Food and Nutrition Sciences
Vol. 3 No. 1 (2012) , Article ID: 17083 , 8 pages DOI:10.4236/fns.2012.31012
Collagen from Tendon of Yezo Sika Deer (Cervus nippon yesoensis) as By-Product
![]()
1Graduate School, Prince of Songkla University, Songkhla, Thailand; 2Mimasaka University, Okayama, Japan; 3Nagoya Research Institute, Toyoake, Aichi, Japan; 4National Fisheries University, Yamaguchi, Japan.
Email: *nandemofreefree@yahoo.co.jp
Received September 5th, 2011; revised October 11th, 2011; accepted October 19th, 2011
Keywords: Collagen; Yezo Sika Deer; Tendon; By-Product; Yield; Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Analysis
ABSTRACT
Collagen from tendon of Yezo sika deer was prepared by limited pepsin digestion. The yield of collagen was very high; 35.7% on the basis of lyophilized dry weight. The secondary structure of this collagen was different from that of porcine skin by ATR-FTIR analysis, although it was the same characteristics, e.g. SDS-PAGE, subunit composition, thermal behavior, as porcine collagen. Since taking up a problem of bovine spongiform encephalopathy infection in land animals such as calf or bovine, collagen from aquatic materials has been used in various industries. However, the present study indicates that tendon of Yezo sika deer as by-product of meat industry will have potential as an important collagen source for use in the foods, cosmetics, and medical fields.
1. Introduction
The collagens are a family of proteins found in most animal tissues, where they play various important structural roles as intracellular matrix constituents. Type I collagen, which consists of two α1 polypeptide chains and one α2, is a prevalent protein in bone, skin, and tendon. So far, for industrial applications as functional foods, biomedical materials, and cosmetics collagen have been extracted from the skins of some vertebrate species such as pig and calf or bovine. However, the outbreak of bovine spongiform encephalopathy (BSE), transmissible spongiform encephalophathy (TSE), the foot-and-mouth disease (FMD), and avian influenza have resulted in anxiety among users of collagen and collagen-derived products from these land animals [1]. Recently, alternatives of land animals as collagen sources are extremely desired. Until now, as a part of a study looking at the effective use of underutilized resources, we tried to prepare and characterize the collagens from aquatic organisms [2-11]. As a result, a large quantity of collagen could be obtained from these aquatic organisms, although the physical and chemical properties of these collagens were different from those of land animals such as porcine.
Many species of wild animals such as Yezo sika deer (Cervus nippon yesoensis), Hokkaido brown bear (Vrsus arctos yesoensis), Hokkaido squirrel (Sciurus vulgaris orientis), and Schrenks red fox (Vulpes vulpes schrencki) lives in the eastern part of Hokkaido, Japan. Particularly, Shiretoko, in the east Hokkaido, has been recorded as one of the World Heritage by United Nations Educational, Scientific and Cultural Organization (UNESCO) in July 15, 2005. While we have no active from the standpoint of nature conservation, the quantity of Yezo sika deer explosively increased and now it is known to live about six hundred of Yezo sika deer in this area. As a result, it is increasing not only any damage to the crops and forest, but also traffic accident to people. To solve the problem as quick as possible, it is necessary to use Yezo sika deer as one of the utilized resources to human life. Fortunately, so far, there are quite report that is likely to be associated with infections such as BSE, TSE, and FMD in Yezo sika deer. The objective of this research was to prepare the collagen from tendon of Yezo sika deer as by-product of meat industry and to compare the characteristics with collagen from other species such as aquatic organisms to investigate whether to have potential as an important collagen source for use in the industries.
2. Materials and Methods
2.1. Sample
Tendon from Yezo sika deer was obtained from Hokusen Development Co. Ltd., Kushiro, Hokkaido, Japan. The tendon was stored at –85˚C until used.
2.2. Preparation of Collagen from Tendon of Yezo Sika Deer
All the preparative procedures were carried out at 4˚C. The frozen tendon was thawed at half and cut into small pieces (0.5 × 1.0 cm) by a scalpel (D-4: As One Co. Ltd., Osaka, Japan). The pieces were extracted with 10 volumes of 10% ethanol to remove fat for 3 days by changing the solution once a day. The resultant matter was washed with distilled water for a day by changing the solution. The matter was extracted with 0.1 M NaOH to remove noncollagenous proteins for 2 days by changing the solution twice a day. The resultant matter was washed with distilled water for 2 days by changing the solution three time a day and lyophilized. The dried matter was extracted with 0.5 M acetic acid for 3 days. The extract was centrifuged at 50,000 × g for 1 h. The supernatants were pooled and salted out to purify the collagen by adding NaCl to a final concentration of 0.7 M and followed by precipitation of the collagen by the addition of NaCl at a final concentration of 2.2 M at a neutral pH in 0.05 M Tris-HCl (pH 7.5). The resultant precipitate was obtained by centrifugation at 50,000 × g for 1 h, and dissolved in a minimum volume of 0.5 M acetic acid, dialyzed against 0.1 M acetic acid, distilled water, and then lyophilized (acid-soluble collagen).
