International Journal of Clinical Medicine
Vol.3 No.6(2012), Article ID:24734,6 pages DOI:10.4236/ijcm.2012.36093
Clinical Analysis of the Pharmacological Interactions of Clopidogrel and Gender Differences: A Case-Control Study*
![]()
Department of Pharmacy, University Hospital of Ferrara, Ferrara, Italy.
Email: s.bianchi@ospfe.it
Received July 29th, 2012; revised August 26th, 2012; accepted September 14th, 2012
Keywords: Antiplatelet Activity; Plus Proton-Pump Inhibitors; Gender
ABSTRACT
Background: Clopidogrel is a prodrug metabolized by cytochrome P450-2C19. Drugs inhibiting this enzyme might reduce its antiplatelet activity. In order to reduce gastrointestinal bleedings, proton-pump inhibitors are usually prescribed in association with clopidogrel. The study aims at assessing the clinical importance of interactions between clopidogrel and inhibitors of CYP2C19. It also aims to evaluate any possible factors that may reduce the therapeutic efficacy of the drug. Particular attention was devoted to possible gender differences in responsiveness to treatment with clopidogrel or clopidogrel plus proton-pump inhibitors. This analysis is a retrospective case-control observational study carried out by the University Hospital of Ferrara. Methods: Subjects were patients who had received clopidogrel from 01-01-2008 to 31-12-2008. For them, we analysed hospital admissions and data of drug prescriptions relative to dispensing of drugs cytochrome P-450-2C19 inhibitors. Patients were subdivided into case and control groups based on the occurrence or not of cardiovascular or cerebrovascular secondary events during therapy with clopidogrel. Results: The study focused on 781 patients, 20.1% of which (n.157) experienced secondary effects. The mean age is 70 years old. Men (67% of the analyzed population) experienced secondary events more than women (OR 1.54; CI 95% 1.04 - 2.28; p < 0.03). 70% of patients took PPIs and we noticed that the risk of secondary events increased by 2.2% with respect to the remaining patients (20.77% vs 18.57%; OR 1.15; CI 0.78 - 1.70; p = NS). Among PPIs, lansoprazole is the most used. For this subgroup the risk is 5.2% higher (risk in those exposed of 23.75% vs 18.57% in those not exposed; or 1.37, 95% CI 0.92 - 2.03; p = NS). The interaction with PPIs is particularly interesting only among women, with a risk 6.3% higher (17.46% exposed, 11.11% non exposed). The risk remains the same among men. Conclusions: Analyzed data show an increase in cardiovascular or cerebral secondary events for patients exposed to PPIs. It also demonstrated the existence of differrent gender in therapeutic response to clopidogrel.
1. Introduction
The prodrug Clopidogrel is a thienopyridine class antiplatelet agent used to treat patients affected by acute coronary artery disease, myocardial infarct, and ischemic stroke. The efficacy of clopidogrel therapy has shown great variability among individuals [1], often correlated to alterations in the cytochrome P450-2C19 activity. In fact, it is the main enzyme which metabolises the drug, is polymorphic and the most common polymorphisms are often responsible for a reduced function of the enzyme.
Since CYP2C19 poor metabolizers have significantly lower plasma levels of the active metabolite, as well as a reduced antiplatelet activity [1] when treatment is required it would be advisable to carry out a genotype test in order to assess whether the patient would respond well to clopidogrel therapy or whether alternative treatment may be more efficacious [2]. The frequency of CYP2C19 polymorphisms vary according to ethnic group, being present in 30% of Caucasians, in 40% of Blacks, and in 55% of Asiatics [3]. One aspect which has been given little attention is that genetic polymorphism may vary between the sexes. This may be due to the fact that gender differences in responsiveness to drugs has only been studied since the early nineties, although it is a subject of research that is arousing increasing interest.
