American Journal of Plant Sciences
Vol.4 No.1(2013), Article ID:27633,7 pages DOI:10.4236/ajps.2013.41012
Antibacterial Activity and Phytochemical Analysis of Mentha piperita L. (Peppermint)—An Important Multipurpose Medicinal Plant
![]()
1Department of Biotechnology, Sri Venkateswara University, Tirupathi, India; 2Department of Biotechnology, Sri Padmavathi Mahila University, Tirupathi, India; 3Department of Biotechnology, Dravidian University, Kuppam, India.
Email: *challagundlav@yahoo.co.in
Received October 15th, 2012; revised November 26th, 2012; accepted December 20th, 2012
Keywords: Mentha piperita; Antibacterial Activity; Phytochemical Analysis
ABSTRACT
Mentha piperita L. (Peppermint) is a perennial glabrous and strongly scented herb belonging to family Lamiaceae. The plant is aromatic, stimulant and used for allaying nausea, headache and vomiting. Its oil is one of the most popular widely used essential oils in food products, cosmetics, pharmaceuticals, dental preparations, mouthwashes, soaps and alcoholic liquors. The antibacterial potential of six extracts from leaf, stem and root of Mentha piperita against pathogenic bacteria such as Bacillus subtilis, Streptococcus pneumonia, Staphylococcus aureus, Escherichia coli, Proteus vulgaris and Klebsiella pneumonia were evaluated by agar well diffusion method. The organic (ethanol, methanol, ethyl acetate, chloroform, hexane and petroleum ether) extracts of the leaves were found to possess strong antibacterial activity against a range of pathogenic bacteria. The ethyl acetate leaf extract of Mentha piperita showed pronounced inhibition than chloroform, petroleum ether and hexane. The leaf extract activity being more on Bacillus subtilis, Staphylococcus aureus and Proteus vulgaris than Escherichia coli, Streptococcus pneumonia and Klebsiella pneumonia. In the present study, we also evaluated the phytochemical analysis for the presence of various secondary metabolites. The analysis revealed the presence of alkaloids, flavonoids, steroids, tannins, and phenols.
1. Introduction
Biologically active compounds from natural sources have always been a great interest for scientists working on infectious diseases. [1]. Higher and aromatic plants have traditionally been used in folk medicine as well as to extend the shelf life of foods, showing inhibition against bacteria, fungi and yeasts [2]. The genus Mentha belonging to family Lamiaceae includes large number of species that differ widely in their characteristics and ploidy level. Mentha species are perennial and could be multiplied both by reproductive and vegetative means. Members of this family possess great pharmacological and commercial significance. Mentha piperita is a perennial plant that is found in various countries of the world both as cultivated and wild. It has been documented in the literature that Mentha piperita is used internally as a tea, tincture, oil or extracts, and applied externally as a rub or liniment. Botanists consider it as an astringent, antiseptic, antipyretic, antispasmodic, anticatarrhal, antimicrobial, rubefacient, stimulant, emmenagogue and anti-aging properties [3].
The principal active constituents of Mentha piperita are the essential oils, which comprise about 1% of the herb. The oils are dominated by monoterpenes, mainly menthol, menthone and their derivatives (e.g., isomenthone, neomenthol, acetyl menthol, pulegone). Less well recognized is peppermint’s potential role in the management of numerous medical problems, including colonoscopy [4]. Menthol and peppermint oil are fungicidal against Candida albicans, Aspergillus albus and Dermatophytic fungi. However, to the best of our knowledge, no serious efforts have been made to test the antibacterial properties of Mentha piperita so far. In the present study we established antibacterial activity of Mentha piperita against pathogenic bacteria. The study confirms that organic solvent leaf extracts possess strong antibacterial properties against various pathogenic bacteria.
2. Materials and Methods
2.1. Preparation of Leaf, Stem and Root Solvent Extracts
Healthy plants were collected, washed thoroughly in tap water and shade dried for 15 days. The leaves, stems and roots were powdered and extracted following the Essawi and Srour procedure with slight modification [5]. The powdered material was soaked in petroleum ether, chloroform, ethyl acetate, ethanol, methanol and hexane in individual beakers by keeping them in a shaker for 3 days. The extracts were filtered through cheese cloth and the extracts were reduced to 10% of its original volume. The filtrate organic solvents were concentrated in vacuum using a rota evaporator, while aqueous extracts were dried using water bath. All the extracts were subjected to antibacterial activity and phytochemical analysis.