The residue produced by the extraction of acid-soluble collagen was washed with distilled water for a day twice a day, suspended in 0.5 M acetic acid, and was digested with 1.0% (w/w) pepsin (EC 3.4.23.1; 2× crystallized; 3085 U/mg protein, Sigma-Aldrich Co., USA) by continuous stirring for 2 days. After centrifugation at 50,000 × g for 1 h, the supernatants were pooled and dialyzed against 0.02 M Na2HPO4 (pH 7.2) for 3 days with the change of solution twice a day to inactivate the pepsin. The precipitate collected by centrifugation in the same conditions was dissolved in 0.5 M acetic acid and salted out by selective salt precipitation at above conditions. The resultant precipitate was obtained by centrifugation at 50,000 × g for 1 h, dissolved in 0.5 M acetic acid, dialyzed against 0.1 M acetic acid for a day, distilled water for 3 days by changing the solution twice a day, and then lyophilized (pepsin-solubilized collagen).
2.3. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Collagen from Tendon of Yezo Sika Deer
SDS-PAGE was performed as described previously [12]. After electrophoresis, the gel was stained with Coomassie brilliant blue R-250 (Fluka Fine Chemical Co. Ltd., Tokyo, Japan) and destained with 7.5% acetic acid and 5% methanol. Myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase B (97 kDa), and bovine serum albumin (66 kDa) were used as marker proteins that were purchased from Sigma-Aldrich Co. (USA).
2.4. Peptide Mapping of Collagen from Tendon of Yezo Sika Deer
Collagen samples (1.0 mg) were dissolved in 0.1 ml of 0.1 M sodium phosphate buffer (pH 7.2) containing 0.5% SDS and boiled for 5 min. The denatured collagen samples were cooled in ice, and then were digested at 37˚C by lysyl endopeptidase (0.24 amidase activity) from Achromobacter lyticus M497-1 (EC 3.4.21.50; Wako Pure Chemical Industries, Ltd., Osaka, Japan). After 30 min, the samples were added SDS to a final concentration of 2%, and boiled for 5 min to stop the digestion. The products were centrifuged at 30,000 × g for 5 min, and the supernatants were pooled and used as the sample solution for SDS-PAGE [13]. Myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), bovine pancreas trypsinogen (24 kDa), and soybean trypsin inhibitor (20.1 kDa) were obtained from Sigma-Aldrich Co., (USA) and used as standards.
2.5. Column Chromatography of Collagen from Tendon of Yezo Sika Deer
Collagen sample (20 mg) was dissolved in 20 mM sodium acetate buffer (pH 4.8) containing 6 M urea at 4˚C for a day by continuous stirring. After denaturation at 45˚C for 30 min, the solution was centrifuged at 50,000 × g at room temperature for 1 h. The supernatants were pooled, pre-incubated at 37˚C, and then applied to a CM-Toyopearl 650 M column (1.0 × 6.0 cm) previously equilibrated with the same buffer. The subunit components were separated and eluted by a linear gradient of 0 - 0.15 M NaCl in the same buffer at a flow rate of 0.8 ml/min. These components were detected at 230 nm and the fractions indicated by the numbers were confirmed by SDS-PAGE using 7.5% gel.
2.6. Denaturation Temperature of Collagen from Tendon of Yezo Sika Deer
Using a Canon-Fenske type viscometer with an average shear gradient of 400 sec-1, denaturation temperature was measured by the method of Nagai [14]. The viscometer containing 5 ml of 0.03% collagen in 0.1 M acetic acid was immersed in a water bath at 20˚C - 50˚C. The viscosities of collagen samples were measured after incubation at each temperature for 30 min. The denaturation temperature was determined as the temperature causing a 50% decrease in viscosity. Each point is the mean of triplicate determinations.
2.7. Amino Acid Composition of Collagen from Tendon of Yezo Sika Deer
Collagen samples were hydrolyzed under reduced pressure in 6 M HCl at 110˚C for 24 h, and the hydrolysates were analyzed on a JASCO liquid-chromatography system by on-line precolumn derivatization with o-phthalaldehyde. This system consisted of a JASCO PU-2080 plus intelligent HPLC-pump, a JASCO FP-2020 plus intelligent fluorescence detector, a JASCO CO-2060 plus intelligent column thermostat, a JASCO DG-2083-53 3-line degasser, a JASCO LG-2080-02 ternary gradient unit, a JASCO AS-2057 plus intelligent sampler, and a JASCO CrestPak C18S (φ4.6 × 150 mm) reversed-phase column. The excitation and emission wavelengths were set at 345 and 455 nm, respectively. Eluents were filtered through Millipore membranefilters (pore size 0.45 μm).
2.8. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy of Collagen from Tendon of Yezo Sika Deer
ATR-FTIR spectra were collected at 20˚C and 40% relative humidity by coupling the ATR accessory (ATR PRO410-S: JASCO Co., Tokyo, Japan) to a JASCO (Tokyo, Japan) FT/IR-4100 type A instrument. The IR spectrometer bench was equipped with a globar source, a KBr bean splitter, and a triglycine sulfate detector. The ATR sampling device utilized a diamond internal reflection element embedded into a ZnSe support/focusing element in a single reflection configuration. Spectra were obtained over the range of 4000 - 650 cm–1 at 4 cm–1 resolution. The resultant spectra were analyzed using an IR Protein Secondary Structure Analysis Program (JASCO Co., Tokyo, Japan).
