Open Journal of Rheumatology and Autoimmune Diseases
Vol.3 No.4(2013), Article ID:39734,4 pages DOI:10.4236/ojra.2013.34039
Profile of Secondary Bone Cancer in Brazzaville
![]()
1Rheumatology Department, University Teaching Hospital of Brazzaville, Brazzaville, Congo; 2Oncology and Radiotherapy Department, University Teaching Hospital of Brazzaville, Brazzaville, Congo.
Email: *hntsiba@yahoo.fr
Copyright © 2013 Honoré Ntsiba et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 22nd, 2013; revised October 22nd, 2013; accepted October 29th, 2013
Keywords: Bone Cancer; Metastasis; Bone Condensing; Osteolytic
ABSTRACT
Objectives: To report an epidemiology study and prognosis for metastatic bone tumor. Methodology: It was a descriptive, transversal study on records of patients hospitalized in Rheumatology and Oncology-Radiotherapy departments of the University Teaching Hospital of Brazzaville, Congo from 1 January 2005 to 31 July 2011 (7 years and 6 months). The diagnosis of bone metastasis was made because of the existence of bone pain, or pathological fracture, or bone swelling and a bone-condensing or mixed or osteolytic radiological image. The anatomo-pathological evidence was made after biopsy of the bone lesion or primary cancer. 3687 patients were hospitalized for active cancer, among them 81 had documented bone metastasis. Results: There were 60 men (74.1%) and 21 women (25.9%) with a sex ratio of 2.85. The average age was 53 years, ranging from 3 to 80 years. 75% of patients were more or equal to 50 years old at the discovery of the bone metastasis. Bone pain was the main mode of discovery (67.9% of cases). However, in 6.2% of cases, it was discovered incidentally. The metastasis was bone condensing in 50.7% of cases, osteolytic in 40.7% and mixed in 8.6%. They were unifocal in 25.9% and multifocal in 74.1% of cases. The Primary cancer most frequently found was that of the prostate in 55.6% of cases, breast in 20.7% and rhabdomyosarcoma in 4.9%. In 6.2% of cases, the primary site of cancer was unknown. The average survival was 25 months. Conclusion: The clinical and radiological presentation remains classic. Cancer of the prostate and breast are the main neoplasia responsible for bone metastasis in our series. The discovery of metastasis remains a major evolutionary step of cancer.
1. Introduction
The appearance of bone metastasis is a common and important event in the development of several types of cancers. It transforms a loco regional disease curable by local treatment into a generalized disease whose treatment is systemic and its prognosis definitively compromised [1]. Secondary bone cancer is the most common bone tumor [2], making of the bone the third site of metastasis of cancer after lung and liver. Its discovery constitutes in one over five cases the mode of revelation of primary cancer. Despite its frequency and severity, secondary bone cancer remains poorly documented in Congo as in other countries in sub-saharan Africa [3]. We have conducted a hospital-based study, whose aim was to report the clinical, radiographic and prognosis of secondary bone cancer observed in our environment.
2. Patients and Methods
This is a descriptive transversal study, on files of patients hospitalized in Rheumatology and Oncology-Radiotherapy departments of the University Teaching Hospital of Brazzaville in Congo, from 1 January 2005 to 31 July 2011, on duration of 7 years and 6 months.
The diagnosis of bone metastasis was made because of the existence of an inflammatory bone pain or spinal pathological fracture of long or flat bones, or bone swelling and the highlighting at the morphological level of an osteolytic, bone condensing or mixed radiological image. The pathological evidence is made after biopsy of the bone lesion or primary cancer when it was found. During this period, 3687 patients were hospitalized for active cancer, of which 81 had documented bone metastasis. Hospital frequency of bone metastasis was 2.32%.
Software EPI INFO version 6 was used to collect data. The statistical analysis of results was performed using SPSS version 12. Test results were considered as statistically significant for p < 5%. The survival curves were compared through the Log RANK test.
