American Journal of Plant Sciences
Vol.4 No.8(2013), Article ID:35465,12 pages DOI:10.4236/ajps.2013.48197
Biomass Accumulation and Nutrient Uptake of Jerusalem Artichoke (Helianthus tuberosus L.)*
![]()
Institute of Environmental Sciences, Faculty of Economics, Agricultural and Health Studies, Szent István University, Szarvas, Hungary.
Email: izsaki.zoltan@gk.szie.hu
Copyright © 2013 Zoltán Izsáki, Gabriella Németh Kádi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 27th, 2013; revised June 30th, 2013; accepted July 15th, 2013
Keywords: Jerusalem Artichoke; Mineral Fertilisation; Biomass Accumulation; Yield; Nutrient Uptake
ABSTRACT
The dynamics of biomass accumulation during the growing period, the yield of leafy stalks and tubers, and the nutrient concentration and nutrient uptake of the yield were investigated for two Jerusalem artichoke varieties (Tápiói Korai and Tápiói Sima) in a field experiment involving mineral fertilisation. Considerable differences were observed between the dynamics of leafy stalk and tuber development in Tápiói Korai which has a short vegetation period and Tápiói Sima where the vegetation period is long. The maximum dry matter ratio between the tuber yield and the leafy stalk yield was 1:1 for Tápiói Korai and 1:4.5 for Tápiói Sima. During the period when the maximum aboveground biomass developed in Tápiói Korai, 100 kg·ha−1 N and P fertiliser resulted in the highest leafy stalk yield (38.34 t·ha−1), while for Tápiói Sima, which developed a much greater leafy stalk mass, the highest aboveground biomass yield (78 - 80 t·ha−1) was given in response to 200 kg·ha−1 N supplemented by P and K fertiliser. Both artichoke varieties produced the greatest tuber yield at a N rate of 200 kg·ha−1, supplemented with P and K fertiliser. The nutrient concentration in the leafy stalks was highest on the 85th day of the vegetation period, prior to intensive dry matter accumulation in the leafy stalks and before tuber formation began. In both varieties the maximum nutrient uptake was recorded on the 155th day. Great differences were observed between the varieties in terms of specific nutrient uptake. For a tuber yield of 10 t, together with the corresponding leafy stalk yield, the specific nutrient uptake of the Tápiói Korai variety amounted to 48 kg N, 10 kg P, 83 kg K, 30 kg Ca and 10 kg Mg, while for Tápiói Sima these figures were 162 kg N, 30 kg P, 300 kg K, 84 kg Ca and 45 kg Mg.
1. Introduction
On a world scale, Jerusalem artichoke (Helianthus tuberosus L.) is a crop of minor importance. Between 2006 and 2010 the growing area averaged 130,000 hectares worldwide, of which 78,000 ha was located in Europe. The major producers in Europe are Italy (50,000 ha), Spain (16,000 ha) and France (9000 ha). Averaged over the last five years, the tuber yield amounted to around 11 t/ha on a world scale and 10 t·ha−1 in Europe (FAOSTAT). Due to its multiple uses and excellent economic value, the Jerusalem artichoke deserves a higher ranking among the world’s crops.
The species has great yield potential, producing 10 - 20 t·ha−1 under poor conditions, 25 - 40 t·ha−1 on moderately fertile areas and 45 - 80 t·ha−1 under intensive conditions. Depending on the end-use and site conditionsthe summer leafy stalk yield ranges from 15 - 50 t·ha−1 and the autumn leafy stalk yield from 20 - 50 t·ha−1. The young leafy stalk yield can be used as fresh fodder or for silaging. Thanks to its good regeneration ability, it can be cut 3 or 4 times when the water supplies are favourable. The autumn leafy stalk yield has poorer fodder value, but can be used as litter, as raw material for the paper Industry or for biogas production, and as fuel. The tubers have a carbohydrate content of 10% - 22%, consisting of 80% - 90% inulin, 7% - 14% sucrose and 3% - 6% reducing sugars. In addition to the carbohydrates they contain 1.2% - 2.0% protein, 0.2% fat and 2.0% - 2.5% fibre. The tubers can be used for human consumption, livestock feeding and bioethanol production, and they can be processed into inulin-rich diabetic sweeteners [1-5].
The development of the above and belowground organs of Jerusalem artichokes, the dynamics of biomass accumulation, the yield of tubers and leafy stalks and their chemical composition are all fundamentally influenced by ecological conditions, variety traits and the technology. Of the many factors involved, the most decisive are the water supplies, the yield potential and vegetation period of the variety, and the nutrient supplies.
In Thailand the effect of the location × genotype interaction on the tuber yield was examined for 15 Jerusalem artichoke clones with diverse genotypes, grown at 9 locations [6]. The tuber yield ranged from 3.0 - 17.2 t·ha−1 under the poorest conditions and from 26.1 - 38.9 t·ha−1 at the best location. Averaged over four years, differences in tuber yield of 15 - 28 t·ha−1 were caused for the same genotypes by differences in the site conditions (water supplies, soil type).
The tubers begin to develop in July and August and growth continues until the foliage withers. The main period of tuber development for varieties with a short vegetation period is in August, while development continues into September and October for those with a longer growing period. Jerusalem artichoke is a relatively drought-tolerant crop, but the size of the tuber yield is determined to a major extent by the water supplies during the tuber formation period. Varieties with a longer vegetation period have greater water requirements and yields. However, the higher yield potential is only manifested compared with earlier maturing varieties if the tuber development period has good water supplies and if harvesting is carried out as late as possible [1,3,7-9].
Jerusalem artichokes are able to accumulate great biomass, for which a large amount of nutrients are required. The crop has good nutrient use efficiency, however, as also shown by Raso [10], who recorded the highest tuber yield (34 t·ha−1) on fertile sandy soil with 50 kg·ha−1 N, while higher N rates (100, 150, 200 kg·ha−1) led to a slight decline in yield. K fertilisation (100, 200 kg·ha−1) had no influence on the yield, and no N × K interaction was observed.
Among the macroelements, the N supplies were most decisive for the size and quality of the tuber yield and for the quantity of aboveground biomass. Excessive N supplies caused a considerable increase in the leafy stalk mass, with a reduction in tuber yield and harvest index. Tuber formation was negatively influenced by P and K deficiencies [11-15].
Little attention has been given in fertilisation experiments to an analysis of the nutrient uptake and nutrient requirements of the crop. Angeli [1] and Izsáki [3] found the specific nutrient requirements of a 10-t tuber yield plus the corresponding leafy stalk yield amounted to 32 - 46 kg N, 13 - 23 kg P and 75 - 100 kg K. There may, however, be considerable deviations in the specific nutrient uptake of Jerusalem artichoke, as the varieties and clones differ in terms of leafy stalk yield, tuber yield potential and the nutrient concentrations of the plant organs even under the same growing conditions. Seiler and Campbell [16,17] examined the nutrient content of the leafy stalk yield of nine wild-growing Jerusalem artichoke populations at flowering and found significant genetic variability in the N, P, K, Ca and Mg concentrations of the leafy stalks of these wild populations. Similar results were reported for the nutrient concentration of the leafy stalks of ten varieties, with values ranging from 0.80% - 1.78% N, 0.10% - 0.25% P, 0.78% - 2.97% K, 1.08% - 3.23% Ca and 0.17% - 0.26% Mg. Genetic variability could also be detected in the nutrient concentrations of the tubers, both for wild populations and for varieties. This great genetic variability in the nutrient contents of the plant organs was found to be suitable for classifying the origin of Jerusalem artichoke varieties and clones [18,19]. In recent years, major sources [19-24] have given the following ranges for the tuber nutrient content in terms of dry matter: 1.00% - 1.70% N, 0.15% - 0.48% P, 0.92% - 2.62% K, 0.13% - 1.48% Ca, 0.06% - 0.24% Mg, 0.02% - 0.04% Na, 16 - 150 mg·kg−1 Fe, 40 - 100 mg·kg−1 Mn, 11 - 35 mg·kg−1 Zn, 2.0 - 9.0 mg·kg−1 Cu and 9 - 13 mg·kg−1 B. The accumulation of nutrients and their concentration and distribution in the various plant organs (stalk, leaves, stolon, tuber) may change substantially during the vegetation period. The nutrient content of the leafy stalk declines to a considerable extent during tuber formation [25-27]. Notable differences in yield potential and in the leafy stalk/tuber ratio were recorded between the varieties.
In the light of the above observation, the nutrient requirements of Jerusalem artichoke cannot be determined independently of the variety or variety group, and cannot be expressed in terms of generally applicable specific nutrient uptake values. The aim of the paper was to determine the dynamics of biomass accumulation in the plant organs, changes in nutrient concentration during the growing period, and the nutrient uptake of two Jerusalem artichoke varieties grown in a fertilisation experiment.