Besides genetic polymorphism, alterations in the pharmacological effect of clopidogrel may also occur as a result of interactions between drugs. This problem has emerged over the past few years particularly in connection with the possible interaction between clopidogrel and proton-pump inhibitors (PPIs) are often prescribed to reduce the occurrence of gastrointestinal bleeding and are enzyme inhibitors of the cytochrome P450-2C19 [4,5]. Many studies [6-8] have revealed a reduction in aggregation of platelets due to PPI interaction in patients taking clopidogrel, but there are conflicting opinions as to whether their concomitant use can reduce the benefits of the antiaggregation therapy and increase the risk of future cardiovascular events [9-16]. The interaction between clopidogrel and proton-pump inhibitors may, however, derive from an effect linked to the single molecule rather than to the class itself [6,7,11,12,17,18]; should the enzymatic inhibition be responsible for the decreased therapeutic effects of clopidogrel, then caution would be advisable when administering other enzymatic inhibitors besides PPIs during antiaggregation therapy.
The aim of the study was to verify the clinical importance of the interaction between clopidogrel and inhibittors of the cytochrome 2C19, by analysing the number of prescriptions dispensed within the University Hospital of Ferrara, which is a provincial hospital serving about 160,000 people. A further aim was to evaluate any possible factors that may reduce the therapeutic efficacy of the drug and expose the patient to a higher risk of secondary events of a cardiovascular or cerebrovascular nature. Particular attention was also devoted to possible gender differences in responsiveness to treatment with clopidogrel.
2. Methods
Data Collection
The collection of data was obtained from:
1) The Pharmacy Dispensary Unit of the University Hospital of Ferrara in relation to the dispensing of clopidogrel (75 mg), PPIs, fluoxetine and sertraline, in the period from 01/01/2008 to 31/12/2008;
2) The computer management system of hospital admissions at the University Hospital of Ferrara. Case history were not available, but we analysed extraction of data relative to admissions of patients affected by cardiovascular and cerebrovascular diseases from 01/01/2007 to 31/12/2008 for the following pathologies correlated to use of clopidogrel [19]:
• Ischemic events such as acute myocardial infarct (AMI) and other acute and subacute forms of ischemic cardiomyopathy, chronic ischemic cardiomyopathy, and angina pectoris;
• Cerebrovascular diseases such as intracranial haemorrhage, occlusion and stenosis of cerebral and precerebral arteries, transient cerebral ischemia and other cerebrovascular diseases;
• Insertion of drug or non-drug eluting coronary artery stent(s).
Data of drug prescriptions in the territory of the Province of Ferrara relative to dispensing of PPIs (esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole), sertraline and fluoxetine over the period 01/01/2008 to 31/12/2008.
3. Study Design and Patient Recruitment
Analysis of the data was carried out by means of retrospective-observational case-control study (Figure 1).
Subjects were patients who had received clopidogrel from the Dispensing Pharmacy Service at the University Hospital of Ferrara, from 01-01 to 31-12 2008. Patients with less than 20% compliance or treatment less than or equal of one month were excluded because their exposure to the drug was too short to reveal any drug interactions.
3.1. Subjects
This study was approved by the Ethics Committee of Ferrara on 30/09/2010 and conducted by applying the legislative decree of June 24, 2003, No. 211 of Good Clinical Practice in clinical trials of medicines for clinical use. The study was conducted in accordance with the Declaration of Helsinki.
