant lutein is, without protection of other antioxidants in atmosphere with oxygen. Our almost 100% extraction is very simple and fast in comparison with the 30 hours extraction procedure described by Liu [26], or four step extraction described by Kopsell and coworkers [24]. When comparing recovery results, there were non-significant differences when comparing all three solvents in air atmosphere, and in nitrogen atmosphere (P < 0.05).
Table 2. Recovery (%) of all-trans-lutein in the spinach samples.
4. Conclusions
In recent years reports suggesting beneficial nutritional and physiological effect of carotenoids have increased interest in vegetables as an important source of bioactive carotenoids. Unfortunately, in most cases not enough attention is paid to methods used for sample preparation and analysis of the content of carotenoids. The method reported above is simple, fast and suitable for the analysis of several free major carotenoids and uses small amounts of the sample and organic solvents.
With our study we present how the degradation of lutein during the extraction procedure can be prevented. Sample preparation must be done under nitrogen, some synthetic antioxidant (TBHQ, BHT) should be added to the solvent and careful manipulation of the test solutions is necessary in order to achieve desired accuracy of alltrans-lutein quantification. The addition of synthetic antioxidants to the extraction solvent is necessary if analysis is done without nitrogen protection, nevertheless they may not totally prevent the undesired reactions of degradation of lutein, but they can improve the recovery.
5. Acknowledgements
Authors thank the Laboratory for Food Chemistry at National Institute of Chemistry Slovenia for the financial support.
REFERENCES
- G. Sandmann, “Carotenoid Biosynthesis and Biotechnological Application,” Archives of Biochemistry and Biophysics, Vol. 385, No. 1, 2001, pp. 4-12. doi:10.1006/abbi.2000.2170
- D. M. Deming and J. W. Erdman Jr., “Mammalian Carotenoid Absorption and Metabolism,” Pure and Applied Chemistry, Vol. 71, No. 12, 1999, pp. 2213-2223. doi:10.1351/pac199971122213
- F. Khachik, J. S. Bertram, M.-T. Huang, J. W. Fahey and P. Talalay, “Dietary Carotenoids and Their Metabolites as Potentially Useful Chemoprotective Agents against Cancer,” In: L. Packer, M. Hiramatsu and T. Yoshikawa, Eds., Antioksidant Food Supplements in Human Health, Academic Press, San Diego, 1999, pp. 203-229. doi:10.1016/B978-012543590-1/50015-9
- F. Khachik, G. R. Beecher and J. C. Smith Jr., “Lutein, Lycopene, and Their Oxidative Metabolites in Chemoprevention of Cancer,” Journal of Cellular Biochemistry, Vol. 59, No. S22, 1995, pp. 236-246. doi:10.1002/jcb.240590830
- D. Ren and S. Zhang, “Separation and Identification of the Yellow Carotenoids in Potamogeton crispus L.,” Food Chemistry, Vol. 106, No. 1, 2008, pp. 410-414. doi:10.1016/j.foodchem.2007.05.074
- A. Alves-Rodrigues and A. Shao, “The Science behind Lutein,” Toxicology Letters, Vol. 150, No. 1, 2004, pp. 57-83. doi:10.1016/j.toxlet.2003.10.031
- W. Stahl and H. Sies, “Bioactivity and Protective Effects of Natural Carotenoids,” Biochimica et Biophysica Acta, Vol. 1740, No. 2, 2005, pp. 101-107. doi:10.1016/j.bbadis.2004.12.006
- R. L. Roberts, J. Green and B. Lewis, “Lutein and Zeaxanthin in Eye and Skin Health,” Clinics in Dermatology, Vol. 27, No. 2, 2009, pp. 195-201. doi:10.1016/j.clindermatol.2008.01.011
- A. Perry, H. Rasmussen and E. J. Johnson, “Xanthophyll (Lutein, Zeaxanthin) Content in Fruits, Vegetables and Corn and Egg Products,” Journal of Food Composition and Analysis, Vol. 22, No. 1, 2009, pp. 9-15. doi:10.1016/j.jfca.2008.07.006
- O. F. O’Connell, L. Ryan and N. M. O’Brien, “Xanthophyll Carotenoids Are More Bioaccessible from Fruits than Dark Green Vegetables,” Nutrition Research, Vol. 27, No. 5, 2007, pp. 258-264. doi:10.1016/j.nutres.2007.04.002
- R. Aman, J. Biehl, R. Carle, J. Conrad, U. Beifuss and A. Schieber, “Application of HPLC Coupled with DAD, APcI-MS and NMR to the Analysis of Lutein and Zeaxanthin Stereoisomers in Thermally Processed Vegetables,” Food Chemistry, Vol. 92, No. 4, 2005, pp. 753-763. doi:10.1016/j.foodchem.2004.10.031