2. Materials and Method
2.1. Experimental Site, Treatments, Crop Management
A mineral fertilisation experiment on Jerusalem artichoke was set up in Szarvas, Hungary (46˚86'N, 20˚55'E) in 2002 on a chernozem meadow soil, calcareous in the deeper layers, with the following major parameters: depth of the humus-containing layer 85 - 100 cm, pH (KCl) of the ploughed layer 5.8, humus content 2.65%, no CaCO3 content, upper limit of plasticity (KA) 46, texture: clay loam. The mean depth of the groundwater was 300 - 350 cm.
According to soil analysis carried out in autumn 2001, before the experiment was set up, the nutrient content of the ploughed layer was as follows (mg·kg−1): AL-P2O5: 121, AL-K2O: 278, AL-Na: 119, KCl-Mg: 955, EDTAMn: 250, EDTA-Cu: 6.8 and EDTA-Zn: 2.6. Based on the methods and limit values recommended by the Plant Protection and Agrochemistry Centre [28,29], the soil has moderate P supplies, good K, Cu and Zn supplies and a high level of Na, Mg and Mn supplies.
The experiment was set up in a split-plot design in four replications. Factor “A” was the two Jerusalem artichoke varieties: Tápiói Korai (short growing period) and Tápiói Sima (long growing period), while factor “B” was the 13 fertiliser rates listed in Table 1. The high rates of P and K fertiliser were applied partly to ensure the phosphorus and potassium required for three growing periods, and partly to create distinct supply levels in the soil. Nitrogen was applied in spring in the form of ammonium nitrate (34% N), while phosphorus and potassium were applied in autumn in the form of super-phosphate (18% P2O5) and potassium chloride (40% K2O). The previous crop was spring barley. The crop was sown with a row distance of 75 cm and a plant distance of 60 cm, with 72 plants per plot (plant density 22,222 plants/ha) on 20 March 2002. The subplot size was 32.4 m2 (4.5 × 7.2 m).
The weather data for the location are presented in Table 2.
2.2. Measurement, Plant Analysis
In order to study the dynamics of biomass accumulation,
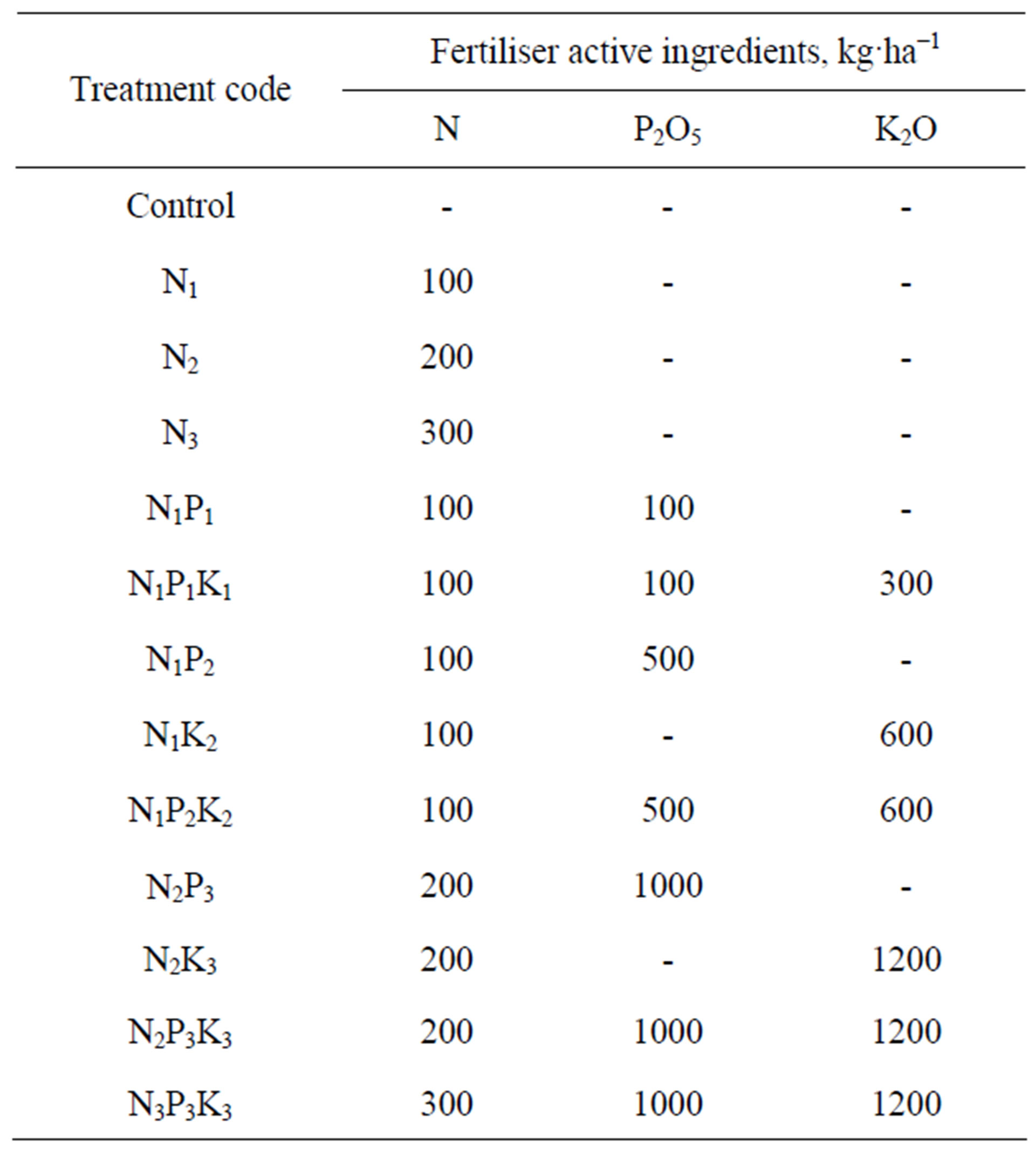
Table 1. Fertiliser treatments applied in the experiment.
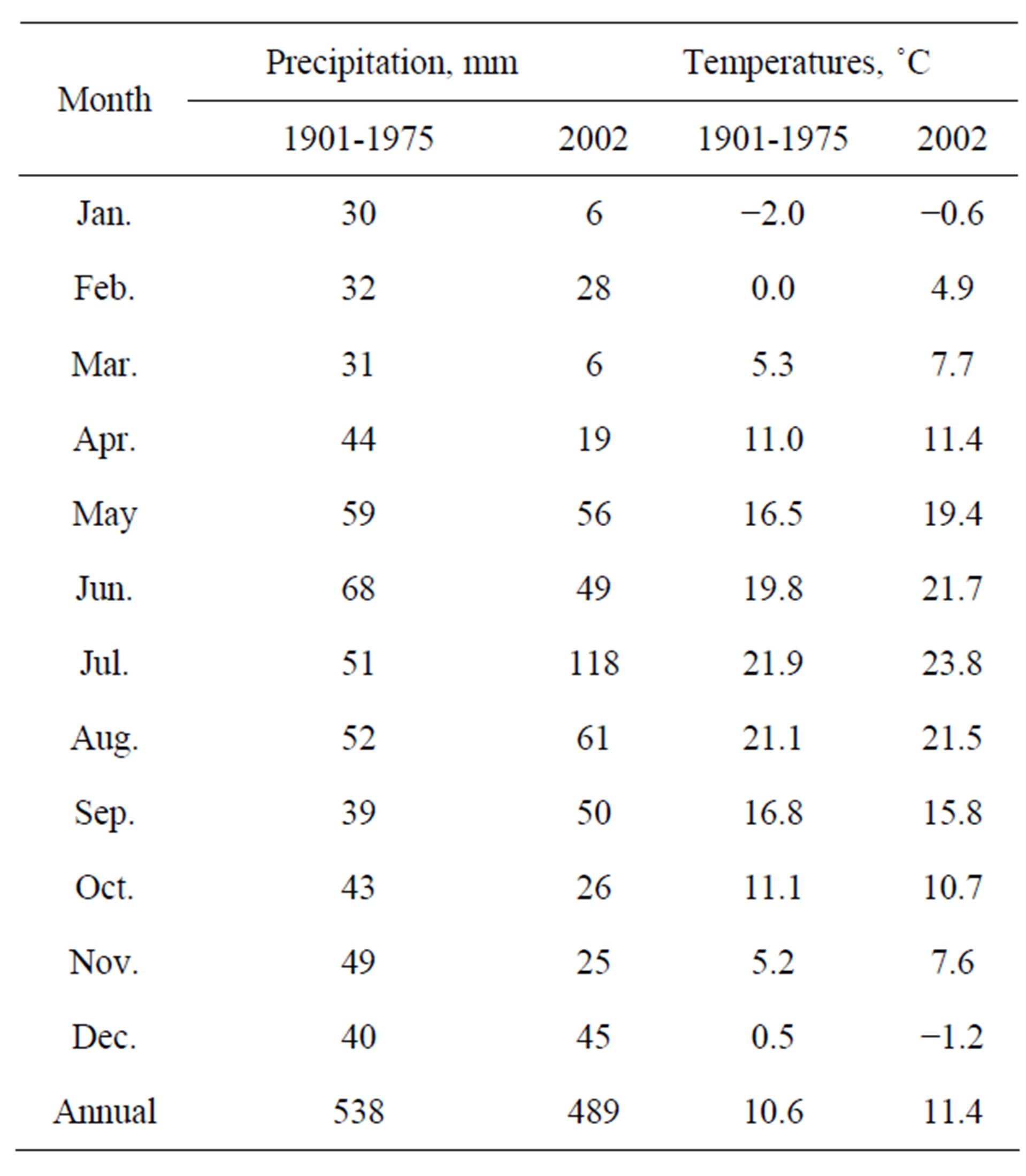
Table 2. Weather data for the experimental location (Szarvas, 1901-1975; 2002).
leafy stalk samples were taken for mass measurements on four (Tápiói Korai) or five (Tápiói Sima) occasions. The tuber mass was recorded twice for Tápiói Korai and three times for Tápiói Sima during tuber development. The sampling dates were on the 85th (12 Jun), 115th (12 Jul), 155th (21 Aug), 195th (30 Sep) and 225th (30 Oct) days after planting. On each sampling date six plants were harvested (2.7 m2), and on the last harvesting date the leafy stalk yield and tuber yield were recorded for the whole plot (24.3 and 21.6 m2). For the analysis of leafy stalks, each sample consisted of one main shoot and the corresponding side-shoots for each plant. In the case of the tubers, five tubers were chosen from each plant. These were cleaned of soil, washed, pulpified and frozen until required for analysis.