3. Results
Our series included 60 men (74.1%) and 21 women (25.9%) with a sex ratio of 2.85. The average age was 53 years, ranging from 3 to 84 years. 75% of the patients were more or equal to 50 years of age at the time of discovery of bone metastasis, with a peak incidence between 60 and 69 years in 34.5% of cases. The average consultation time was estimated at 22 months with extremes of 1 and 37 months. However, in 54.3% of cases, patients consulted relatively early, from 1 to 6 months after the onset of symptoms. Bone pain was the main mode of discovery of metastasis at the clinical level (67.9% of cases), followed by compressive neurological manifestations (17.1%). Only 8.6% of cases were revealed by a pathological fracture. Although hypercalcemia was found in 71.6% of cases, it does not constitute a mode of discovery bone metastasis and in 6.2% of cases, the discovery was accidental. The site of the bone metastasis was unifocal in 25.9% of cases, interesting mainly the lumbar spine (33.3%), femur (19.0%), pelvis (14.3%) and thoracic spine (9.5%). The disease was multifocal in 74.1% of cases. It touched the pelvis and spine in 35% of cases and in 33.3% of cases it was multi spine.
In X-ray, it was bone condensing metastasis in 50.7% of cases (Figure 1), osteolytic in 40.7% (Figure 2) and mixed in 8.6% of cases. The primary cancer most frequently found was that of the prostate, representing 55.6% of bone metastasis and breast in 20.7%, rhabdomyosarcoma in 4.9% of cases. In 6.2% of patients, the primary site of cancer was unknown. Bone metastasis was the first symptom of the primary tumor in 48.1% of cases. The average time of survival of our patients was estimated at 25 months (Figure 3). There was a significant association between the survival of patients developing bone metastasis after the detection of the primary tumor and those that developed them before (p = 0.001) in contrast, there were no association between survival of the patients and the number of bone metastasis (p = 0.5).
4. Discussion
The occurrence of bone metastasis in the development of cancer appears to be relatively low with regard to our results and the importance of cancer malignancy in hospitalization at the University Teaching Hospital of Brazzaville. The high cost of investigations and the lack of an efficient technical platform make the diagnosis uncertain and likely to affect the hospital frequency of this condition that seems underestimated in our environment [4]. Indeed, previous studies in our department [3] found a
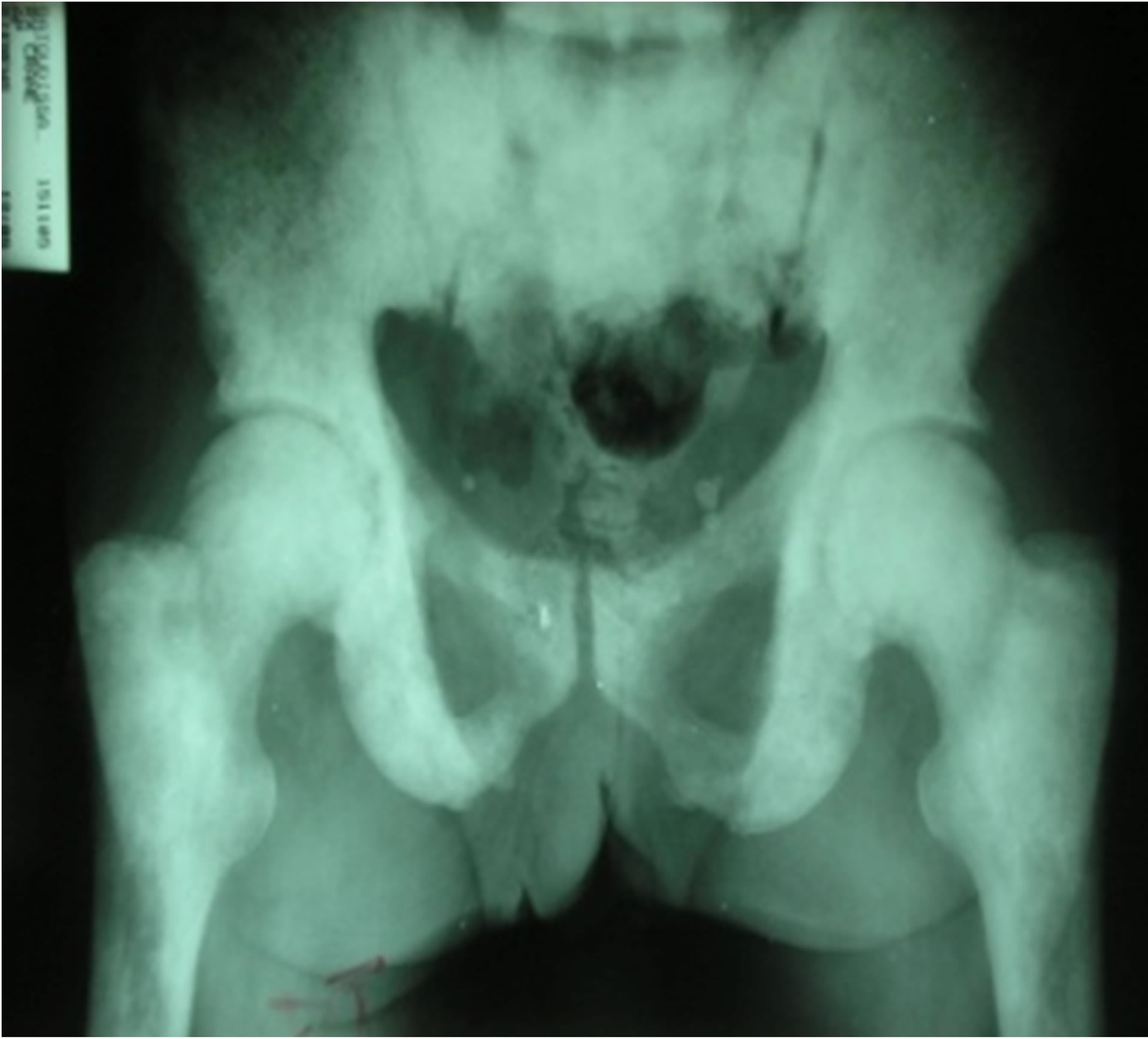
Figure 1. Bone condensing metastasis of the pelvis and upper ends of both femurs.
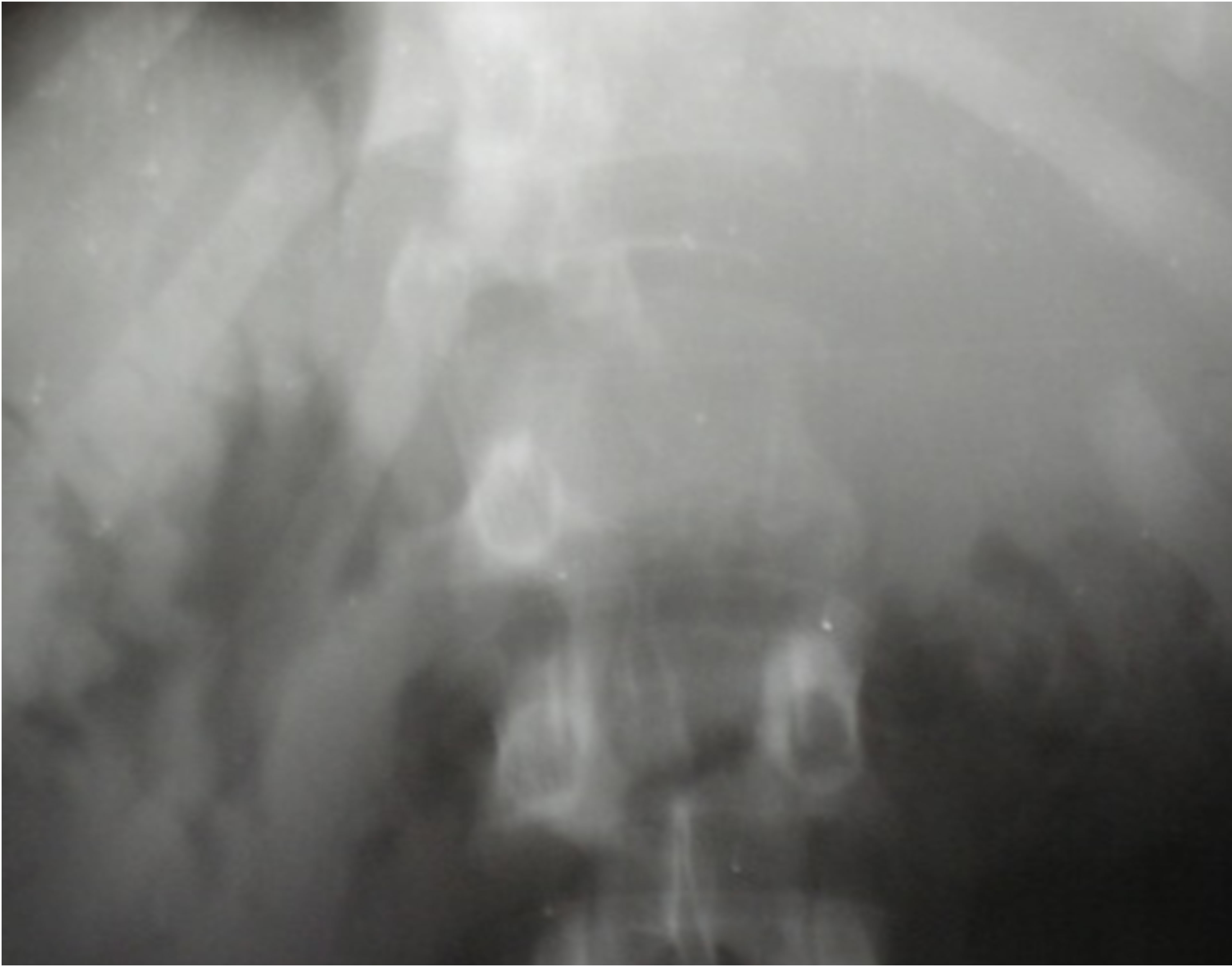
Figure 2. Osteolytic vertebral metastasis of L1.