2.2. Inoculums
The test microorganisms Bacillus subtilis, Streptococcus pneumonia, Staphylococcus aureus, Escherichia coli, Proteus vulgaris and Klebsiella pneumonia were obtained from culture repository of Best Biotech culture collection, Bangalore. The organisms were inoculated onto NB (Nutrient Broth), (0.5% Peptone, 0.5% Sodium Chloride, 0.15% Yeast extract; pH 7.4) 0and incubated at 37˚C for overnight for the growth of the bacteria.
2.3. Determination of Antibacterial Activity
The antibacterial activity of the leaf, stem and root extracts was determined using agar well diffusion method with slight modification of Perez [6]. Nutrient agar was inoculated with the given microorganisms by spreading the bacterial inoculums on the media. Wells (5 mm diameter) were punched in the agar with a cork borer and filled with plant extracts. Control wells containing neat solvents (negative control) and standard antibiotic solution (positive control) viz., Chloromphenicol (100 μg/ml) were also run parallel in the same plate. The plates were incubated at 37˚C for 24 h. The antibacterial activity was assessed by measuring the diameter of the zone of inhibition for the respective drug. The antibacterial activity of different extracts of leaf, stem and roots of Mentha piperita was made in triplicate. The diameter of the zones of inhibition in the triplicate was measured by calculating the difference between cork borer (5 mm) and the diameters of inhibition.
2.4. Phytochemical Analysis
Phytochemical analysis of all the evaporated solvent extracts was conducted following the procedure of Indian Pharmacopoeia (1985). To test for alkaloids, (200 mg plant material in 10 ml methanol, filtered); a 2ml filtrate + 1% HCl + steam, 1 ml filtrate + 6 drops of Mayer’s reagents/Wagner’s reagent/Dragendroff reagent, creamish precipitate/brownish-red precipitate/orange precipitate indicated the presence of respective alkaloids. For tannins (200 mg plant material in 10 ml distilled water, filtered); a 2 ml filtrate + 2 ml FeCl3, blue-black precipitate indicated the presence of tannins. For flavonoids (200 mg plant material in 10 ml ethanol, filtered); a 2 ml filtrate + conc. HCl + magnesium ribbon pink-tomato red colour indicated the presence of flavonoids. Steroids (Liebermann-Burchard reaction: 200 mg plant material in 10 ml chloroform, filtered); a 2 ml filtrate + 2 ml acetic anhydride + conc. H2SO4. Blue-green ring indicated the presence of steroids. For phenols, 1ml of each solvent extracts dissolved in alcohol or water was separately treated with a few ml of neutral ferric chloride solution. The change in colour indicated the presence of phenols.
3. Results
The various crude extracts of Mentha piperita showed significant activity against all the bacteria tested. The antibacterial activity of the Mentha piperita was assessed using the agar well diffusion method by measuring the diameter of growth inhibition zones and its subsequent concentration was tabulated and represented in Table 1 and Figure 1. Among all the extracts, ethyl acetate, chloroform and ethanol leaf extracts showed high activity (7.2 - 15.3 mm of zone of inhibition) on all organisms. All the leaf extracts (ethanol, methanol, ethyl acetate and chloroform) except hexane and petroleum ether showed high activity (6 - 12 mm) when compared to the stem extracts (ethanol, methanol, ethyl acetate, chloroform and hexane) which showed a moderate activity (5 - 10 mm). The root extracts (chloroform, hexane and petroleum ether) did not show any activity on Streptococcus pneumonia, Staphylococcus aureus, Escherichia coli, Proteus vulgaris and Klebsiella pneumonia. Ethanol extract of root showed activity (3 - 6 mm) against Bacillus subtilis, Streptococcus pneumonia, Escherichia coli, and Proteus vulgaris. Hexane extract of root showed a less activity on Streptococcus pneumonia (2.4 mm) and it did not show any effect on other organisms. The obtained results of the crude extracts are comparable with the standard antibiotics such as kanamycin, penicillin, cefotaxime and tetracycline. All the tested organisms are highly sensitive to the ethyl acetate leaf extract of Mentha piperita (5.8 - 14.7 mm) than the standard penicillin, cefotaxime and tetracycline antibiotics. Kanamycin showed more or less similar activity (9.0 - 21.2 mm) on the tested organisms when compared with the ethyl acetate leaf extract (7.2 - 15.3 mm).
