American Journal of Plant Sciences
Vol.4 No.6(2013), Article ID:33550,6 pages DOI:10.4236/ajps.2013.46153
Identification of Potential Species Croton bonplandianum, Sedges and Balanitis aegyptiaca for the Application of Phytoremediation
![]()
B R D School of Biosciences, Sardar Patel University, Vallabh Vidya, India.
Email: *asp.fus@gmail.com
Copyright © 2013 Pritesh Parmar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received November 10th, 2012; revised April 3rd, 2013; accepted May 10th, 2013
Keywords: Balanites aegyptiaca; Croton bonplandianum; Hyperaccumulator; Phytoremediation; Screening; Sedges
ABSTRACT
Heavy metal contamination to the environment is a serious problem in the developing countries due to anthropogenic activities, a threat needs to remediate to sustain the life on earth, technology includes use of microorganisms and different plant species. In consideration of biomass, phytoremediation is a very useful techniques above all, can be exploit by identification of hyperaccumulator, which accumulates the heavy metal under metal stress condition. In view of constraints of efficient plant species in present study thirty seven different plant species were screened for the identification of heavy metal accumulators. Croton bonplandianum, sedges and Balanites aegyptiaca amongst the all exhibit superior potential of heavy metal accumulation. This is the first report to unravel the heavy metal accumulation property of three different plant species which can be exploited for the bioremediation of heavy metals.
1. Introduction
The contamination of the environment by toxic metals poses a threat for “Man and Biosphere”, reducing agricultural productivity and damaging the health of ecosystem. Natural processes such as volcanic eruptions, continental dusts and anthropogenic activities like mining, combustion of fossil fuel, phosphate fertilizers, military activities, metal working industries etc. lead to emission and accumulation of heavy metals in ecosystem. These heavy metals are toxic because they cause DNA damage and their carcinogenic effects in animals and humans are probably caused by their mutagenic ability [1,2].
Remediation of polluted sites generally involves clean up or making safe of a site or water body contaminated by toxic substances whether they are natural or manmade. Remediation also includes using various technologies to either degrade contaminants or remove them via other chemical or biological means. The following are among the choices available for environmental consultants for remediating heavy metal contamination of soil [3].
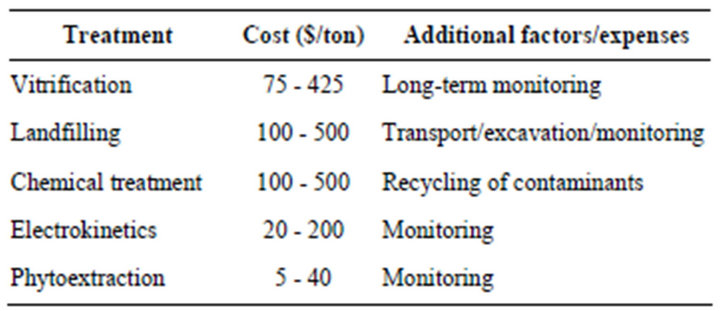
Various engineering-based methods such as soil excavation, soil washing or burning or pump and treat systems as shown in below table are already being used to remediate metal contaminated soils.
However, these non biological techniques are not fully acceptable as they destroy the biotic components of soil and are technically difficult and expensive to implement. Since last decade phytoremediation has emerged as a new, low cost and effective technology that uses plants and their associated microbial flora for environmental clean up [4-6].
Phytoremediation involves growing or encouraging the growth of plants in a contaminated matrix, either artificially (constructed wetlands) or naturally for a required growth period, to remove contaminants from the matrix, or facilitate immobilization (binding/containment) or degradation (detoxification) of the pollutants. The plants can be subsequently harvested processed and disposed. The main difference between constructed and natural wetlands is that while the size of the former might be small and remain constant, the latter can be large and increase in size with time, which thus, affect the intensity and efficiency of phytoremediation capability of both systems.
2. Types of Phytoremediation
Several types of phytoremediation can be defined according to Schwitzguébel [7] as:
2.1. Phytoextraction
It utilizes pollutant-accumulating plants to remove pollutants like metals or organics from soil by concentrating them into the plant parts that are harvestable.
2.2. Phytotransformation
The degradation of complex organic molecules to simple molecules or the incorporation of these molecules into plant tissues.
2.3. Phytostimulation
Plant-assisted bioremediation, the stimulation of microbial and fungal degradation by release of exudates/enzymes into the root zone (Rhizosphere).
2.4. Phytovolatilization
It involves the use of plants to take up contaminants from the soil, transforming them into volatile form and transpiring them into the atmosphere.
2.5. Rhizofiltration
The use of plant roots to adsorb pollutants, mainly metals, but also organic pollutants, from water and aqueous waste streams.
2.6. Pump and Tree (Dendroremediation)
The use of trees to evaporate water and thus to extract pollutants from the soil.
2.7. Phytostabilisation
The use of plants to reduce the mobility and bioavailability of pollutants in the environment, thus preventing their migration to groundwater or their entry into the food chain.