frequency of 3.5% over a period of 6 years and 2 months. Male dominance remains classic as described by many authors [3,5,6]. It seems to be explained by the frequency of prostate cancer, a cancer of easy diagnosis in our professional context. The same is true for the average age of onset at 53 years, at least in Africa [6,7]. The appearance of metastasis seems later in western series as shown by Vandecandelaere et al. [8] and Houze et al. [9] who reported an average age of 63 and 77 years respectively. The average time before consultation for bone metastasis remains long, 22 months. Medical wandering of patients before specialist consultation is the main cause [4,10], as well as the lack of effective means of investigation for early diagnosis. This long period before consultation suggests a predominant mode of revelation of bone metastasis by bone complications, neurological or metabolic.
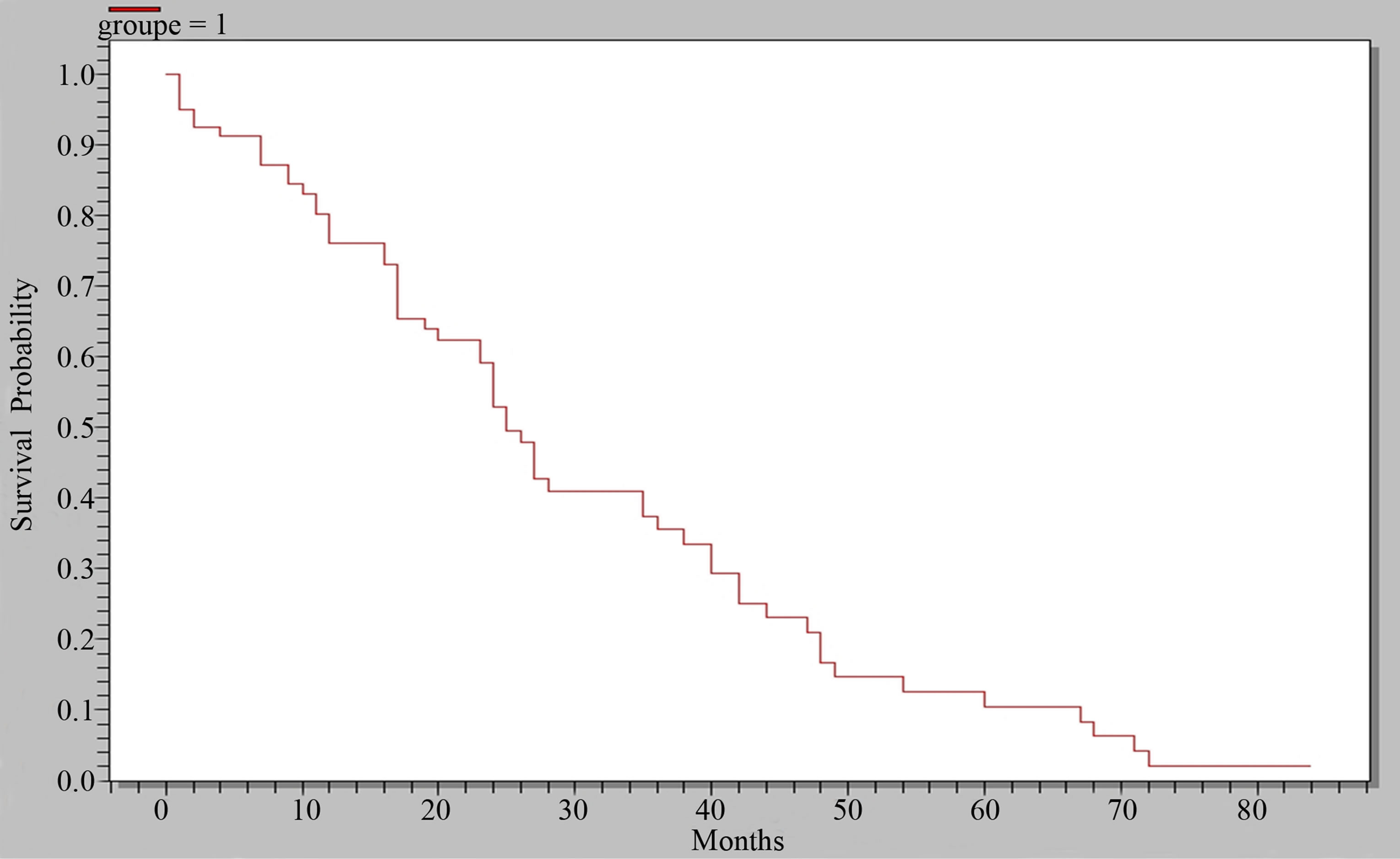
Figure 3. Curve of overall survey.
The mode of clinical revelation remains dominated by bone pain, joining results from Kagohashi in Japan [11] and Belaksir in Morocco [6]. It is present in 67.9% of patients in our series, of variable intensity ranging from simple discomfort to excruciating pain, debilitating, rebellious to the usual analgesics. It should however be noted that although hypercalcemia is frequent (71.6%); it is most often asymptomatic and is not a current mode of revelation in our study. This seems surprising given the pejorative nature of the existence of hypercalcemia during bone metastasis [12,13]. The exclusive measuring of total calcium in our series without adjustment to albumin, and the lack of systematic determination of ionized calcium, that is an active and responsible part of the usual clinical signs, may account for the false or elevated calcium levels or minimal levels.