3. Results and Discussion
3.1. Preparation of Collagen from Tendon of Yezo Sika Deer
The tendon of Yezo sika deer was treated with ethanol and NaOH to remove fat and noncollagenous proteins. The yield of lyophilized matter was about 41.8% on a wet weight basis. Acid-soluble collagen was extracted from lyophilized matter using acetic acid, but was hardly solubilized (yield: about 0.08% on a dry weight basis of lyophilized matter). On the other hand, the resultant matter from acid-soluble collagen was used for the extraction of pepsin-solubilized collagen. As a result, the collagen was successfully solubilized and was effectively purified by differential salt precipitation. The yield was high about 35.7% on a dry weight basis of lyophilized matter. In our previous papers, it reported the preparation and characterization of collagens from aquatic organisms. The yields of these collagens were as follows: fish skin [acid-soluble collagen from Japanese sea bass, chub mackerel, and bullhead shark: 44.7% - 51.4% [8]; pepsinsolubilized collagen from ocellate puffer fish: 44.7% [5]; acid-soluble collagen (25.8%) and pepsin-solubilized collagen (38.4%) from channel catfish [15]; acid-soluble collagen (47.5%) and pepsin-solubilized collagen (92.2%) from deep-sea redfish [16]; pepsin-solubilized collagen from grass carp: 46.6% [17], fish bone (40.7-53.6%) from Japanese sea bass, horse mackerel and ayu [10], fish scale (pepsin-solubilized collagens from Japanese sea bass, red sea bream, and sardine: 37.5% - 50.9%) [7], octopus (pepsin-solubilized collagens from Callistoctopus arakawai arm: 62.9% [6], paper nautilius outer skin: 50.0% [11]), cuttlefish outer skin (pepsin-solubilized collagens from Sepia lycidas: 35.0% [4] and from diamondback squid: 35.6% [14]), test (pepsin-solubilized collagen from purple sea urchin: 35.0%) [9], and jellyfish (pepsin-solubilized collagens from edible jellyfish exumbrella: 46.4% [2] and from rhizostomous jellyfish mesogloea: 35.2% [3]), respectively. Collagen is the protein that is found in the highest concentration, about 30%, in the living body. In particular, it is well known that a large amount of collagen contains in skin and bones of organisms. From this investigation it suggests that collagen obtained from tendon of Yezo sika deer has potential use as an alternative to land animal collagen such as bovine and pig in foods, cosmetics, and biomedical materials as well as aquatic organisms collagen.
3.2. SDS-PAGE of Collagen from Tendon of Yezo Sika Deer
By SDS-PAGE using 7.5% gel, the pepsin-solubilized collagen from tendon of Yezo sika deer was investigated. As a result, two distinct α chains corresponding to α1 and α2 were detected for their different positions in mobility (Figure 1). On the other hand, the existence of α3 chain could not identified to be detected in the same position to α1 under this electrophoretic conditions employed. Small amounts of β- and γ- chains were detected in this collagen. These positions of collagen from tendon of Yezo sika deer were similar to those of porcine skin. From these results, this collagen may have a chain composition of (α1)2α2 heterotrimer similar to that of mammalian collagen such as porcine skin. Cliche et al. [18] examined the chicken skin collagen extracted by pepsin digestion and reported that this collagen consisted of two distinct α chains such as α1 and α2.
3.3. Peptide Mapping of Collagen from Tendon of Yezo Sika Deer
By SDS-PAGE using 10% gel, the denatured acid-soluble and pepsin-solubilized collagens from tendon of Yezo sika deer were investigated to direct compare the pattern of peptide fragments with porcine skin collagen. These collagens were not perfectly hydrolyzed by lysyl endopeptidase and the peptide fragments of collagen were detected in molecular weight ranging from 100 to 20 kDa (Figure 2). The electrophoretic patterns of acidsoluble
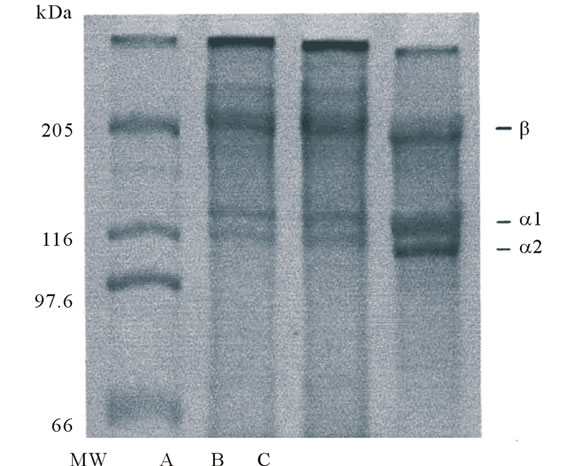
Figure 1. SDS-polyacrylamide gel electrophoresis of collagens from porcine skin and tendon of Yezo sika deer on 7.5% gels containing 3.5 M urea. (MW): high molecular marker: (A): acid-soluble collagen from tendon of Yezo sika deer; (B): pepsin-solubilized collagen from tendon of Yezo sika deer; (C): porcine skin collagen.