The leafy stalk and tuber samples were analysed for the following nutrients: N, P, K, Na, Ca, Mg, Fe, Mn, Zn and Cu. For the determination of N, P, K and Na the samples were digested first with sulphuric acid and then with hydrogen peroxide, after which N and P were measured photometrically and Na and K using a flame photometer. The Ca, Mg, Fe, Mn, Zn and Cu contents were determined with an atomic absorption spectrometer (AAS) after hydrolysis with 2 mol·dm–3 KCl. The nutriaent concentrations are given in terms of dry matter, averaged over the fertilisation treatments.
2.3. Statistical Analysis
The data of the leafy stalk and the tuber yield were statistically processed using single-factor analysis of variance for each variety, according to the method given by Sváb [30]. The least significant differences (LSD) were given at the P = 0.05 level.
3. Results and Discussion
3.1. Leafy Stalk Yield
The accumulation of fresh and dry matter in the leafy stalks of the Tápiói Korai variety is presented in Tables 3 and 4.
Averaged over the fertiliser treatments, 60% of the total fresh leafy stalk mass was accumulated by the 85th day of the growing season, and 84% of the total aboveground biomass by the 115th day. The maximum fresh leafy stalk mass was recorded on the 155th day, so 40% of the total aboveground fresh mass was formed in the course of 70 days, between the 85th and 155th days of the vegetation period. The leafy stalks of this early maturing variety gradually withered from the last ten days of August, the leaves dropped, and only 22% of the aboveground biomass could be harvested on the 195th day of the season (Table 3).
Averaged over the fertilisation treatments, the dynamoics of dry matter accumulation in the leafy stalks

Table 3. Fresh leafy stalk yield of the Tápiói Korai Jerusalem artichoke variety, t·ha−1 (Szarvas, 12 Jun-30 Sep 2002).

Table 4. Dry matter yield of the leafy stalks of the Tápiói Korai Jerusalem artichoke variety, t·ha−1 (Szarvas, 12 Jun- 30 Sep 2002).
differed from that of the fresh mass, due to the fact that the dry matter content of the leafy stalks gradually increased throughout the growing period. On the 85th day the dry matter content of the leafy stalks was 8%, while this increased to 18%, 33% and 38%, respectively by the 115th, 155th and 195th days; 15% of the total aboveground dry matter had formed by the 85th day of the vegetation period, and 46% by the 115th day. The most dynamic dry matter incorporation was detected between the 115th and 155th days, when the maximum value was recorded. So 54% of the aboveground dry matter was formed over the course of 40 days. Only 26% of the maximum leafy stalk dry matter could be harvested on the 195th day of the growing season (Table 4).
The maximum values of fresh and dry mass were achieved in the leafy stalks of the Tápiói Korai variety on the 155th day of the growing period, when the fresh mass was around 33 t·ha−1 and the dry matter around 11 t·ha−1, averaged over the fertiliser treatments. When the effect of fertilisation was examined over the maximum biomass formation period, it was found that without fertilisation a fresh mass of 24.07 t·ha−1 was achieved, which was significantly increased by N fertiliser alone. The application of the 300 kg·ha−1 rate of N fertiliser (N3) resulted in a fresh mass of 37.56 t·ha−1, and none of the fertiliser treatments caused a significant increase compared with this yield. The 100 kg·ha−1 rate of N fertiliser combined with 100 kg·ha−1 P fertiliser (N1P1) gave a very similar yield (38.34 t·ha−1) to that achieved with N3. As the soil had good K supplies, the addition of 100 kg·ha−1 K fertileiser (N1P1K1) had no significant influence on the fresh leafy stalk mass compared with N1P1. High rates of P and K fertiliser (P2, K2) combined with 100 kg·ha−1 N led to a significant reduction in the fresh mass compared with N1P1. However, this negative effect of replenishment rates of P and K was not observed at the 200 and 300 kg·ha−1 N supply levels. In the early stages of the growing period the fertiliser effects were similar to those observed during the period of maximum fresh mass accumulation. When maximum dry matter accumulation was observed in the leafy stalks, the dry matter mass of the aboveground plant organs was 7.97 t·ha−1 without fertileiser. The effect of the various fertiliser levels on the dry matter content of the leafy stalks was very similar to that described for the fresh mass (Tables 3 and 4).
The accumulation of fresh and dry matter in the leafy stalks of the Tápiói Sima variety is presented in Tables 5 and 6.

Table 5. Fresh leafy stalk yield of the Tápiói Sima Jerusalem artichoke variety, t·ha−1 (Szarvas, 12 Jun-30 Oct 2002).
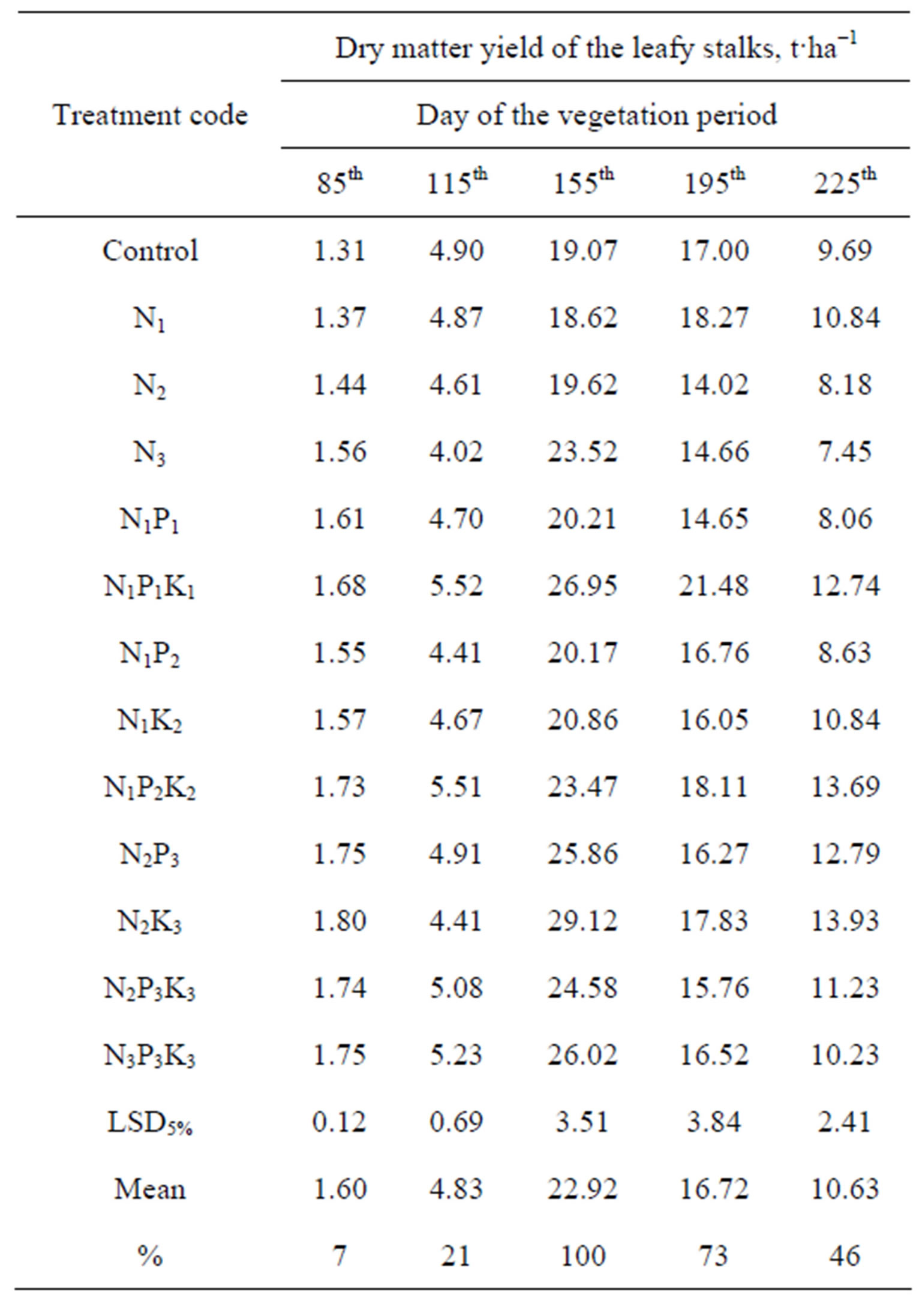
Table 6. Dry matter yield of the leafy stalks of the Tápiói Sima Jerusalem artichoke variety, t·ha−1 (Szarvas, 12 Jun- 30 Oct 2002).