3.2. Data Processing
Data was organised into files containing patient card number, date of birth, age at the end of the year 2008, and sex. Patients prescribed clopidogrel were subdivided into two groups: those who experienced a recurrence of cardiovascular or cerebrovascular events (case group) and those who did not (control group). In fact these events could be correlated to a reduction in or loss of the drug’s antiaggregation effect of clopidogrel. The recurrence was defined by both hospital admission and drug prescription. The case group was made up of patients with:
• Drug prescriptions for acute coronary syndromes (ACS) or stent surgery, both medicated and unmedicated, occurring during therapy with clopidogrel;
• Hospitalisation during therapy with clopidogrel for the following pathologies:
♦ Stent surgery;
♦ Acute myocardial infarction;
♦ Intermediate coronary syndrome;
♦ Occlusion and stenosis of precerebral arteries, only
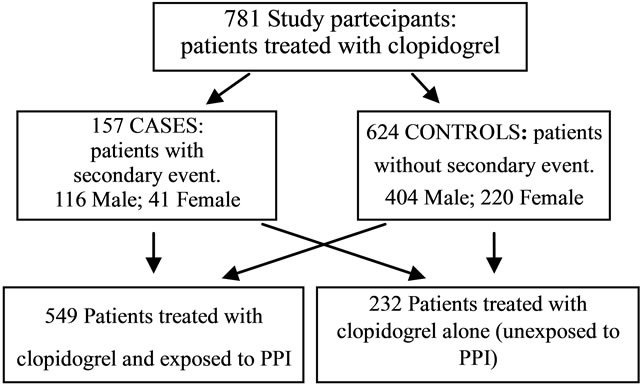
Figure 1. Characteristics of study patients.
with mention of cerebral infarction;
♦ Occlusion of cerebral arteries, only with mention of cerebral infarction;
♦ Transient cerbral ischemia;
♦ Lower extremity emboli and deep vein thrombosis.
Both groups were further divided into subgroups of patients exposed or not exposed to CYP 2C19 inhibitors. The exposure of interest was prolonged therapy (more than one packet of drugs) with PPIs (omeprazole, lansoprazole, esomeprazole, rabeprazole and pantoprazole), fluoxetine and sertraline. In fact, it was assumed that prolonged therapy could be taken at the same time as treatment with clopidogrel and, therefore, could cause interactions between drugs. Exposure to these drugs was evaluated in both case and control groups. Firstly, all the P450-2C19 cytochrome inhibitors were assessed since they have the same mechanism of interaction. Secondly, a further analysis was carried out considering both the number of concomitant therapies and the single drugs administered in order to verify whether the percentage of exposures to CYP2C19 inhibitors varied between the case and control groups in relation to drug type. The purpose of this analysis was to reveal any possible differences in interaction with the cytochrome on the part of the inhibitors and possible clinical complications of differing importance.