- B. Stephen Inbaraj, J. T. Chien and B. H. Chen, “Improved High Performance Liquid Chromatographic Method for Determination of Carotenoids in the Microalga Chlorella pyrenoidosa,” Journal of Chromatography A, Vol. 1102, No. 1-2, 2006, pp. 193-199. doi:10.1016/j.chroma.2005.10.055
- B. Gandul-Rojas, M. R. Cepero and M. I. Mínguez-Mosquera, “Chlorophyll and Carotenoid Patterns in Olive Fruits, Olea europaea Cv. Arbequina,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 6, 1999, pp. 2207-2212. doi:10.1021/jf981158u
- J. Oliver and A. Palou, “Chromatographic Determination of Carotenoids in Foods,” Journal of Chromatography A, Vol. 881, No. 1-2, 2000, pp. 543-555. doi:10.1016/S0021-9673(00)00329-0
- S. Orset, G. C. Leach, R. Morais and A. J. Young, “SprayDrying of the Microalga Dunaliella salina: Effects on Beta-Carotene Content and Isomer Composition,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 11, 1999, pp. 4782-4790. doi:10.1021/jf990571e
- A. M. Nuutila, K. Kammiovirta and K. M. Oksman-Caldentey, “Comparison of Methods for the Hydrolysis of Flavonoids and Phenolic Acids from Onion and Spinach for HPLC Analysis,” Food Chemistry, Vol. 76, No. 4, 2002, pp. 519-525. doi:10.1016/S0308-8146(01)00305-3
- P.-P. Hao, J.-R. Ni, W.-L. Sun and W. Huang, “Determination of Tertiary Butylhydroquinone in Edible Vegetable Oil by Liquid Chromatography/Ion Trap Mass Spectrometry,” Food Chemistry, Vol. 105, No. 4, 2007, pp. 1732-1737. doi:10.1016/j.foodchem.2007.04.058
- K. Cernelic, B. Simonovska and I. Vovk, “The Influence of Adding Antioxidants TBHQ and BHT to Ethanol at Extraction of Lutein from Spinach,” Abstract Book and Final Programme of 10th International Nutrition & Diagnostics Conference, Prague, 4-7 September 2010, p. 101.
- N. E. Craft and J. H. Soares Jr., “Relative Solubility, Stability, and Absorptivity of Lutein and Beta-Carotene in Organic Solvents,” Journal of Agricultural and Food Chemistry, Vol. 40, No. 3, 1992, pp. 431-434. doi:10.1021/jf00015a013
- M. Dachtler, T. Glaser, K. Kohler and K. Albert, “Combined HPLC-MS and HPLC-NMR On-Line Coupling for the Separation and Determination of Lutein and Zeaxanthin Stereoisomers in Spinach and in Retina,” Analytical Chemistry, Vol. 73, No. 3, 2001, pp. 667-674. doi:10.1021/ac000635g
- A. Bunea, M. Andjelkovic, C. Socaciu, O. Bobis, M. Neacsu, R. Verhe and J. Van Camp, “Total and Individual Carotenoids and Phenolic Acids Content in Fresh, Refrigerated and Processed Spinach (Spinacia oleracea L.), Food Chemistry, Vol. 108, No. 2, 2008, pp. 649-666. doi:10.1016/j.foodchem.2007.11.056
- U. Kidmose, P. Knuthsen, M. Edelenbos, U. Justesen and E. Hegelund, “Carotenoids and Flavonoids in Organically Grown Spinach (Spinacia oleracea L.) Genotypes after Deep Frozen Storage,” Journal of the Science of Food and Agriculture, Vol. 81, No. 9, 2001, pp. 918-923. doi:10.1002/jsfa.902
- R. Tsao and R. Yang, “Lutein in Selected Canadian Crops and Agri-Food Processing by-Products,” Journal of Chromatography A, Vol. 1112, No. 1-2, 2006, pp. 202-208. doi:10.1016/j.chroma.2005.09.088
- D. A. Kopsell, M. G. Lefsrud, D. E. Kopsell, A. J. Wenzel, C. Gerweck and J. Curran-Celentano, “Spinach Cultigen Variation for Tissue Carotenoid Concentrations Influences Human Serum Carotenoid Levels and Macular Pigment Optical Density Following a 12-Week Dietary Intervention,” Journal of Agriculture and Food Chemistry, Vol. 54, No. 21, 2006, pp. 7998-8005. doi:10.1021/jf0614802
- F. Böhm, R. Edge and T. G. Truscott, “Interactions of Dietary Carotenoids with Singlet Oxygen (1O2) and Free Radicals: Potential Effects for Human Health,” Acta Viochimica Polonica, Vol. 59, No. 1, 2012, pp. 27-33.
- Y. Liu, C. O. Perera and V. Suresh, “Comparison of Three Chosen Vegetables with Others from South East Asia for Their Lutein and Zeaxanthin Content,” Food Chemistry, Vol. 101, No. 4, 2007, pp. 1533-1539. doi:10.1016/j.foodchem.2006.04.005