The Tápiói Sima variety has a longer vegetation period, develops greater aboveground biomass and has somewhat different growth dynamics compared with the Tápiói Korai variety. Averaged over the fertilisation treatments, only 30% of the total fresh leafy stalk mass was accumulated by the 85th day, and this figure only increased to 40% by the 115th day. The maximum fresh leafy stalk mass was recorded on the 155th day of the growing period. The most dynamic growth period was between the 115th and 155th days, when 41% of the total aboveground fresh mass was formed over a period of 40 days. The foliage gradually began to wither from the beginning of September, and 50% of the fresh leafy stalk mass could be harvested at the end of October (225th day) (Table 5).
Averaged over the fertiliser treatments the maximum quantity of leafy stalk dry matter was recorded on the 155th day. Dry matter accumulation was only at the 7% and 21% level, respectively, on the 85th and 115th day of the vegetation period, when the dry matter content of the fresh mass had reached 8% and 18%, respectively. The most dynamic dry matter accumulation was observed between days 115 and 155, when more than 18 t dry matter was formed per hectare in the course of 40 days, equivalent to around 80% of the total dry matter. At this time the dry matter content of the leafy stalks averaged 34%, and this did not change substantially until the end of October (225th day). On average, 46% of the maximum dry matter could be harvested at the end of the growing period (Table 6).
The maximum fresh and dry matter yield of the leafy stalks of the Tápiói Sima variety amounted to around 68 and 23 t·ha−1, respectively, averaged over the treatments. When the effect of fertilisation was examined during the period when the greatest aboveground biomass was accumulated (155th day), the fresh mass of the leafy stalks was found to be 57.78 t·ha−1 without fertilisation. Only the highest rate (300 kg/ha) of N fertiliser gave a significant increase in fresh mass (71.30 t·ha−1) compared to the control when applied alone, and a similar yield (68.34 t·ha−1) was achieved when the 100 kg·ha−1 N rate was supplemented with P and K fertiliser (N1P1K1). Further significant increases in aboveground fresh mass were recorded when the higher (200, 300 kg·ha−1) N rates were applied in combination with P and K fertiliser. In the earlier part of the growing season (115th day) only the N1P1K1 level of fertilisation resulted in a significantly higher fresh mass than in the control, while at a later stage (195th day) the highest leafy stalk mass was obtained in the N1P1K1 and N1P2K2 treatments. The effect of the nutrient supplies on the dry matter mass of the leafy stalks was very similar to that described for the fresh mass; in this case, too, the best fertiliser level was found to be N1P1K1 (Tables 5 and 6).
3.2. Tuber Yield
The tuber yield data of the two Jerusalem artichoke varieties are presented in Table 7.
At the end of September (195th day), when the Tápiói Korai variety was harvested, the tuber yield without fertilisation was 40.43 t·ha−1. When N fertiliser was applied alone, the 100 kg·ha−1 rate resulted in a significant yield surplus (8.59 t·ha−1) compared to the control, while the higher N rates (200, 300 kg·ha−1) led to a significant yield reduction compared to the 49.02 t·ha−1 yield achieved with 100 kg·ha−1 N. When this N rate was combined with P and K fertilisation, no significant increase in yield was recorded, while the 200 kg·ha−1 N rate only led to a significantly higher tuber yield when supplemented with P and K. Compared with the maximum yield (60.01 t·ha−1, N2P3) the highest, 300 kg·ha−1 N rate (N3P3K3) led to a significant reduction in the tuber yield.
The rainfall (137 mm) during the tuber formation period of the Tápiói Sima variety (August-October) was equivalent to the long-term mean, but was nevertheless unable to satisfy the water requirements of the crop in

Table 7. Tuber yield of the Tápiói Korai és Tápiói Sima Jerusalem artichoke varieties, t·ha−1 (Szarvas, 21 Aug-30 Oct 2002).
this stage of development. For this reason, the tuber yield of this variety was only 46% of that achieved for Tápiói Korai, averaged over the treatments. Without fertilisation, the tuber yield was 18.92 t·ha−1 at harvesting in late October (225th day). This yield level was only significantly increased by the 200 kg·ha−1 N rate when supplemented with P and K. On this soil, which has moderate P and good K supplies, the yield-enhancing effect of P fertiliser is greater than that of K at the 200 kg·ha−1 N level. No further significant rise in the tuber yield was achieved at the 300 kg·ha−1 N rate combined with P and K fertiliser (N3P3K3).
3.3. Total Biomass
The total biomass data for the two Jerusalem artichoke varieties, averaged over the fertiliser treatments, are presented in Table 8.
Both the changes in the nutrient concentrations in the various plant organs during the growing season and the quantity of nutrients absorbed also depend on the biomass accumulation. The accumulation of fresh and dry
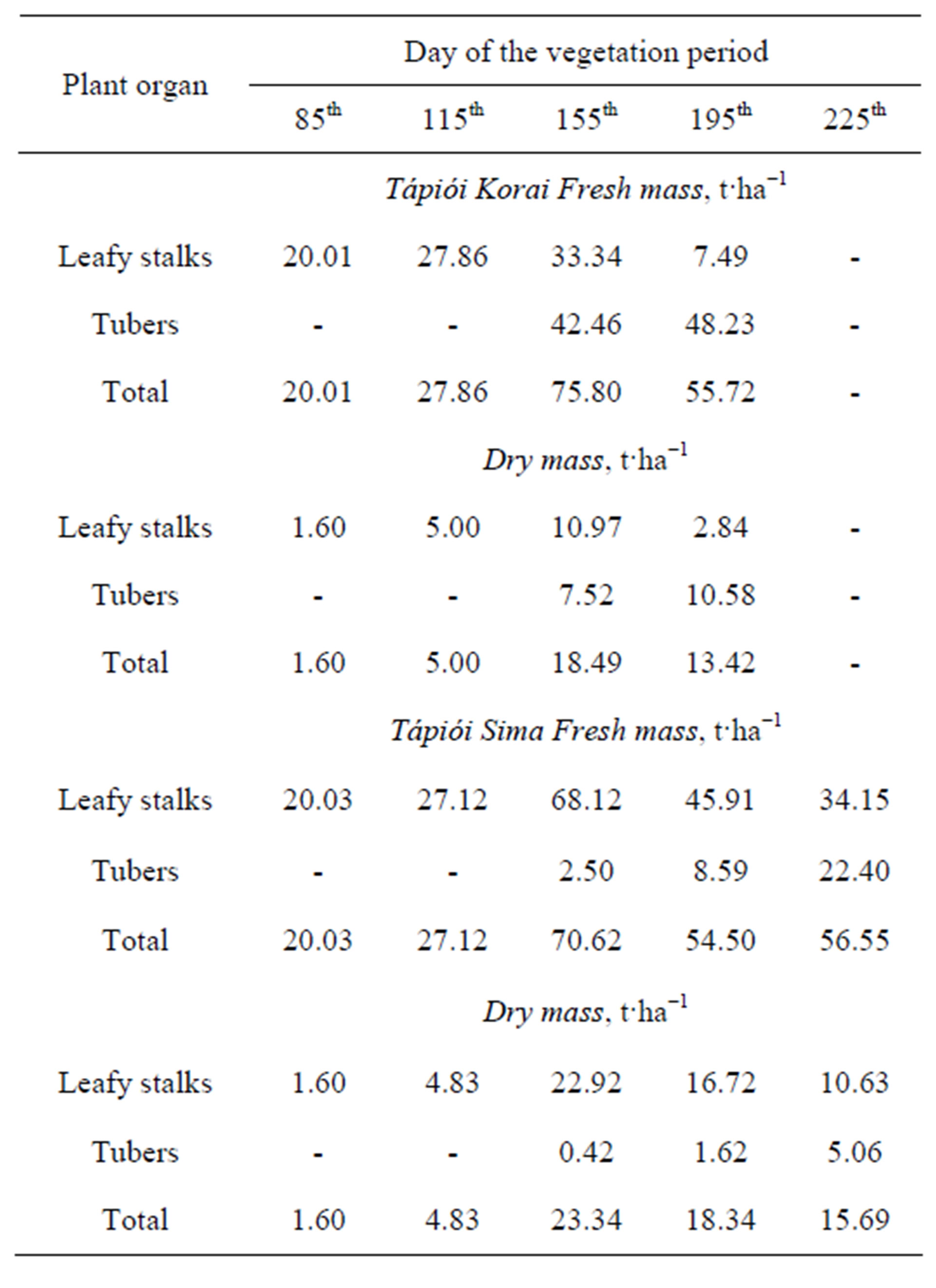
Table 8. Biomass of the Tápiói Korai and Tápiói Sima Jerusalem artichoke varieties during the growing season, averaged over the fertiliser treatments, t·ha−1 (Szarvas, 2002).
matter in the leafy stalks and tuber yield of the two varieties during the vegetation period can be seen in Table 8.