Define abbreviations and acronyms the first time they are used in the text, even after they have been defined in the abstract. Abbreviations such as IEEE, SI, MKS, CGS, sc, dc, and rms do not have to be defined. Do not use abbreviations in the title or heads unless they are unavoidable.
3.3. Statistical Method
Data analysis was carried out using the Odds Ratio and the Chi-Square test.
4. Result
The study population was composed of 781 patients who had taken clopidogrel from 01-01-2008 to 31-12-2008 (Table 1).
The mean treatment period was calculated as 291 days in relation to the length of prescribed treatment proposed, the mean compliance was 87% (dev.st. ±25%). Of the 781 patients included in the study, 157 made up the group of cases and 624 the controls, therefore 20.1% of patients treated with clopidogrel had recurrence of car-
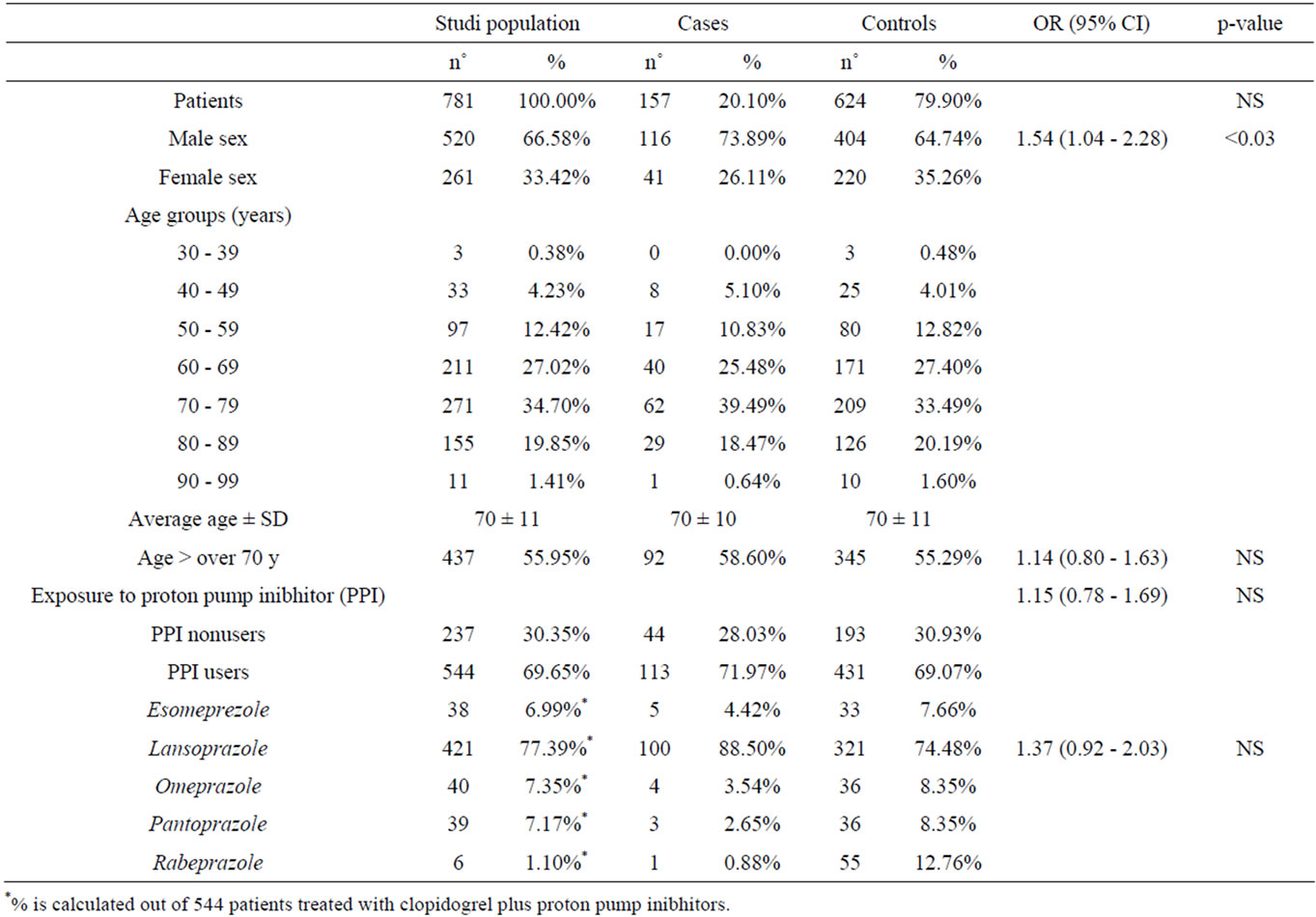
Table 1. Characteristic of patients.
diovascular or cerebrovascular events during antiaggregation therapy.
The mean age of patients in the study was 70 years, for both the study population as a whole and for the separate analysis by case and control groups. The distribution of patients into the various age groups showed how treatment with clopidogrel was greater in elderly patients, with over half of the population falling within an age range of between 60 and 79 years. The study group was composed of 67% males and 33% females.
Exposure to PPI during clopidogrel terapy concerned 70% of the patients recruited in the study. This patient group was noticeably older (mean age 71 years) compared to the patients not taking PPIs during treatment with clopidogrel (mean age 68 years). Population distribution in relation to drug type (Figure 2) revealed greater use of lansoprazole compared to other PPIs.