2.8. Hydraulic Control
The control of the water table and the soil field capacity by plant canopies. As highlighted above, there are several ways in which plants are used to clean up or remediate contaminated sites. To remove pollutants from soil, sediment and/or water and air plants can break down, or degrade organic pollutants or contain and stabilize inorganic contaminants by acting as filters or traps. It is amendable to a broad range of organic and inorganic contaminants including many metals with limited alternative options.
During the last two decades, Gujarat has emerged as one ofthe major players in the country’s industrial arena. An entire industrial belt gradually developed from Mehsana in the North, to Vapi in the South, which was later christened as the Golden Corridor of growth. Naturally, this means a heavy concentration of chemical industry, both from the public sector and the private sector.
In VV Nagar, there is a large Industrial Estate called Vitthal Udyognagar Industrial Estate. Vitthal Udyognagar GIDC Industrial Association is actively involved in the development activities of industry. The major type of industry are Hosiery and Readymade Clothes, Engineering, Carpentry Wooden, Chemical and products, Rubber and plastic, Metal Alloy, Minerals, Metal Works and Electric, Machinery & Parts. The total investment is Rs. 31990.99 lacs and employing 24,693 people.
In Anand Taluka, main industrial area is GIDC estate of V. V. Nagar. There are around 630 units in the estate. Majority are engineering units. Other industries are chemical, plastic, rubber, electric and electronics. The detailed breakup is as under Table 1.
The Gujarat State Fertilizers & Chemicals Limited (GSFC) at Bajwa, a company with annual turnover of over Rs. 3000 crores, is situated on National Highway No. 8 near Village Chhani, Dist. Vadodara, Gujarat State. The company has set up a number of plants producing fertilizers, chemicals, petrochemicals, agricultural & Biotechnological products. For operating these plants, many raw materials (both solids & liquids), consumables,

Table 1. Type of industries (Vitthal Udyognagar) of GIDC units.
intermediates, chemicals, solvents etc are required. Some of these products are transported on regular basis and some of them on occassional basis depending upon the need & requirement of plants.
The Nandesari GIDC Industrial Estate was developed in 1968-69 as a general industrial estate and later on converted to a chemical estate The Nandesari Industrial Estate (Nandesari) is on 220 hectares of fertile land adjoining the river Mini that joins river Mahi at a distance of 30 kms. Nandesari, coordinates: 22˚25'5"N 73˚5'33"E has a large notified industrial area consisting of large amount of chemical factories situated in Vadodara district of Gujarat state.
Ahmadabad is located on the banks of the River Sabarmati in the Northern part of Gujarat and the Western part of India. It is located at 23.03˚N 72.58˚E spanning an area of 310 km² and is the centre for industrial, institutional and political activities of the Gujarat state, India. Vatva GIDC phase IV (22˚58'11"N 72˚39'11"E) an industrial site discharge their partially treated effluent in Kharicut canal.
In view of geological survey of India for the mineral exploration, the area extend over two sq. km to the north and north west of Ambaji village (24020'N:72051'E, Survey of India top sheet No. 45D/15) in Dantataluka of Banaskantha district Gujarat [8].
Groundwater in Ankleshwar Industrial Estate in Bharuch district in Gujarat is highly contaminated. The contamination is a result of more than 3000 industrial units in the estate: around 270 million litres of liquid waste and 50,000 tonnes of solid waste is generated annually. This often becomes a problem for people living in villages around the industrial estate because they depend on groundwater to meet their daily needs. Even crops growing on contaminated soil absorb the pollutants.
Until now numbers of plant species have been identified to be used as a hyperaccumulator in terms of heavy metals all over the world but the technology is still not used effectively due to constraints of plant species available to be apply for phytoremediation. In view of requirement of novel plant spp for the potential application of phytoremediation with desirable properties screening was done in present investigation.
3. Materials and Methods
3.1. Sample Collection
Thirty seven different dicot and monocot plant species used in the study were collected from different metal contaminated areas of Gujarat, India includes GIDCs situated at Vitthal Udyognagar, Anand district, Nandesari in Vadodara district, Vatva in Ahmadabad district, Ankleshwar; Bajwa; Gujarat State Fertilizer Corporation (GSFC) Vadodara and Ambamata mining site at Ambaji.
3.2. Sample Preparation and Estimation of Heavy Metals Content by ICP-AAS (Table 2)
The collected samples were washed thoroughly in tap water followed by distilled water and allowed to dry in a hot air oven at 80˚C for three days. 250 mg of dry plant material (root, stem and leaf) of heavy metals plants were digested in an acid mixture of nitric acid:sulphuric acid: perchloric acid (Qualigens) (2:1:1v/v). Acid mixture was evaporated and metal residues were dissolved in 25 ml of 0.1 N HCl (Qualigens). Analysis of treated and control plant samples in terms of heavy metals was done by Atomic absorption spectroscopy (AAS, GBC-911) on dry weight basis [9].