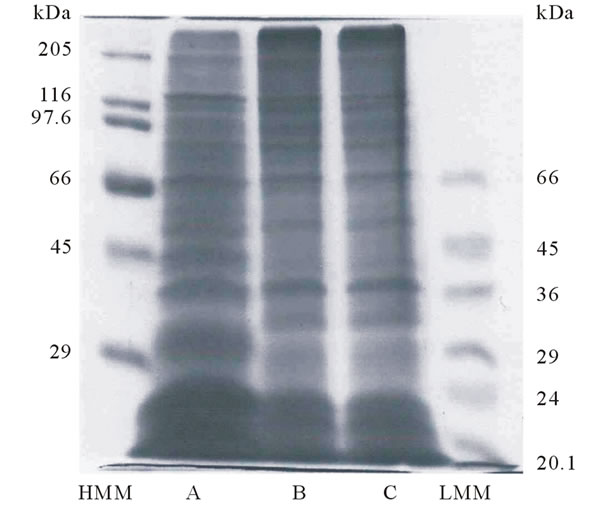
Figure 2. Peptide mapping of lysyl endopeptidase digests of collagens from porcine skin and tendon of Yezo sika deer. (HMM): high molecular marker; (A): porcine skin collagen; (B): acid-soluble collagen from tendon of Yezo sika deer; (C): pepsin-solubilized collagen from tendon of Yezo sika deer; (LMM): low molecular marker.
and pepsin-solubilized collagens were very similar, but these were slightly different from that of porcine skin collagen: the protein bands having molecular weights about 40, 33, 29 kDa were only shown in collagens from tendon of Yezo sika deer.
3.4. Subunit Composition of Collagen from Tendon of Yezo Sika Deer
To separate the subunit components of pepsin-solubilized collagen from tendon of Yezo sika deer, the denatured collagen was applied to a CM-Toyopearl 650M column. Although complete resolution of the peaks was not achieved, it assumed that the large peak having the shoulder on the right and left contained an α chain as a major component in this collagen (Figure 3).
To identify each of α chains, several chromatographic fractions, as indicated by the numbers, were analyzed by SDS-PAGE using 7.5% gel. The result suggest that this collagen consists of two α chains. An α1 chain was found
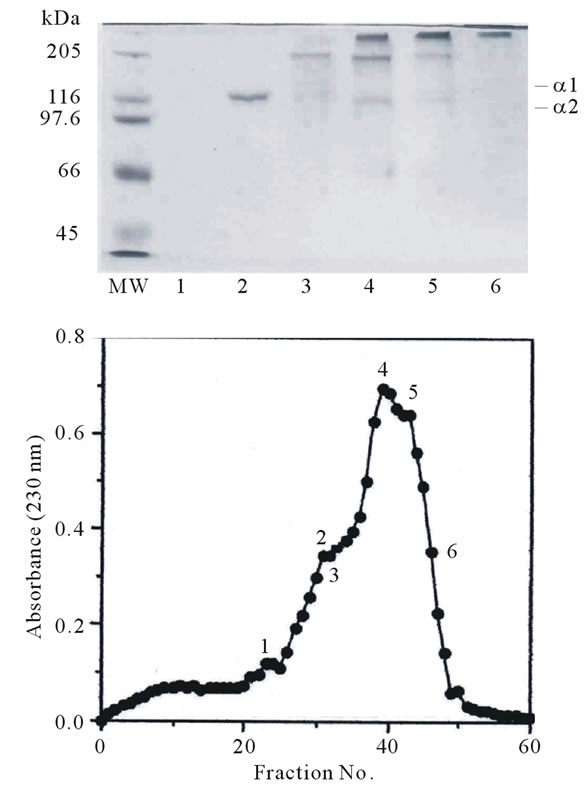
Figure 3. CM-Toyoperal 650 M column chromatography of denatured pepsin-solubilized collagen from tendon of Yezo sika deer. A 1.0 × 6.0 column of CM-Toyopearl 650 M was equilibrated with 20 mM sodium acetate buffer (pH 4.8) containing 6 M urea, and maintained at 37˚C. The collagen sample (20.0 mg) was dissolved in 5 ml of the same buffer, denatured at 45˚C for 30 min, and then eluted from the column with a linear gradient of 0 to 0.15 M NaCl at a flow rate of 0.8 ml/min. The fractions indicated by the numbers were examined by SDS-PAGE using 7.5% gel.