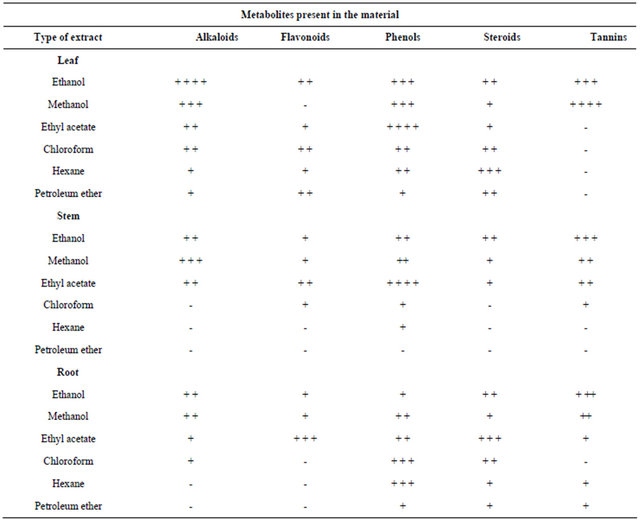
Preliminary phytochemical analysis of crude organic extracts of tissue cultured and field grown plants of Mentha piperita was carried out. The results of the phytochemical analysis of different extracts showed that
Table 1. Susceptibility of test bacterial strains to leaf, stem and root extracts of Mentha piperita and standard antibiotics.

(-) No inhibition.
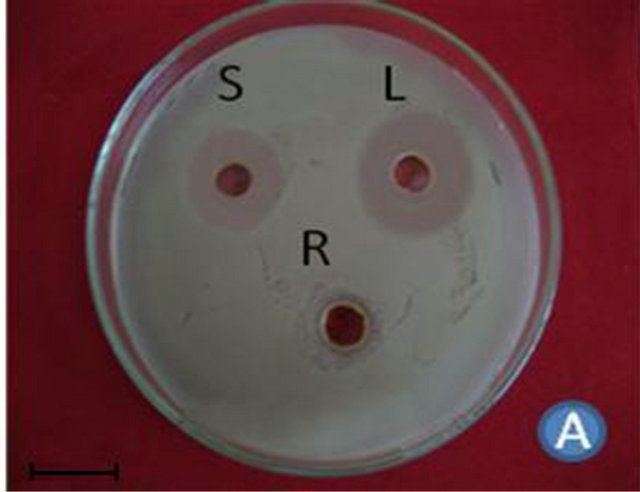

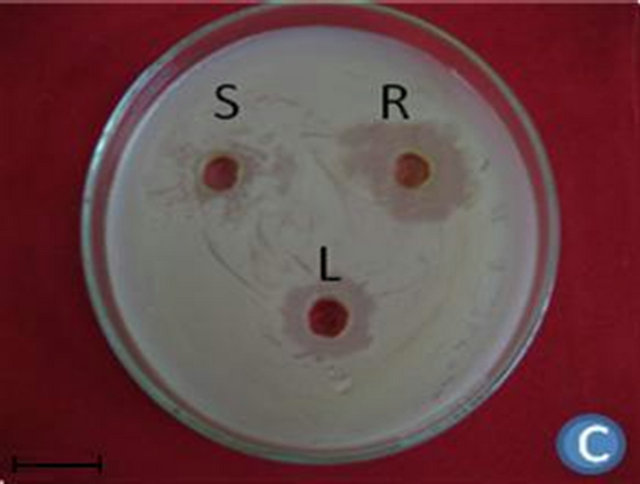
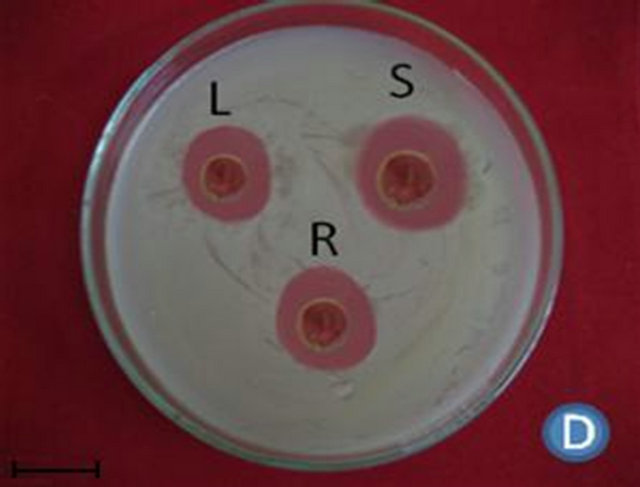

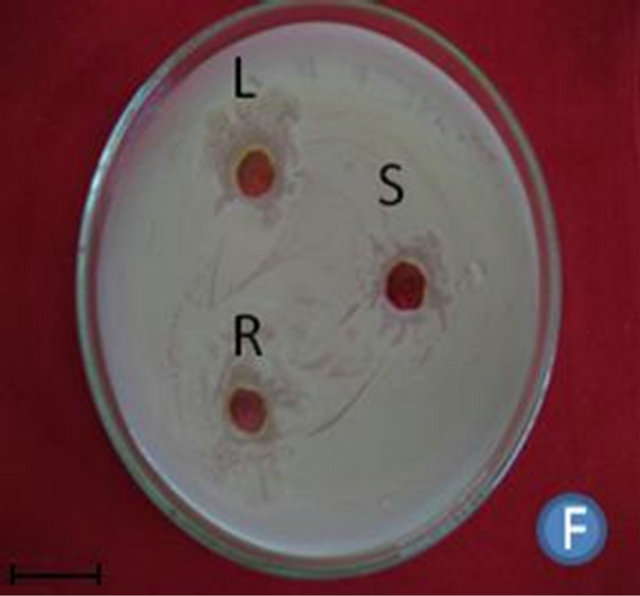
Figure 1. (A) Highest antibacterial activity exhibited by ethyl acetate leaf extract against Bacillus subtilis; (B) Highest antibacterial activity exhibited by ethanol and chloroform stem extracts against Escherichia coli; (C) Highest antibacterial activity exhibited by ethanol and ethyl acetate root extracts against Bacillus subtilis and Streptococcus pneumonia; (D) Least antibacterial activity shown by hexane leaf extract against Klebsiella pneumonia; (E) Least antibacterial activity shown by petroleum ether stem extract against Proteus vulgaris; (F) Least antibacterial activity shown by hexane root extract against Streptococcus pneumonia.
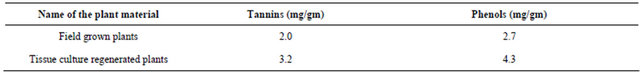
each of the plant part tested are rich in at least one of alkaloids, flavonoids, phenols, tannins and steroids (Table 2). Higher concentrations of alkaloids were present in the ethanol, methanol and ethyl acetate leaf extracts when compared to the stem and root extracts. Quantitative analysis of tannins and phenols revealed that more phenols (4.3 mg/gm) and tannins (3.2 mg/gm) were present in tissue cultured plants followed by the field grown plants (Table 3).
4. Discussion