In accordance with the aim of the study, the data analysis firstly concerned the comparison between the incidence of recurrences in patients undergoing treatment with clopidogrel alone and those on therapy with clopidogrel + PPIs. Exposure to proton-pump inhibitors was revealed in 72% of patients (n.113) in the case group, and in 69% (n.431) of controls, or rather among patients in whom no recurrences had been revealed while on therapy with clopidogrel. Statistical analysis of these data showed an increased risk of recurrences of 2.207% (risk in those exposed of 20.77% vs 18.57% in those not exposed; OR 1.15; 95% CI 0.78 - 1.69; p = NS).
Later, analysis of the subgroups in relation to type of concomitant therapy was performed. In the study population, lansoprazole was taken by 77% of patients undergoing a concomitant therapy to clopidogrel and it was the only subgroup in which it was possible to make an in depth analysis: exposure to lansoprazole concerned 89% of patients in the case group and 74% in the control group. The statistical analysis was carried comparing patients undergoing therapy with clopidogrel and lansoprazole with those taking clopidogrel alone. Patients being treated with other proton-pump inhibitors were excluded in order to reduce the possibility of bias. The analysis revealed a difference in the risk, following exposure to lansoprazole, of 5.188% (risk in those exposed of 23.75% vs 18.57% in those not exposed; OR 1.37, 95% CI 0.92 - 2.03; p = NS).
The data were further analysed in order to verify the possibility of other variables which might be correlated to a loss of the therapeutic effect of clopidogrel. Age was not shown to affect the drug’s efficacy, whereas interesting data emerged regarding gender differences in responsiveness to clopidogrel therapy. The case group was composed of 74% males and 26% females, such percentages differ from those of the control group (Figure 3).
Statistical analysis of the frequency of recurrences in
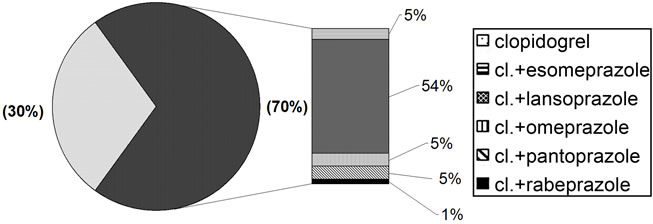
Figure 2. Drugs taken by the study population.
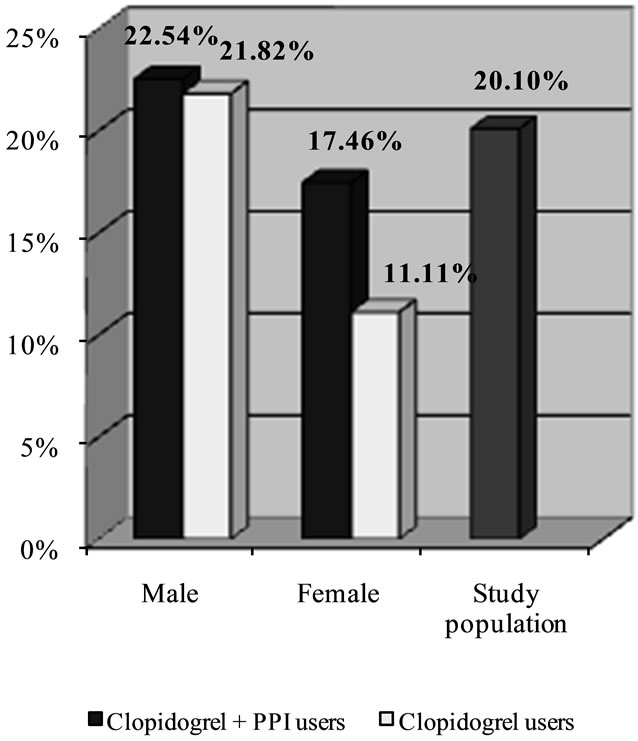
Figure 3. Percentage of risk of secondary cardiovascular events or celebrovascular disease in patients treated with clopidogrel plus PPIs versus clopidogrel alone.
the females as compared to the males revealed that the former had a lower incidence of cardiovascular or cerebrovascular secondary events while undergoing therapy with clopidogrel (OR 1.54; 95% CI 1.04 - 2.28; p < 0.03; Table 2).