The dry matter content of the leafy stalks and tubers gradually increased as the growing season progressed. At sampling dates between the 85th and 195th days of the season the dry matter content of the leafy stalks of the Tápiói Korai variety amounted to 8%-18%-33%-38%, while that of the tubers was found to be 17.6% and 21.9% on the 155th and 195th days respectively. In the case of Tápiói Sima, the dry matter content of the leafy stalks was 8%-18%-34%-36%-31% between days 85 and 225, while that of the tubers was 16.8%-18.8%-22.5% at the three sampling dates.
The maximum values of fresh and dry matter mass were recorded on the 155th day in the early maturing Tápiói Korai variety. At this date 59% of the total dry matter mass (18.49 t·ha−1) was found in the leafy stalks and 41% in the tuber yield, but the proportion shifted to 79:21% in favour of the tuber yield by harvest. In this variety the dynamics of dry biomass accumulation showed the following pattern on the 85th-115th-155th- 195th days of the growing season: 9%-27%-100%-73%.
The late maturing Tápiói Sima variety also exhibited maximum values of fresh and dry matter mass on the 155th day of the growing period. At this date 98% of the total dry matter was found in the leafy stalks and only 2% in the tubers, but by harvest this ratio had changed to 68:32%. The dynamics of total dry matter accumulation for this variety showed a pattern of 7%-21%-100%- 79%-67% on the 85th-115th-155th-195th-225th days of the vegetation period.
A comparison of the two varieties revealed a substantial difference in the accumulation dynamics of the leafy stalk and tuber yields and in the mass ratio of the yield components.
3.4. Nutrient Concentration
The nutrient concentrations in the leafy stalks and tubers of the Tápiói Korai Jerusalem artichoke variety are presented in Tables 9 and 10.
With the exception of Ca the highest nutrient concentrations in the leafy stalks were recorded on the 85th day of the growing period (12 Jun), when 9% of the total dry matter content had formed and tuber formation had not yet begun. Over the next 30 days the aboveground shoot system grew intensively, resulting in dilution, especially for the N, P and K concentrations. The reduction in the nutrient concentration was 20% for N, 17% for P and 33% for K, while only moderate dilution could be detected for the other nutrients. The tuber formation of the Tápiói Korai variety began in the middle ten days of July (115th day), and the most intensive leafy stalk and tuber formation was recorded over the next 40 days (between days 115 and 155), when 73% of the total dry matter was accumulated. The greatest decline in the nutrient concentrations was observed during this growth period.
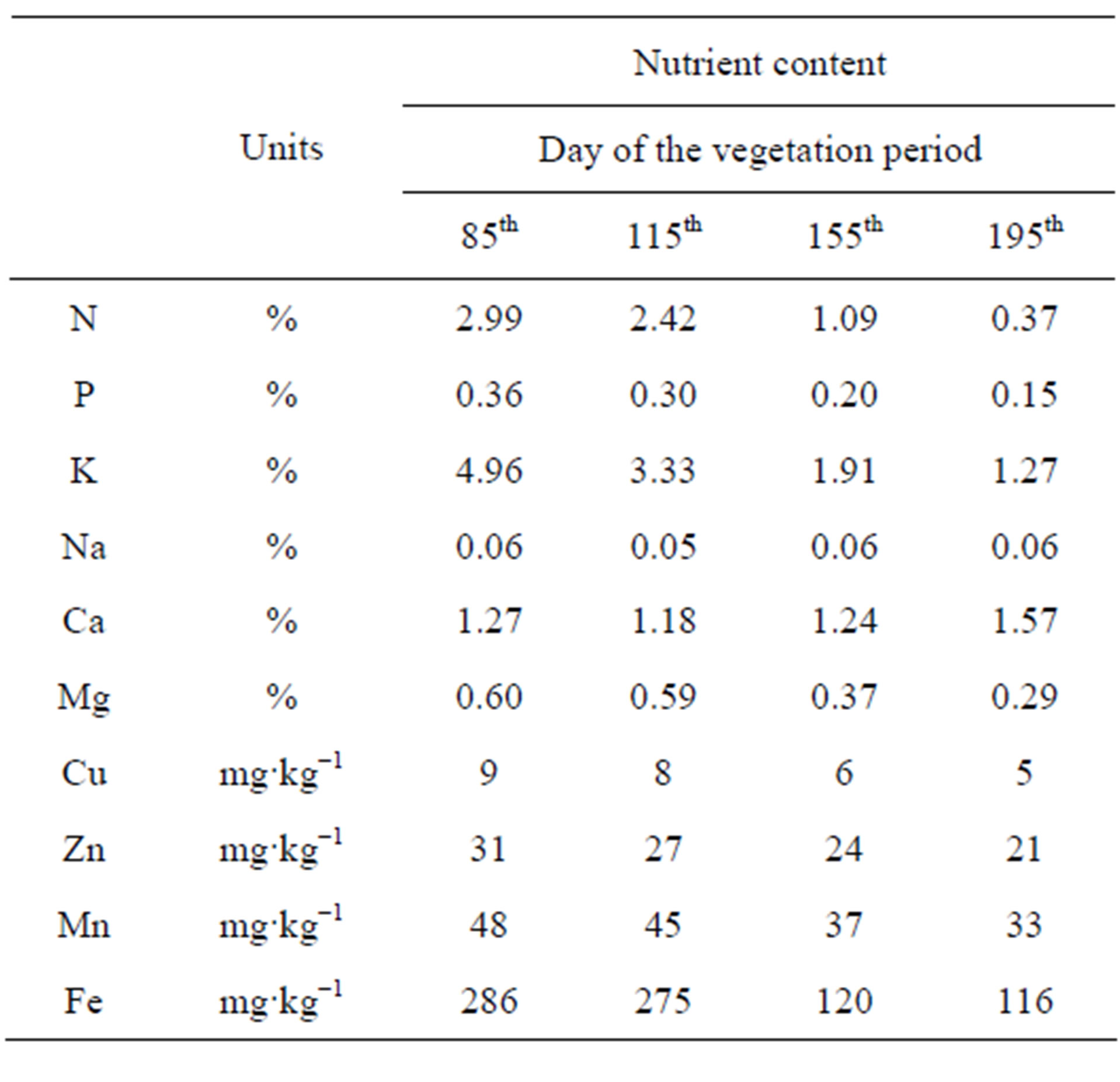
Table 9. Nutrient content in the leafy stalks of the Tápiói Korai Jerusalem artichoke variety during the growing season (Szarvas, 2002).

Table 10. Nutrient content in the tubers of the Tápiói Korai and Tápiói Sima Jerusalem artichoke varieties during the gorwing season (Szarvas, 2002).
Compared with the initial concentrations (100%, 85th day) the concentrations in the leafy stalks dropped to 36% for N, 56% for P, 39% for K, 62% for Mg, 66% for Cu, 77% for Zn and Mn and 42% for Fe. Little change was observed in the Na concentration during the growing period, while the Ca concentration was greatest at the end of the growing season. The leafy stalks started withering from the end of August, which was associated with a further decrease in the nutrient concentrations by harvest (Table 9).
The tuber yield of the Tápiói Korai variety was analysed on days 155 and 195. The N content of the tubers was highest at harvest (1.66%), while the P content did not change (0.37%) and the K content dropped steeply (2.09%). The Ca and Mg contents of the tubers were exemely low compared to those of the other macroelements (0.09% - 0.14%). The microelement contents of the tubers declined up to harvesting, with the exception of the Fe content which increased (Table 10).
The nutrient concentration data for the leafy stalks of the Tápiói Sima variety are presented in Table 11.
On the 85th day of the growing period (12 Jun) both Jerusalem artichoke varieties had similar dry matter mass in the leafy stalks (1.60 t·ha−1). Nevertheless, the N, P, K and Ca contents of the Tápiói Sima variety were considerably lower than those of Tápiói Korai. In the Tápiói Sima variety, 7% of the total dry matter had accumulated by the 85th day of the vegetation period and 21% by the 115th day, with no significant change in the nutrient concentration of the leafy stalks. The greatest extent of dry

Table 11. Nutrient content in the leafy stalks of the Tápiói Sima Jerusalem artichoke variety during the growing season (Szarvas, 2002).
matter incorporation was detected between the 115th and 155th days, when 79% of the total dry matter was accumulated in the course of 40 days and a considerable dilution of the nutrient concentrations was observed, amounting to 26% for N, 12% for K, 11% for Ca and 28% for Mg. Tuber formation did not begin until the second half of August in the Tápiói Sima variety, and the tuber yield made up 2% of the total dry matter mass on the 155th day of the growing season. The intensive period of tuber formation occurred between the 155th and 225th days, when the translocation of nutrients into the tuber began, accompanied by the withering and collapse of the leafy stalks. This process resulted in a considerable decline in the nutrient concentration of the leafy stalks, which meant that only 23% of the initial nutrient concentration (85th day) could be detected for N, 37% for P, 36% for K, 70% for Ca, 75% for Mg, 66% for Mn and 72% for Fe.