On overall, leaf extracts are very active against all the tested bacterial strains followed by stem extracts and root extracts. Antibacterial properties of leaf and stem extracts against these bacteria suggests that the leaf and stem extracts can be used for wound healing and septicemia. Similar results were also reported in Adhatoda vasica as wound healing agent [7,8]. Staphylococcus
Table 2. Phytochemical analysis of secondary metabolites such as alkaloids, flavonoids, phenols, steroids, tannins in different plant parts of Mentha piperita
+ Less; + + Moderate; + + + High; + + + + very high.
Table 3. Estimation of total tannins and phenols present in field grown plants and tissue culture regenerated plants of Mentha piperita.
aureus is one of the causative bacterium in community acquired pneumonia and blood sepsis. Escherichia coli and Staphylococcus aureus are one of the causative organisms of hospital acquired pneumonia [9]. The obtained results suggest the presence of an active principle which is active against these microbes. Singh reported the antimicrobial action of natural products from Sphaeranthus indicus [10]. From these studies it is obvious that the active principles are confined to the aerial parts of this plant especially in leaves. Even the presence of alkaloids, flavonoids, steroids, phenols and tannins were recorded in the root extracts but failed to kill the test organisms to the most extent. The presence of some of the secondary metabolites reported by the earlier workers from the leaves such as eudesmanoids [11], isoflavone glycosides [12] and essential oils may be the cause of the antibacterial activity of this plant. It is evident from the literature that, the phenols [13], tannins
[14,15], terpenoids [16], flavonoids and flavonoid glycosides [17] are active against a wide range of microbes.