in fractions 30 to 43 as indicated by the number. A small amount of α2 chain was found in fractions 38 to 43. Judging from the electrophoretic pattern, it is suggested that pepsin-solubilized collagen from tendon of Yezo sika deer is a heterotrimer with a chain composition of (α1)2α2. The result was similar to that of collagen such as porcine, bovine, and chicken skin [18]. Nagai and coresearchers have been investigated the characteristics of collagen from aquatic organisms. They reported that the diversity existed in the subunit composition of collagen from these species: ocellate puffer fish skin [(α1)2α2] [5], fish bone [Japanese sea bass: (α1)2α2; horse mackerel: (α1)3; ayu: α1α2α3] [10], fish scale from sardine, red sea bream, and Japanese sea bass [(α1)2α2] [7], caudul fin from Japanese sea bass [(α1)3] [19], paper nautilus outer skin [11] and Callistoctopus arakawai arm α1α2α3 [6], cuttlefish [(α1)2α2] [4], diamondback squid (α1α2α3) [1], purple sea urchin test [(α1)2α2] [9], edible jellyfish exumbrella (α1α2α3) [2], and rhizostomous jellyfish mesogloea (α1α2α3α4) [3], respectively.
3.5. Denaturation Temperature of Collagen from Tendon of Yezo Sika Deer
The heat transformation of collagen is interpreted as disintegration of triple helical structure of collagen into random coils. Among some measurements, viscosity measurement is most useful for learning about the loss of viscosity with heating, attributed to denaturation of collagen. The fractional changes with increasing temperature in collagen samples were shown in Figure 4. The denaturation temperature of pepsin-solubilized collagen
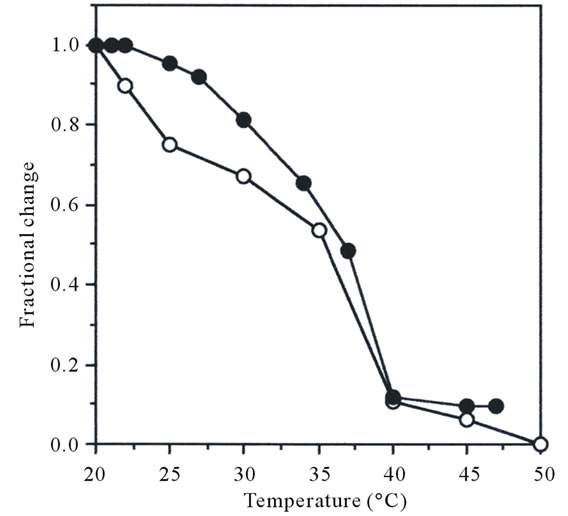
Figure 4. Thermal denaturation curve of pepsin-solubilized collagen from tendon of Yezo sika deer. The denaturation temperature was measured by viscosity in 0.1 M acetic acid. The incubation time at each temperature was 30 min. Collagen concentration 0.03%; (●): porcine skin collagen; (о): pepsin-solubilized collagen from tendon of Yezo sika deer.
from tendon of Yezo sika deer was calculated from the thermal denaturation curve. For comparison, denaturation temperature of porcine skin collagen was similarly measured. It was calculated that denaturation temperature of pepsin-solubilized collagen from tendon of Yezo sika deer was about 35.4˚C (Figure 4). This value was just a little lower than that of porcine skin collagen (37.0˚C). In general, the denaturation temperature of collagen from land animals was higher that those of aquatic organisms: this is correlated with their environmental and body temperatures [20]. Nagai and co-researchers has been investigated the denaturation temperatures of collagens from aquatic organisms. These values were reported as follows: fish skin (28.0˚C) [5], fish scale (28.0˚C - 28.5˚C) [7], caudul fin (28.0˚C) [19], octopus outer skin and arm (27.0˚C - 28.0˚C) [6,11], cuttlefish outer skin (27.0˚C - 27.5˚C) [4,14], purple sea urchin test (28.0˚C) [9], exumbrella and mesogloea from jellyfishes (26.0˚C - 28.8˚C) [2,3], respectively.
3.6. Amino Acid Composition of Collagen from Tendon of Yezo Sika Deer
The amino acid composition, expressed as residues per 1000 total residues, is shown in Table 1. As a result,
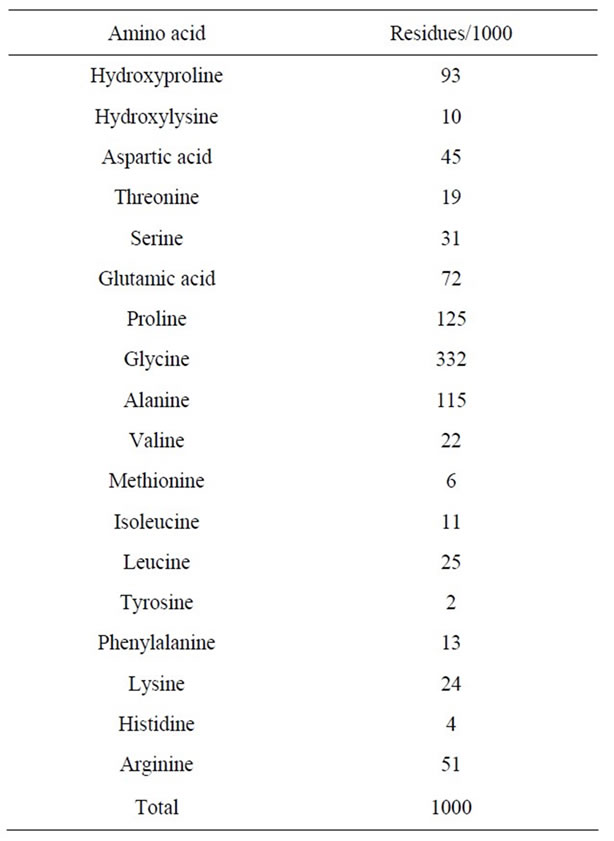
Table 1. Amino acid composition of pepsin-solubilized collagen from tendon of Yezo sika deer.