The final analysis concerned the possible clinical relevance of the interaction between clopidogrel and PPIs in the male and female groups, separately. Differences were also observed here: in females, exposure to the protonpump inhibitors was linked to an increased risk of recurrences (17.46% in women exposed vs 11.11% in those not exposed; p = NS), whereas the percentage of risk was more or less unchanged in males (22.54% vs 21.82%; p = NS).
5. Discussion
The first relevant data in the study is the percentage of recurrence in patients treated with clopidogrel, which is of 20.1%, as confirmed by other studies [10,20]. This
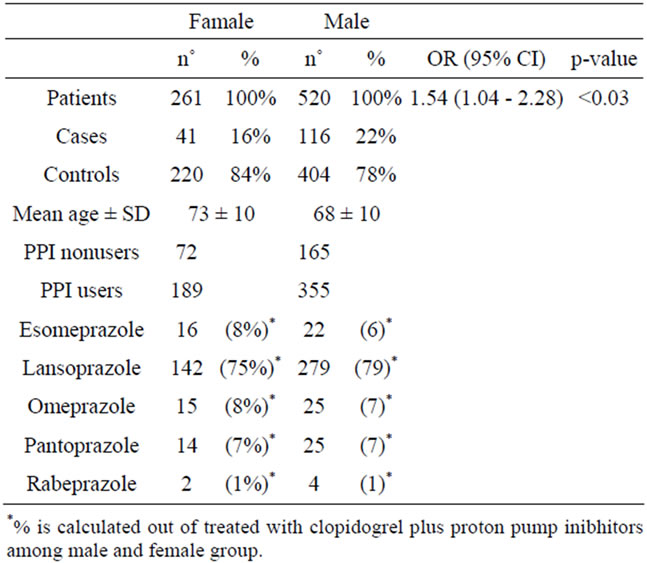
Table 2. Characteristics of study patients.
may mean a failed therapeutic effectiveness of the drug and it is therefore important, in the clinical practice, to discover the possible generating factors.
Exposure to PPI drugs concerned a remarkable percentage of patients recruited in the study (70%) because in the existing conditions (age and antiaggregation therapy) gastroprotective treatment was needed.
This work has highlighted the increased risk of cardiovascular and cerebrovascular recurrences in patients undergoing therapy with clopidogrel who are exposed to proton pump inhibitors as compared to those who are not exposed to PPIs It is still discussed whether or not the interaction between clopidogrel and PPIs is a class effect, for this reason a subgroups analysis has been carried following the type of concomitant therapy. The purpose was to verify whether there were any differences in the level of interaction between clopidogrel and the various PPIs because the various proton-pump inhibitors present a different affinity towards hepatic cytochromes [17].
The prevailing use of lansoprazolo (70% study population) compared to the other inhibitors was due to the fact that the drug is included in the Provincial Pharmaceutical Reference, and is, therefore, dispensed to patients on discharge from hospital and later repeated by their G.Ps. Analysis of this hypothesis produced interesting data concerning the prescription of PPIs in patients being treated with clopidogrel in the territory of Ferrara: of all the patients included in the study, 457 (59%) received a prescription of lansoprazole for a longer period of time than that covered by a single packet of the drug, 314 (69%) of whom had received a hospital prescription for the drug in 2008, and 270 continued therapy with PPI at home. In particular, 257 (95%) patients continued to be prescribed lansoprazole, whereas in 5% of cases the drug was substituted with other PPI. Finally, 143 patients undergoing treatment with lansoprazole were prescribed the drug exclusively within the territory. Analysis of patients on clopidogrel and lansoprazole therapy confirmed the results of the overall analysis.