Changes in the nutrient contents in the tubers of the Tápiói Sima Jerusalem artichoke variety during the growing season can be seen in Table 10.
The nutrient concentrations in the tubers were highest on the 155th day of the vegetation period, at the beginning of tuber formation, with the exception of Na and Fe.
By harvest (225th day) the nutrient contents decreased, by 13% for N, 20% for P and 33% for K. The contents of Ca, Mg, Cu, Zn and Mn were 40% - 50% lower at this stage than at the start of tuber formation. The tuber Fe concentration declined to the greatest extent (by almost two-thirds), while the Na content increased. A compareson of the nutrient contents in the tubers of the two varieties at harvest revealed that the N content was higher in the Tápiói Sima variety than in the Tápiói Korai variety, while the Mn and Fe concentrations were lower. No great differences were observed for the other nutrients.
3.5. Nutrient Uptake
Data on the nutrient uptake of the Tápiói Korai Jerusalem artichoke variety are presented in Table 12.


Table 12. Nutrient uptake of the Tápiói Sima Jerusalem artichoke variety during the growing season, kg·ha−1 (Szarvas, 2002).
The most intensive accumulation of the nutrients N, P, K, Mg, Ca, Cu and Fe in the leafy stalks was observed between the 85th and 115th days of the growing season.
The maximum incorporation of nutrients in the leafy stalks was recorded on the 155th day. The most dynamic nutrient accumulation period for the tubers was between days 115 and 155 of the vegetation period, after whichnutrient incorporation into the tubers became less intense. The maximum nutrient uptake of the whole plant (leafy stalks + tubers) was observed on the 155th day. These values were used in calculating specific nutrient uptake, as this was the nutrient quantity required for yield formation.
The specific nutrient uptake data for the Tápiói Korai Jerusalem artichoke variety are presented in Table 13. The specific nutrient uptake required for 10 t tuber yield plus the corresponding leafy stalks was calculated on the basis of the maximum nutrient uptake and the tuber yield at harvest as: 48 kg N, 10 kg P, 83 kg K, 30 kg Ca and 10 kg Mg.
The nutrient uptake of the Tápiói Sima Jerusalem artichoke variety during the growing season can be seen in Table 14.
The most intensive period of dry matter and nutrient accumulation in the leafy stalks was observed between the 115th and 155th days. At the end of this period the dry matter mass of the leafy stalks was around 23 t·ha−1, nutrient uptake had reached a maximum, and a total of 355 kg N, 66 kg P, 660 kg K, 188 kg Ca and 99 kg Mg had been incorporated. In this later maturing variety tuber formation began later, in the second half of August, and the greatest nutrient accumulation in the tuber yield was observed in October. The maximum nutrient uptake for
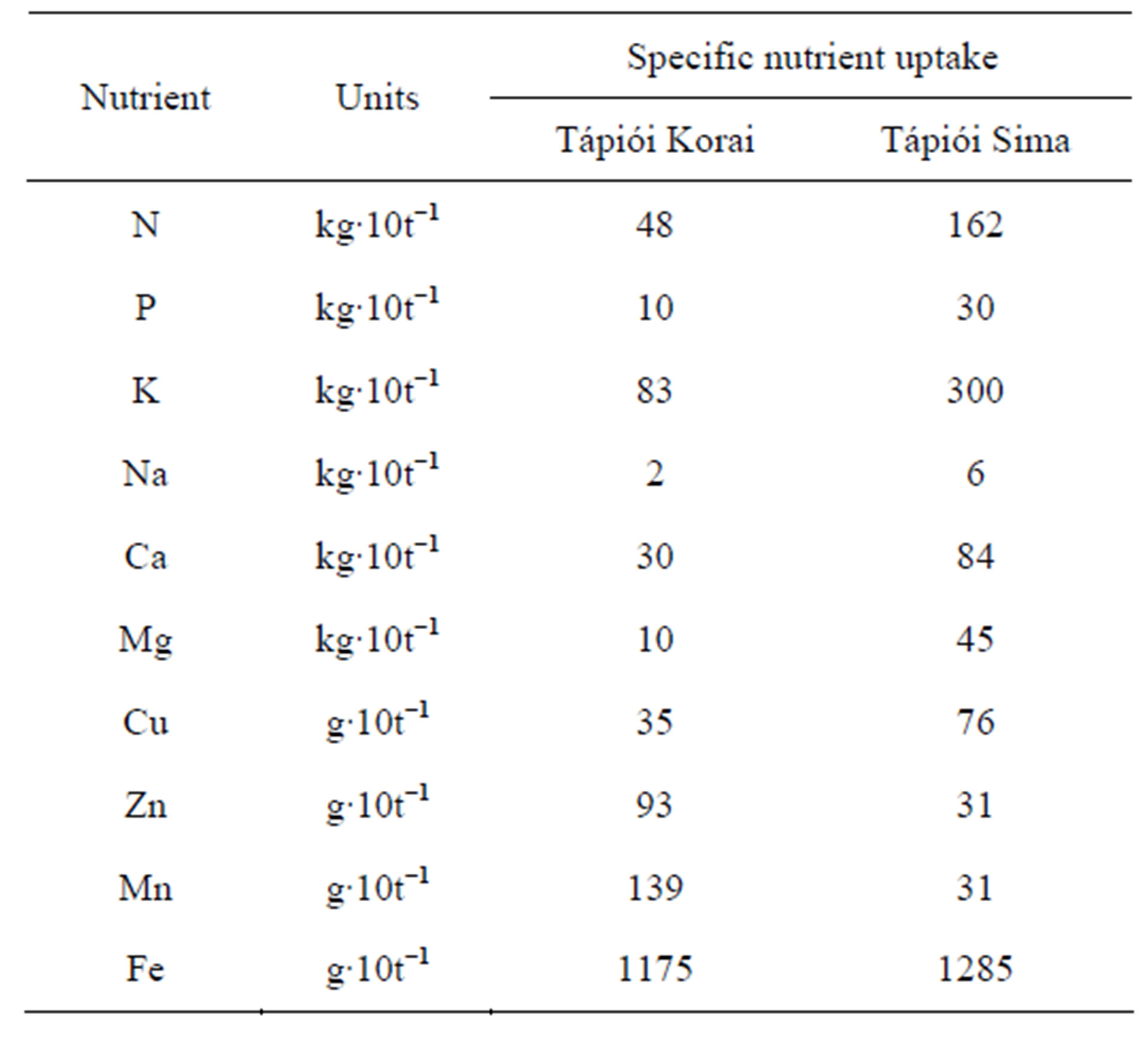
Table 13. Specific nutrient uptake of the Tápiói Korai and Tápiói Sima Jerusalem artichoke varieties, kg 10·ha−1 tuber yield + the corresponding leafy stalks (Szarvas, 2002).