glycine having 332 residues was the most abundant amino acid in collagen from tendon of Yezo sika deer, and followed by proline (125 residues), alanine (115 residues), hydroxyproline (93 residues), and glutamic acid (72 residues) in the order. On the other hand, tyrosine, histidine, methionine, hydroxylysine, isoleucine, and phenylalanine were very low about 2 - 13 residues. It was not detected tryptophan and cysteine at al in this collagen. The characteristic amino acid in collagen, imino acid, were also detected and were calculated to 218 residues (proline and hydroxyproline): the total contents of imino acid in collagen of aquatic organisms as follows: skin from ocellate puffer (17.0%) [5], scale from sardine, red sea bream, and Japanese sea bass (19.3% - 19.7%) [7], caudul fin from Japanese sea bass (19.3%) [19], arm from Callistoctopus arakawai (17.8%) [6], outer skin from cuttlefish (18.6% - 18.8%) [4,14], test from purple sea urchin (17.9%) [9], exumbrella from edible jellyfish (12.1%) [2], respectively. Moreover, other researchers also reported total contents of imino acid in collagen from fish skin: bigeye snapper (19.3%) [21], channel catfish (17.0%) [15], deep-sea redfish (16.0%) [16], grass carp (18.6%) [17], walleye pollack (18.4%) [22], respectively. That is, total contents of imino acid in pepsin-solubilized collagen from tendon of Yezo sika deer were much higher than those from fishes and shellfishes. The higher imino acid content, the more stable are the helices of collagen. The result of the experiment gave good agreement with the tendency that had been obtained by the measurement of denaturation temperature.
Next, it was calculated the degree of hydroxylation of proline residues in collagen samples. As a result, the degree in pepsin-solubilized collagen from tendon of Yezo sika deer was about 42.7%. On the other hand, the degree of hydroxylation of proline residues in collagen samples from aquatic organisms was reported as follows: skin from ocellate puffer (39.4%) [5], scales from sardine, red sea bream, and Japanese sea bass (43.7% - 44.4%) [7], caudual fin from Japanese sea bass (42.5%) [19], arm from Callistoctopus arakawai (51.7%) [6], outer skin from cuttlefish (47.8% - 47.9%) [4,14], test from purple sea urchin (48.0%) [9], and exumbrella from edible jellyfish (32.8%) [2], respectively. Moreover, it was reported the degree of hydroxylation of proline residues in collagen samples from skins of other fish species: bigeye snapper (39.9%) [21], channel catfish (42.9%) [15], deep-sea redfish (38.1%) [16], grass carp (34.9%) [17], and walleye Pollack (37.5%) [22], respectively. It suggests that the reason for the lower denaturation temperature in collagen samples is that was the lower imino acid content rather than the extent of hydroxylation of imino acid, although it is known that the degree of hydroxylation of proline influences the thermal stability of collagen: a higher degree of hydroxylation is associated with higher denaturation temperature of collagen.
3.7. ATR-FTIR Spectroscopy Analysis of Collagen from Yezo Sika Deer
Figure 5 represents the ATR-FTIR spectrum of pepsin-solubilized collagen from tendon of Yezo sika deer.
The result was similar spectrum pattern to those of collagens from other species [15,16,22,23]. The amide I band position was found at 1640 cm–1. In general, the amide I band with characteristic frequencies in the range of 1600 to 1700 cm–1 was mainly associated with the stretching vibrations of the carbonyl group along the polypeptide backbone [24], and was sensitive marker of the peptide secondary structure [25]. The amide II and III bands were observed at 1541 and 1235 cm–1: it showed N-H bending vibrations and C-H stretching, respectively [24]. It is shown that the amide A band was associated with N-H stretching vibration and hydrogen bands presented in collagen. The amide A band position was found at 3306 cm–1. Doyle and others reported that a free N-H stretching vibration occurs in the range 3400 to 3440 cm–1, and when the NH group of a peptide is involved in hydrogen bond, the position is shifted to lower frequencies, usually 3300 cm–1 [26]. That is, more NH groups of collagen from tendon of Yezo sika deer were involved in hydrogen bond. The amide B band was found at 2964 cm–1, which is related to asymmetrical stretch of CH2 [23]. From these results it suggests that the existence of herical arrangements of pepsin-solubilized collagen from tendon of Yezo sika deer.