The gender differences revealed by the study were found to be significant: the possibility that therapy with clopidogrel may be more efficacious, or rather may determine a lower incidence of cardiovascular or cerebrovascular recurrences in females as compared to males (p < 0.03) would warrant further study. This finding may be due to the link between the genetic polymorphism of the P450-2C19 cytochrome with a higher frequency of poor metabolizers in male population compared to the female population. The lack of available data regarding the genetic make-up of the study population did not allow us to confirm this hypothesis.
Females, moreover, showed marked differences in treatment with clopidogrel whether they were on concomitant therapy with PPIs or not. This may depend on the different metabolic capacity between females and males.
6. Conclusions
In spite of the intrinsic limitation posed by an observational type of study design, this work highlights the prescriptive and clinical condition at the University Hospital of Ferrara.The small population in relation with the large number of variables which may interfere with the therapeutic effectiveness of the drug can explain the failed statistical significance of the results. However, a careful analysis of the patient before choosing both the antiaggregation therapy and a possible related gastroprotective treatment becomes important.
The gender difference in the antiaggregation response to clopidrogrel is the most relevant and meaningful data in the study and should be further investigated.
7. Authors’ Contributions
SB defined the design of this study, establishing the number of patients to be enrolled, the number of prescriptions by therapeutic analysis, and the type of data needed to achieve the defined objectives. SB submitted the study to the Ethics Committee of Ferrara, established the protocols for statistical data analysis, continuously monitored the data obtained, and contributed to the writing and revision of the article. SB defined operating modes, introducing the DDD for comparison of treatments in the dosage given. CC analyzed the requirements of patients and processed the data to obtain patient characteristics, types of drugs prescribed, dosages used in therapy, changes of drug or dosing and possible associations. EB participated in drafting and revising the article.
PS evaluated the data obtained and the execution of the study, also participating in the drafting and revision of the article.
All authors read and approved the final manuscript.
REFERENCES
- J. L. Mega, S. L. Close, S. D. Wiviott, et al., “Cytochrome P-450 Polymorphisms and Response to Clopidogrel,” New England Journal of Medicine, Vol. 360, 2009, pp. 354-362. doi:10.1056/NEJMoa0809171
- FDA Drug Safety Communication, “Reduced Effectiveness of Plavix (Clopidogrel) in Patients Who Are Poor Metabloizers of the Drug,” 2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm
- FDA, “Early Communication about an Ongoing Safety Review of Clopidogrel Bisulfate,” 2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformation forHeathcareProfessionals/ucm079520.htm
- Clopidogrel RCP, 2010. http://www.ema.europa.eu/humandocs/Humans/EPAR/clopidogreltevapharma/clopidogreltevapharma.htm
- P450 Drug Interaction Table. Division of Clinical Pharmacology, Indiana University Department of Medicine, 2010. http://medicine.iupui.edu/clinpharm/DDIs/table.asp
- M. Gilard, B. Arnaud, G. Le Gal, J. F. Abgrall and J. Boschat, “Influence of Omeprazole on the Antiplatelet Action of Clopidogrel Associated with Aspirin,” Journal of Thrombosis and Haemostasis, Vol. 4, No. 11, 2006, pp. 2508-2509. doi:10.1111/j.1538-7836.2006.02162.x