Diterpenoid alkaloids, commonly isolated from the plants of Ranunculaceae or buttercup family [15], are commonly found to have antimicrobial properties. Flavonoids were present in higher concentrations in ethyl acetate root extracts, when compared to stem and leaf. Flavonoids are known to be synthesized by plants in response to microbial infection and should not be surprising that they have been found in vitro to be effective antimicrobial substances against a wide array of microorganisms. Their activity is probably due to their ability to complex with extracellular and soluble proteins and to complex with bacterial cell walls. More lipophilic flavonoids may also disrupt microbial membranes [17]. Higher concentrations of phenols were recorded in the ethanol, methanol and ethyl acetate leaf extracts followed by chloroform and hexane root extracts when compared to the stem extracts. High amount of steroids were detected in hexane leaf extracts. Moderate steroid concentration was seen in chloroform, hexane and petroleum ether root extracts, whereas steroids were absent in the chloroform, hexane and petroleum ether extracts of stem. Tannins were absent in ethyl acetate, chloroform, hexane and petroleum ether extracts of leaf followed by hexane and petroleum ether extracts of stem. But higher concentrations were seen in ethanol and methanol leaf extracts, followed by ethanol stem extracts and root extracts.
Tannins have received a great deal of attention in recent years, since it was suggested that the consumption of tannin-containing beverages, especially green teas and red wines, can cure or prevent a variety of ills [18]. Scalbert reviewed the antimicrobial properties of tannins in 1991. According to these studies, tannins can be toxic to filamentous fungi, yeast, and bacteria. Condensed tannins have been determined to bind cell walls of ruminal bacteria, preventing growth and protease activity [15].
The present work shows that the compounds from Mentha piperita possess potent antimicrobial activity and suggesting that the Mentha piperita leaf extracts contain the effective active constituents responsible for eliminating the bacterial pathogens and also the present study reveals that more phenols and tannins were present in the tissue cultured plants followed by field grown plants. Same types of results were observed in Bacopa monnieri [19].
5. Conclusion
As the work for the development of herbal medicines is in progress worldwide, the present report will help in the isolation of new products/drugs. Finally, it can be concluded that the active chemical compounds present in Mentha piperita should certainly find place in treatment of various bacterial infections and indicate this herb should be studied more extensively to explore its potential in the treatment of infectious diseases as well.
6. Acknowledgements
Ms. P.Sujana would like to thank UGC, New Delhi for granting major research project and giving financial assistance in the form of fellowship.
REFERENCES
- R. P. Samy and S. Ignacimuthu, “Antibacterial Activity of Some Medicinal Plants from Eastern Ghats, South India, Solai Bull,” Ethanopharmacology, Vol. 72, No. 1, 2000, pp. 39 - 41.
- V. Hulin, A. G. Mathot, R. Mafart and L. Dufosse, “Les Proprieties Anti Microbiennes des Huiles Essentielles et Composes Daromes. (Antimicrobial Properties of Essential Oils and Flavour Compounds),” Science Aliments, Vol. 18, No. 6, 1998, pp. 563-582.
- M. A. Ali, M. Saleem, W. Ahmad, M. Parvez and R. Yamdagni, “A Chlorinated Monoterpene Ketone, Acylated-Sitosterol Glycosides and a Flavanone Glycoside from Mentha longifolia (Lamiaceae),” Phytochemistry, Vol. 59, No. 8, 2002, pp. 889-895. doi:10.1016/S0031-9422(01)00490-3
- L. I. Spirling and I. R. Daniels, “Botanical Perspectives on Health Peppermint: More than Just an After-Dinner Mint,” Perspectives in Public Health, Vol. 121, No. 1, 2001, pp. 62-63. doi:10.1177/146642400112100113
- T. Essawi and M. Srour, “Screening of Some Palestian Medicinal Plants for Antibacterial Activity,” Journal of Ethanopharmacology, Vol. 70, No. 3, 2000, pp. 343-349. doi:10.1016/S0378-8741(99)00187-7
- C. Perez, M. Pauli and P. Bazerque, “An Antibiotic Assay by Agar Well Diffusion Method,” Acta Biological et Medica Experimentalis, Vol. 15, No. 1, 1990, pp. 113- 115.