4. Results and Discussion
4.1. Screening and Identification of a Hyperaccumulator
Thirty seven different plant species collected from many parts of Gujarat state were identified by Prof. A. S. Reddy, Head of the Herbarium section, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India. (The Herbarium of Sardar Patel University, established in late 1950s, perhaps, is the largest herbarium in Gujarat having more than 50,000 specimens collected from different corners of Gujarat State as well as North-West parts of Maharashtra State) and screened for their metal accumulating capacity using ICP analysis technique. Table 2 shows the quantity of heavy metals present in the roots of the different plant species. The amount of heavy metals uptake, specifically cadmium (Cd) was found to be highest with a concentration of 1185.6 ppm in grass collected from Ambamata mines, Ambaji followed by 1051.2 ppm in Balanites aegyptiaca collected from Ambamata mines, Ambaji and 74.44 ppm in Croton bonplandianum, collected from the waste water drainage line passing from GIDC, Vitthal Udyognagar compared to other plant species. Parmar et al. [10] showed higest accumulation of lead in leaves of Colocasia esculenta in comparison to heavy metlas As, Ni, Cr and Cd in roots of Colocasia esculenta.
4.2. Acclimatization Study
As one of the essential features of any hyper accumulator plant is, its ability to flourish in any physical conditions, the plant reporting highest accumulation, Croton bonplandianum and grass was cultivated in pot containing soil collected from surroundings of the Bioscience department, Sardar Patel University, Vallabh Vidyanagar,

Table 2. Heavy metals intake of different plant species evaluated by ICP-AAS analysis.

Figure 1. Acclimatization study on Croton bonplandianum (a) and grass-sedge (b) after three weeks of cultivation.
Gujarat, India. Figure 1 represents the survival of the plant with well developed stem and young leaves after three weeks of cultivation.
The outcome of this study directs that Croton bonplandium, sedges and Balanitisae gyptiacaare suitable candidates to be used for phytoremediation of heavy metals from contaminated soil. This is the first report on theses plants which focuses on its ability to accumulate heavy metals to a reasonable level to be applied for the phytoremediation technology.
5. Acknowledgements
This work was supported by grants for project work from Department of Biotechnology (DBT), Government of India.
REFERENCES
- S. E. Knasmuller, H. Gottmann, A. Steinkellner, C. Fomin, A. Pickl, R. G. Paschke and M. Kundi, “Detection of Genotoxic Effects of Heavy Metal Contaminated Soils with Plant Bioassays,” Mutation Research, Vol. 420, No. 1, 1998, pp. 37-48. doi:10.1016/S1383-5718(98)00145-4
- C. Baudouin, M. Charveron, R. Tarrouse and Y. Gall, “Environmental Pollutants and Skin Cancer,” Cell Biology and Toxicology, Vol. 18, No.5, 2002, pp. 341-348. doi:10.1023/A:1019540316060
- D. J. Glass, “Economic Potential of Phytoremediation,” In: I. Raskin and B. D. Ensley, Eds., Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment, John Wiley & Sons Inc, New York, 1999, pp.15-31.
- I. Raskin, P. B. A. N. Kumar, S. Dushenkov and D. E. Salt, “Bioconcentration of Heavy Metals by Plants,” Current Opinion in Biotechnology, Vol. 5, No. 3, 1994, pp. 285-290. doi:10.1016/0958-1669(94)90030-2
- D. E. Salt, R. C. Prince, I. J. Pickering and I. Raskin, “Mechanisms of Cadmium Mobility and Accumulation in Indian Mustard,” Plant Physiology, Vol. 109, No. 4, 1995, pp. 1427-1433.
- D. E. Salt, R. D. Smith and I. Raskin, “Phytoremediation,” Annual Review of Plant Physiology and Plant Molecular Biology, Vol. 49, No. 1, 1998, pp. 643-668. doi:10.1146/annurev.arplant.49.1.643
- J. Schwitzguebel, “Potential of Phytoremediation, an Emerging Green Technology. Ecosystem Service and Sustainable Watershed Management in North China,” Proceedings of International Conference, Beijing, 23-25 August 2000, p. 5.
- N. C. Shekar, “Antiquity of Mining and Metallurgical Activities at Ambaji, Kumbaria and Deri, Gujarat and Rajasthan,” Indian Journal of History of Science, Vol. 18, No. 2, 1983, pp. 176-183.
- T. J. Ganje and A. L. Page, “Rapid Acid Dissolution of Plant Tissue for Cadmium Determination by Atomic Absorption Spectrophotometry,” Atomic Absorption Newsletters, Vol. 13, 1974, pp. 131-134.
- P. Parmar, M. Patel, B. Dave and R. B. Subramanian, “Identification of Colocassiae sculentuma Novel Plant spp for the Application of Phytoremediation,” African Journal of Basic and Applied Sciences, Vol. 4, No. 3, 2012, pp. 67-72.
NOTES
*Corresponding author.