Sarver and Krueger reported that the protein secondary structure was investigated using FTIR and the resultant spectra were analyzed as a data base [27]. As an application obtained from the literature, an IR protein secondary structure analysis program was developed by JASCO Co., Tokyo, Japan. As a result of analysis of the resultant spectra using this technique, the percentage of secondary
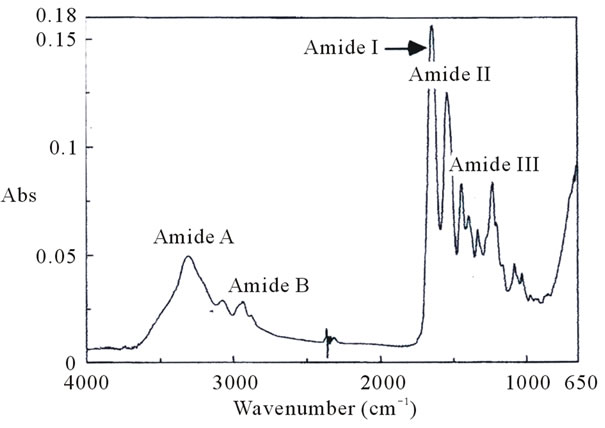
Figure 5. Fourier transform infrared spectra of pepsin-solubilized collagen from tendon of Yezo sika deer.
structural components such as α-helix, β-sheet, β-turn, and others (random coil structure) of pepsin-solubilized collagen from tendon of Yezo sika deer were calculated in the order: α-helix, 15%; β-sheet, 33%; β-turn, 22%; and others, 24%, respectively. The result was apparently different from those of porcine skin (α-helix, 9%; β-sheet, 51%; β-turn, 13%; and others, 15%, respectively). That is, it suggests that the triple helical structure well held in pepsin-solubilized collagen from tendon of Yezo sika deer different from porcine skin collagen.
4. Conclusions
In this study, collagen was extracted and characterized from tendon of Yezo sika deer. The secondary structure of this collagen was different from those of porcine skin by ATR-FTIR analysis, although it was the same characteristics, e.g. SDS-PAGE, subunit composition, thermal behavior, as porcine collagen. Since taking up a problem of BSE infection in land animals such as calf or bovine, collagen from aquatic materials has been used in various industries. However, the present study indicates that tendon from Yezo sika deer will have potential to be not associated with infection such as BSE, TSE, and FMD as an important collagen source for use in the foods, cosmetics, and medical fields.
REFERENCES
- A. Jongjareonrak, S. Benjakul, W. Visessanguan, T. Nagai and M. Tanaka, “Isolation and Characterisation of Acid and Pepsin-Solubilised Collagens from the Skin of Brownstripe Red Snapper (Lutjanus vitta),” Food Chemistry, Vol. 93, No. 3, 2005, pp. 475-484. doi:10.1016/j.foodchem.2004.10.026
- T. Nagai, T. Ogawa, T. Nakamura, T. Ito, H. Nakagawa, K. Fujiki, M. Nakao and T. Yano, “Collagen of Edible Jellyfish Exumbrella,” Journal of the Science of Food Agriculture, Vol. 79, No. 6, 1999, pp. 855-858. doi:10.1002/(SICI)1097-0010(19990501)79:6<855::AID-JSFA299>3.0.CO;2-N
- T. Nagai, W. Worawattabamateekul, N. Suzuki, T. Nakamura, T. Ito, K. Fujiki, M. Nakao and T. Yano, “Isolation and Characterization of Collagen from Rhizostomous Jellyfish (Rhopilema asamushi),” Food Chemistry, Vol. 70, No. 2, 2000, pp. 205-208. doi:10.1016/S0308-8146(00)00081-9
- T. Nagai, E. Yamashita, K. Taniguchi, N. Kanamori and N. Suzuki, “Isolation and Characterisation of Collagen from the Outer Skin Waste Material of Cuttlefish (Sepia lycidas),” Food Chemistry, Vol. 72, No. 4, 2001, pp. 425- 429. doi:10.1016/S0308-8146(00)00249-1
- T. Nagai, Y. Araki and N. Suzuki, “Collagen of the Skin of Ocellate Puffer Fish (Takifugu rubripes),” Food Chemistry, Vol. 78, No. 2, 2002, pp. 173-177. doi:10.1016/S0308-8146(01)00396-X
- T. Nagai, K. Nagamori, E. Yamashita and N. Suzuki, “Collagen of Octopus Callistoctopus arakawai Arm,” International Journal of Food Science and Technology, Vol. 37, No. 3, 2002, pp. 285-289.