- M. K. Bhargava, H. Singh and A. Kumar, “Evalution of Adhatoda vasica as a Wound Healing Agent in Buffaloes—Clinical Mechanical and Biochemical Studies,” Indian Veterinary Journal, Vol. 65, No. 1, 1988, pp. 33-38.
- S. R. Thaakur, “Antibacterial Activity of Adhatoda vasica Leaf Extract,” Asian Journal of Microbiology, Biotechnology and Environmental Science, Vol. 8, No. 2, 2006, pp. 287-289.
- S. J. Pedler and A. W. Berington, “Respiratory Infections,” In: R. Walker and C. Edwards, Eds., Clinical Pharmacy and Therapeutics, Churchill Livingstone Publications, Philadelphia, 2003, pp. 519-529.
- S. K. Singh, K. Saroj, U. J. Tirupathi, A. K. Singh and R. H. Singh, “An Antimicrobial Principle from Sphaeranthus indicus,” International Journal of Crude Drug, Vol. 26, No. 4, 1998, pp. 235-239.
- P. P. Prasad, D. O. Sawaikar, S. R. Rojatkar and B. A. Nagasampagi, “Eudesmanoids from Sphaeranthus indicus,” Fitoterapia, Vol. 71, No. 3, 2000, pp. 264-268. doi:10.1016/S0367-326X(99)00157-4
- M. S. Shekhani, P. M. Shah, A. Yasmin, R. Siddiqui, S. Perveen, K. M. Khan, S. U. Kazmia and Atta-Ur-Rahman, “An Immunostimulant Sesquiterpene Glycoside from Sphaeranthus indicus,” Phytochemistry, Vol. 29, No. 8, 1990, pp. 2573-2576. doi:10.1016/0031-9422(90)85191-H
- T. L. Mason and B. P. Wasserman, “Inactivation of Red Beet Betaglucan Synthase by Native and Oxidized Phenolic Compounds,” Phytochemistry, Vol. 26, No. 8, 1987, pp. 2197-2202. doi:10.1016/S0031-9422(00)84683-X
- A. Scalbert, “Antimicrobial Properties of Tannins,” Phytochemistry, Vol. 30, No. 12, 1991, pp. 3875-3883. doi:10.1016/0031-9422(91)83426-L
- G. A. Jones, T. A. Mc Allister, A. D. Muir and K. J. Cheng, “Effects of Sainfoin (Onobrychis viciifolia scop.) Condensed Tannins on Growth and Proteolysis by Four Strains of Ruminal Bacteria,” Applied Environmental Microbiology, Vol. 60, No. 4, 1994, pp. 1374-1378.
- M. Scortichini and M. Pia Rossi, “Preliminary in Vitro Evaluation of the Antimicrobial Activity of Terpens and Terpenoids towards Erwinia amylovora (Burrill),” Jounal of Applied Bacterialogy, Vol. 71, No. 2, 1991, pp. 109- 112. doi:10.1111/j.1365-2672.1991.tb02963.x
- H. Tsuchiya, M. Sato, T. Miyazaki, S. Fujiwara, S. Tanigaki, M. Ohyama, T. Tanaka and M. Linuma, “Comparative Study on the Antibacterial Activity of Phytochemical Flavanones against Methicillin-Resistant Staphylococcus aureus,” Journal of Ethnopharmacology, Vol. 50, No. 1, 1996, pp. 27-34. doi:10.1016/0378-8741(96)85514-0
- M. Serafini, A. Ghiselli and A. Ferro-Luzzi, “Red Wine, Tea and Anti-Oxidants,” Lancet, Vol. 344, No. 8922, 1994, pp. 344-626.
- D. Tejavathi and N. N. Rao, “Comparative Studies between Normal and Tissue Cultured Plants of Bacopa monnieri (L.) Pennel,” In: I. A. Khan and A. Khanum, Eds., Role of Biotechnology in Medicinal and Aromatic Plants, Ukaaz Publications, Hyderabad, 1998, pp. 239- 247.
NOTES
*Corresponding author.