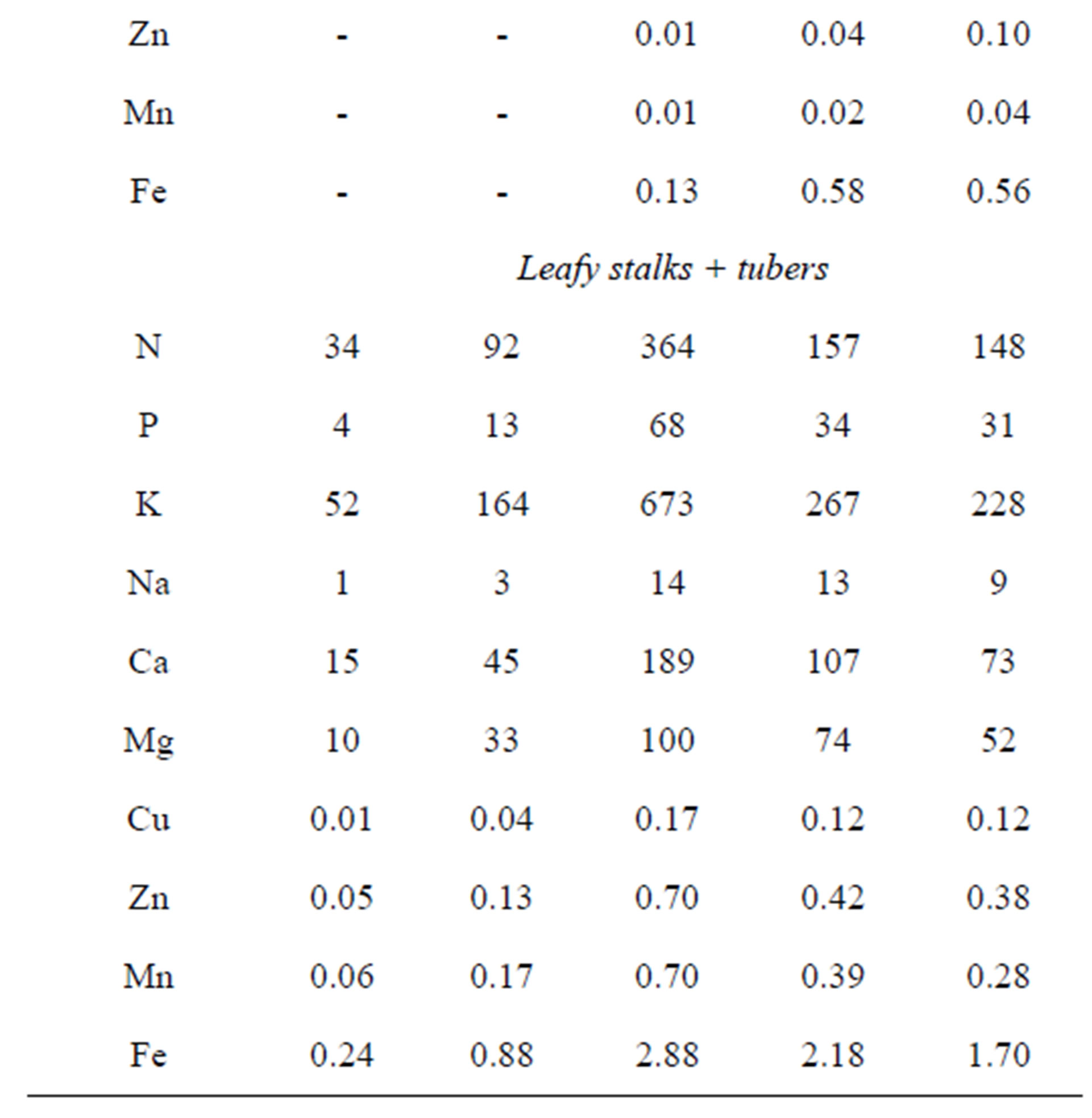
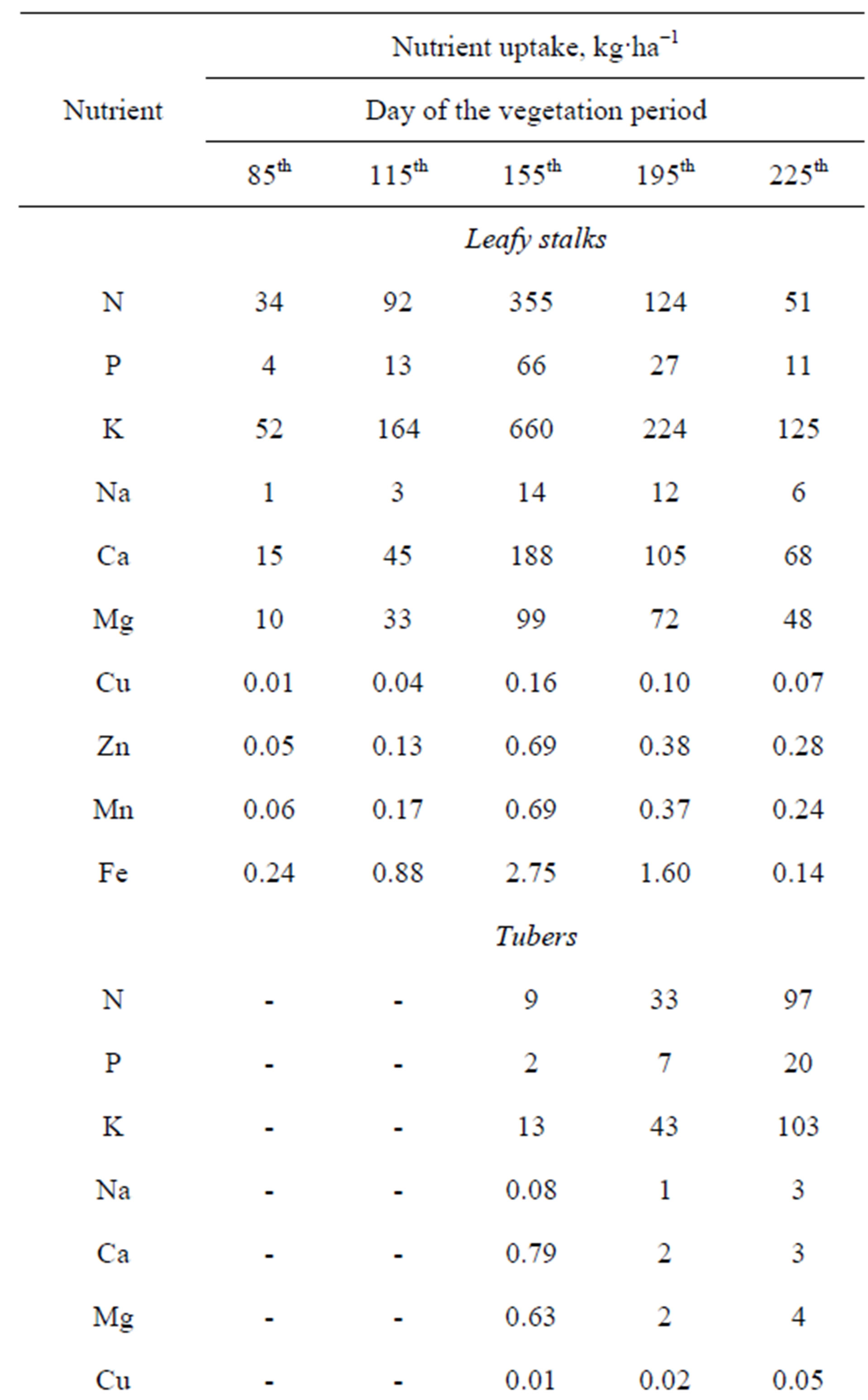
Table 14. Nutrient uptake of the Tápiói Sima Jerusalem artichoke variety during the growing season, kg·ha−1 (Szarvas, 2002).
the whole plant (leafy stalks + tubers) was recorded on the 155th day of the growing period, and was almost equivalent to the quantity of nutrients incorporated into the leafy stalks, as tuber formation had only just begun. By the end of the growing period (225th day) 40% of the maximum nutrient uptake could be detected for N, 45% for P, 34% for K, 39% for Ca and 52% for Mg.
The maximum quantity of nutrients absorbed and the tuber yield at harvest (22.4 t·ha−1) were used to calculate the specific nutrient uptake. In the case of the Tápiói Sima variety, the specific nutrient uptake for 10 t tuber yield plus the corresponding leafy stalks amounted to 162 kg N, 30 kg P, 300 kg K, 84 kg Ca and 45 kg Mg.
A comparison of the two varieties shows that the specific three times that of the Tápiói Korai variety (Table 12). This could be explained by the fact that the leafy stalk yield of Tápiói Sima was twice that of Tápiói Korai, while the tuber yield was only half as great. There was thus a substantial difference in the proportion of the maximum dry matter mass in the tuber yield and the leafy stalks, which was 1:1 for Tápiói Korai and 1:4.5 for Tápiói Sima.
4. Conclusions
Substantial differences could be observed between the dynamics of growth, biomass accumulation and tuber formation in the early maturing Jerusalem artichoke variety Tápiói Korai and the late maturing variety Tápiói Sima. The most intensive period of main stalk growth was between the 85th and 115th days for Tápiói Korai and between the 115th and 155th days for Tápiói Sima.
Averaged over the fertiliser treatments, the maximum total biomass of both varieties was recorded on the 155th day, but there were considerable differences in the share of the leafy stalks and tubers in the total biomass. At this stage the tuber yield made up 56% of the biomass in Tápiói Korai and 4% in Tápiói Sima. This ratio rose to 87% and 40%, respectively, by harvest. The ratio of tubers to leafy stalks in the maximum dry matter mass was 1:1 for Tápiói Korai and 1:4.5 for Tápiói Sima.
During the period when the maximum aboveground biomass was formed, 100 kg·ha−1 rates of N and P fertileiser resulted in the greatest leafy stalk yield (38.34 t·ha−1) for Tápiói Korai. In the case of Tápiói Sima, which produced a substantially greater leafy stalk mass, the highest quantity of aboveground biomass (78 - 80 t·ha−1) was obtained with the 200 kg·ha−1 N rate, supplemented with P and K fertiliser.
The excessive (300 kg·ha−1) N rate without P or K led to a decrease in the tuber yield of Tápiói Korai. The highest tuber yield for both varieties was achieved with 200 kg·ha−1 N supplemented with P and K fertiliser.
The highest nutrient concentration in the leafy stalks was recorded on the 85th day of the growing season, prior to intensive dry matter accumulation in the stalks and before tuber formation began. The greatest reduction in the nutrient content of the leafy stalks was observed during tuber formation.