- M. Gilard, B. Arnaud, J. C. Cornily, G. Le Gal, K. Lucut, G. Le Calvez, J. Mansourati, D. Mottier, J. F. Abgrall and J. Boschat, “Influence of Omeprazole on the Antiplatelet Action of Clopidogrel Associated with Aspirin: The Randomized, Double-Blind OCLA Study,” Journal of the American College of Cardiology, Vol. 51, No. 3, 2008, pp. 256-260. doi:10.1016/j.jacc.2007.06.064
- S. D. Wiviott, D. Trenk, A. L. Frelinger, et al., “Prasugrel Compared with High Loading and Maintenance Dose Clopidogrel in Patients with Planned Percutaneous Coronary Intervention: The Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 Trials,” Circulation, Vol. 116, 2007, pp. 2923-2932. doi:10.1161/CIRCULATIONAHA.107.740324
- M. L. O’Donoghue, et al., “Pharmacodynamic Effect and Clinical Efficacy of Clopidogrel and Prasugrel with or without a Proton-Pump Inhibitor: An Analysis of Two Randomised Trials,” Lancet, Vol. 374, No. 9694, 2009, pp. 989-997. doi:10.1016/S0140-6736(09)61525-7
- P. M. Ho, et al., “Risk of Adverse Outcomes Associated with Concomitant Use of Clopidogrel and Proton Pump Inhibitors Following Acute Coronary Syndrome,” Journal of the American Medical Association, Vol. 301, No. 9, 2009, pp. 937-944. doi:10.1001/jama.2009.261
- D. N. Juurlink, T. Gomes, D. T. Ko, et al., “A PopulationBased Study of the Drug Interaction between Proton Pump Inhibitors and Clopidogre,” CMAJ, Vol. 180, No. 7, 2009, pp. 1228-1229. doi:10.1503/cmaj.082001
- D. Sibbing, T. Morath, J. Stegherr, S. Braun, W. Vogt, M. Hadamitzky, A. Schvmig, A. Kastrati, N. von Beckerath, “Impact of Proton Pump Inhibitors on the Antiplatelet Effects of Clopidogrel,” Journal of Thrombosis and Haemostasis, Vol. 101, No. 4, 2009, pp. 714-719.
- J. A. Rassen, N. K. Choudhry, J. Avorn and S. Schneeweiss, “Cardiovascular Outcomes and Mortality in Patients Using Clopidogrel with Proton Pump Inhibitors after Percutaneous Coronary Intervention or Acute Coronary Syndrome,” Circulation, Vol. 120, 2009, pp. 2322- 2329. doi:10.1161/CIRCULATIONAHA.109.873497
- S. D. Wiviott, et al., “Evaluation of Prasugrel Compared with Clopidogrel in Patients with Acute Coronary Syndromes: Design and Rational for the Trial Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38),” American Heart Journal, Vol. 152, No. 4, 2006, pp. 627-635. doi:10.1016/j.ahj.2006.04.012
- S. D. Wiviott, et al., “Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes,” New England Journal of Medicine, Vol. 357, 2007, pp. 2001-2015. doi:10.1056/NEJMoa0706482
- D. Sibbing and A. Kastrati, “Risk of Combining PPIs with Thienopyridines: Fact or Fiction? Lancet, Vol. 374, No. 9694, 2009, pp. 952-954. doi:10.1016/S0140-6736(09)61562-2
- X.-Q. Li, T. B. Andersson, M. Ahlstrom and L. Weidolf, “Comparison of Inhibitory Effects of the Proton PumpInhibiting Drugs Omeprazole, Esomeprazole, Lansoprazole, Pantoprazole, and Rabeprazole on Human Cytochrome P450 Activities,” Drug Metabolism and Disposition, Vol. 32, No. 8, 2004, pp. 821-827.
- EMA, “Public Statement. Interaction between Clopidogrel and Proton Pump Inhibitors,” 2010. www.ema.europa.eu/humandocs/PDFs/EPAR/Plavix/17494810en.pdf
- ICD-9 CM, “International Classification of Diseases, 9th Revision, Clinical Modification,” Italian Version, 2002.
- Y. B. Pride, S. D. Wiviott, J. L. Buros, C. Zorkun, M. U. Tariq, E. M. Antman, E. Braunwald and C. M. Gibson, “Effect of Prasugrel versus Clopidogrel on Outcomes among Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention without Stent Implantation: A Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel (TRITON)-Thrombolysis in Myocardial Infarction (TIMI) 38 Substudy,” American Heart Journal, Vol. 158, No. 3, 2009, pp. e21-e26. doi:10.1016/j.ahj.2009.06.021
NOTES
*The authors declare that they have no competing interests.