- T. Nagai, M. Izumi and M. Ishii, “Fish Scale Collagen. Preparation and Partial Characterization,” International Journal of Food Science and Technology, Vol. 39, No. 3, 2004, pp. 239-244. doi:10.1111/j.1365-2621.2004.00777.x
- T. Nagai and N. Suzuki, “Isolation of Collagen from Fish Waste Material-Skin, Bone and Fins,” Food Chemistry, Vol. 68, No. 3, 2000, pp. 277-281. doi:10.1016/S0308-8146(99)00188-0
- T. Nagai and N. Suzuki, “Partial Characterization of Collagen from Purple Sea Urchin (Anthocidaris crassispina) Test,” International Journal of Food Science and Technology, Vol. 35, No. 5, 2000, pp. 497-501. doi:10.1046/j.1365-2621.2000.00406.x
- T. Nagai and N. Suzuki, “Preparation and Characterization of Several Fish Bone Collagens,” Journal of Food Biochemistry, Vol. 24, No. 5, 2000, pp. 427-436. doi:10.1111/j.1745-4514.2000.tb00711.x
- T. Nagai and N. Suzuki, “Preparation and Partial Characterization of Collagen from Paper Nautilus (Argonauta argo, Linnaeus) Outer Skin,” Food Chemistry, Vol. 76, No. 2, 2002, pp. 149-153. doi:10.1016/S0308-8146(01)00255-2
- T. Nagai, N. Suzuki and T. Nagashima, “Collagen from Common Minke Whale (Balaenoptera acutorostrata) Unesu,” Food Cshemistry, Vol. 111, No. 2, 2008, pp. 296- 301. doi:10.1016/j.foodchem.2008.03.087
- U. K. Laemmli, “Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4,” Nature, Vol. 227, 1970, pp. 680-685. doi:10.1038/227680a0
- T. Nagai, “Collagen from Diamondback Squid (Thysanoteuthis rhombus) Outer Skin,” Zeitschrift für Naturforschung, Vol. 59c, 2004, pp. 271-275.
- H. Y. Liu, D. Li and S. D. Guo, “Studies on Collagen from the Skin of Channel Catfish (Ictalurus punctaus),” Food Chemistry, Vol. 101, No. 2, 2007, pp. 621-625. doi:10.1016/j.foodchem.2006.01.059
- L. Wang, X. An, Z. Xin, L. Zhao and Q. Hu, “Isolation and Characterization of Collagen from the Skin of DeepSea Redfish (Sebastes mentella),” Journal of Food Science, Vol. 72, No. 8, 2007, pp. E450-455. doi:10.1111/j.1750-3841.2007.00478.x
- Y. Zhang, W. Liu, G. Li, B. Shi, Y. Miao and X. Wu, “Isolation and Partial Characterization of Pepsin-Soluble Collagen from the Skin of Grass Carp (Ctenopharyngodon idella),” Food Chemistry, Vol. 103, No. 3, 2007, pp. 906-912. doi:10.1016/j.foodchem.2006.09.053
- S. Cliche, J. Amiot, C. Avezard and C. Gariépy, “Extraction and Characterization of Collagen with or without Telopeptides from Chicken Skin,” Poultry Science, Vol. 82, No. 3, 2003, pp. 503-509.
- T. Nagai, “Characterization of Collagen from Japanese Sea Bass Caudal Fin as Waste Material,” European Food Research and Technology, Vol. 218, No. 5, 2004, pp. 424- 427. doi:10.1007/s00217-004-0892-7
- B. J. Rigby, “Amino-Acid Composition and Thermal Stability of the Skin Collagen of the Antarctic Ice-fish,” Nature, Vol. 219, 1968, pp. 166-167. doi:10.1038/219166a0
- P. Kittiphattanabawon, S. Benjakul, W. Visessanguan, T. Nagai and M. Tanaka, “Characterisation of Acid-Soluble Collagen from Skin and Bone of Bigeye Snapper (Priacanthus tayenus),” Food Chemistry, Vol. 89, No. 3, 2005, pp. 363-372. doi:10.1016/j.foodchem.2004.02.042
- M. Yan, B. Li, X. Zhao, G. Ren, Y. Zhuang, H. Hou, X. Zhang, Li. Chen and Y. Fan, “Characterization of AcidSoluble Collagen from the Skin of Walleye Pollack (Theragra chalcogramma),” Food Chemistry, Vol. 107, No. 4, 2008, pp. 1581-1586. doi:10.1016/j.foodchem.2007.10.027
- J. H. Muyonga, C. G. B. Cole and K. G. Duodu, “Characterisation of Acid Soluble Collagen from Skins of Young and Adult Nile Perch (Lates niloticus),” Food Chemistry, Vol. 85, No. 1, 2004, pp. 81-89. doi:10.1016/j.foodchem.2003.06.006
- K. J. Payne and A. Veis, “Fourier Transform IR Spectroscopy of Collagen and Gelatin Solutions: Deconvolution of the Amide I Band for Conformational Studies,” Biopolymers, Vol. 27, No. 11, 1988, pp. 1749-1760. doi:10.1002/bip.360271105
- W. K. Surewicz and H. H. Mantsch, “New Insight into Protein Secondary Structure from Resolution Enhanced Infrared Spectra,” Biochimica Biophysica Acta, Vol. 952, 1988, pp. 115-130. doi:10.1016/0167-4838(88)90107-0
- B. B. Doyle, E. G. Bendit and E. R. Blout, “Infrared Spectroscopy of Collagen and Collagen-like Polypeptides,” Biopolymers, Vol. 14, No. 5, 1975, pp. 937-957. doi:10.1002/bip.1975.360140505
- R. W. Sarver Jr. and W. C. Krueger, “Protein Secondary Structure from Fourier Transform Infrared Spectroscopy: A Data Base Analysis,” Analytical Biochemistry, Vol.194, No. 1, 1991, pp. 39-100.
NOTES
*Corresponding author.