Maximum nutrient uptake was recorded for both varieties on the 155th day, when 230 kg N, 50 kg P, 398 kg K, 146 kg Ca and 50 kg Mg had been incorporated into the total dry matter (18.49 t·ha−1) of Tápiói Korai, and 364 kg N, 68 kg P, 673 kg K, 189 kg Ca and 100 kg Mg into that of Tápiói Sima (23.34 t·ha−1).
Great differences were observed in the specific nutriaent uptake of the two varieties, as there was a substantial deviation in the ratio of the tubers and leafy stalks in the maximum dry matter mass. The specific nutrient uptake required for 10 t tuber yield plus the corresponding leafy stalks was 48 kg N, 10 kg P, 83 kg K, 30 kg Ca and 10 kg Mg for Tápiói Korai, and 162 kg N, 30 kg P, 300 kg K, 84 kg Ca and 45 kg Mg for Tápiói Sima.
REFERENCES
- I. Angeli, J. Barta and L. Molnár, “A Gyógyító Csicsóka,” Mezőgazda Kiadó, Budapest, 2000.
- E. László and I. Réczey, “Megújuló Nyersanyagok nem Élelmiszeripari Felhasználása,” NF-2000 Magyarország Információ Szolgáltató Rendszer, Budapest, 2000.
- Z. Izsáki, “Csicsóka,” In: J. Antal, Ed., Növénytermesztéstan, Mezőgazda Kiadó, Budapest, 2005, pp. 93-102.
- J. Barta and G. Pátkai, “Chemical Composition and Storability of Jerusalem Artichoke Tubers,” Acta Alimentaria, Vol. 36, No. 2, 2007, pp. 257-267. doi:10.1556/AAlim.36.2007.2.13
- J. Stanley, F. Kays and Stephen, “Biology and Chemistry of Jerusalem Artichoke,” CRC Press, Nottingham, 2007.
- W. Pimsaen, S. Joglay, B. Suriharn, T. Kesmala, V. Pensuk and A. Patanothai, “Genotype by Environment (G × E) Interactions for Yield Components of Jerusalem Artichoke (Helianthus tuberosus L.),” Asian Journal of Plant Sciences, Vol. 9, No. 1, 2010, pp. 11-19. doi:10.3923/ajps.2010.11.19
- D. G. Dorell and B. B. Chubey, “Irrigation, Fertilizer, Harvest Dates and Storage on the Reducing and Fructose Concentrations of Jerusalem Artichoke Tubers,” Canadian Journal of Plant Science, Vol. 57, No. 2, 1977, pp. 591-596. doi:10.4141/cjps77-084
- P. Denoroy, “The Crop Physiology of Helianthus tuberosus (L.), a Model Oriented View,” Biomass Bioenerg, Vol. 11, No. 1, 1996, pp. 11-32. doi:10.1016/0961-9534(96)00006-2
- W. J. Mclaurin, Z. C. Somda and S. J. Kays, “Jerusalem Artichoke Growth, Development, and Field Storage. I. Numerical Assessment of Plant Part Development and Dry Matter Acquisition and Allocation,” Journal of Plant Nutrition, Vol. 22, No. 8, 1999. pp. 1303-1313. doi:10.1080/01904169909365714
- E. Raso, “Jerusalem Artichoke. Effect of Nitrogen-Potassium Fertilizing,” Terra e Sole, Vol. 45, No. 575-576, 1990, pp. 431-433.
- G. Soja, G. Dersch and W. Praznick, “Harvest Dates, Fertilizer and Varietal Effect on Yield, Concentration and Molecular Distribution of Fructan in Jerusalem Artichoke (Helianthus tuberosus L.),” Journal of Agronomy and Crop Science, Vol. 165, No. 2, 1990, pp. 181-189. doi:10.1111/j.1439-037X.1990.tb00849.x
- G. Soja and E. Haunold, “Leaf Gas Exchange and Tuber Yield in Jerusalem Artichoke (Helianthus tuberosus L.) Cultivars,” Field Crop Research, Vol. 26, No. 3, 1991, pp. 241-252. doi:10.1016/0378-4290(91)90002-D
- B. Sawicka, “Changes in chemical composition of Helianthus tuberosus L. under Differentiated Nitrogen Fertilization,” Zeszyty Problemove Postepow Nauk Rolniczych, No. 484, 2002, pp. 573-579.
- M. A. Rodrigues, L. Sousa, J. E. Cabanas and M. Arrobas, “Tuber Yield and Leaf Mineral Composition of Jerusalem Artichoke (Helianthus tuberosus L.) Grown under Different Cropping Practices,” Spanish Journal of Agricultural Research, Vol. 5, No. 4, 2007, pp. 545-553.
- K. Gao, T. Zhu and G. Han, “Water and Nitrogen Interactively Increased the Biomass Production of Jerusalem Artichoke (Helianthus tuberosus L.) in Semi Arid Area,” African Journal of Biotechnology, Vol. 10, No. 34, 2011, pp. 6466-6472.
- G. J. Seiler and L. G. Campbell, “Genetic Variability for Mineral Element Concentrations of Wild Jerusalem Artichoke Forage,” Crop Science, Vol. 44, No. 1, 2004, pp. 289-292.
- G. J. Seiler and L. G. Campbell, “Genetic Variability for Mineral Concentration in the Forage of Jerusalem Artichoke Cultivars,” Euphytica, Vol. 150, No. 1-2, 2006, pp. 281-288. doi:10.1007/s10681-006-9119-2
- S. Terzic, M. Zoric, J. Atlagic, I. Maksimovic, T. Zeremski and B. Dedic, “Classification of Jerusalem Artichoke Accessions by Linear Discriminant Analysis of Mineral Concentration in Tubers and Leaves,” Helia, Vol. 34, No. 55, 2011, pp. 83-90. doi:10.2298/HEL1155083T
- H. Danilcenko, E. Jariene, M. Gajewski, J. Cerniauskiene, J. Kulaitiene, B. Sawicka and P. Aleknaviciene, “Accumulation of Elements in Some Organically Grown Alternative Horticultural Crops in Lithuania,” Hortorum Cultus—Acta Scientiarum Polonorum, Vol. 10, No. 2, 2011, pp. 23-31.
- E. Cieslik,”Mineral Content of Jerusalem Artichoke New Tubers,” Zeszyty Naukowe Akademii Rolniczej, Vol. 342, No. 10, 1998, pp. 23-30.
- V. Tamas, D. Belala, F. Ionescu, M. Popescu and M. Neagu, “Research for Obtaining from Affordable Natural Mineral Sources, Agreed to Replace Some Synthetic Animal Feed Additives,” Lucrari Stiintifice, Seria Agronomie, Vol. 52, 2009, pp. 75-79.
- S. Terzic and J. Atlagic, “Nitrogen and Sugar Content Variability in Tubers of Jerusalem Artichoke (Helianthus tuberosus L.),” Genetika, Vol. 41, No. 3, 2009, pp. 289- 295. doi:10.2298/GENSR0903289T
- X. Y. Ma, L. H. Zhang, H. B. Shao, G. Xu, F. Zhang, F. T. Ni and M. Brestic, “Jerusalem Artichoke (Helianthus tuberosus L.), a Medical Salt-Resistant Plant Has High Adaptability and Multiple-Use Values,” Journal of Medical Plants Research, Vol. 5, No. 8, 2011, pp. 1272-1279.
- M. Harmankaya, F. A. Juhaimi and M. M. Özcan, “Mineral Contents of Jerusalem Artichoke (Helianthus tuberosus L.) Growing Wild in Turkey,” Analytical Letters, Vol. 45, No. 15, 2012, pp. 2269-2275. doi:10.1080/00032719.2012.686131
- G. Németh and Z. Izsáki, “Macroand Micro-Element Content and Uptake of Jerusalem Artichoke (Helianthus tuberosus L.),” Cereal Research Communications, Vol. 34, No. 1, 2006, pp. 597-600. doi:10.1556/CRC.34.2006.1.149
- M. A. Rodrigues, L. Sousa, J. E. Cabanas and M. Arrobas, “Tuber Yield and Leaf Mineral Composition of Jerusalem Artichoke (Helianthus tuberosus L.) Grown under Different Cropping Practices,” Spanish Journal of Agricultural Research, Vol. 5, No. 4, 2007, pp. 545-553.
- Z. C. Somda, Z. C. McLaurin and S. J. Kays, “Jerusalem Artichoke Growth, Development, and Field Storage. II. Carbon and Nutrient Element Allocation and Redistribution,” Journal of Plant Nutrition, Vol. 22, No. 8, 2008, pp. 1315-1334. doi:10.1080/01904169909365715
- MÉM NAK, “A TVG Tápanyagvizsgáló Laboratórium Módszerkönyve,” MÉM Növényvédelmi és Agrokémiai Központ, Budapest, 1978.
- MÉM NAK, “Műtrágyázási Irányelvek és Üzemi Módszer,” MÉM Növényvédelmi és Agrokémiai Központ, Budapest, 1979.
- J. Sváb, “Biometrical Methods in Research Work,” Mezőgazdasági Kiadó, Budapest, 1981.
NOTES
*This research was funded from a Széchenyi grant (No. NKFP 3/012, 2001-2004).