The origin of bone metastasis is dominated by prostate cancer and breast cancer. This concerns two forms of cancer known to be osteophilic. This dominance is caused by the high frequency of these forms and their easy diagnosis in our professional context [3,5]. The osteophilic nature of the rhabdomyosarcoma is not exceptional; cases of bone metastasis have been reported by RakotoRatsimba et al. in Madagascar [14] and Nesa et al. in Belgium [15]. Two (2) patients are singled out by the presence of bone metastasis of cancer of the endometrium. This rare location for lymphophilic cancer has been reported by several authors [3,6,16].
In our series, metastases are preferentially multifocal (74.1%). The predominant axial seat of bone metastasis is well established [11,17]. Indeed, tumor cells preferably metastasize, but not exclusively, to the most richly vascularized segments of the skeleton, especially hematopoietic bone marrow of the axial skeleton, but also to the ends of the humerus, femur and tibia. The lack of recourse to more sophisticated imaging techniques, such as CT scan, magnetic resonance imaging in our series does not affect this result. Indeed, scintigraphy in addition to allowing early diagnosis of metastasis, also allows a complete mapping of locations of bone metastasis [9]. Thus, scintigraphic studies also find an axial predominance of the metastatic bone affections [18].
In X-rays, the presentation of bone metastasis remains classic, condensing, osteolytic or mixed. The predominance of bone condensing images is correlated with the frequency of bone metastasis of prostate cancer, first osteophilic responsible for such images [3,19,20]. However it does not appear to be the only responsible in our series, since 6.2% of condensing images were present in breast cancer.
The presence of metastasis at diagnosis is a wellknown poor prognosis factor. Although it widely burdens the evolution of cancerous disease in our study (statistically significant association), it cannot be considered as the only unfavorable factor. It is likely that the difficulties of management at the time of discovery including access to treatment with bisphosphonates and biotherapy (Denosumab) in our country, as well as the overall cost of cancer treatment, largely favor the evolution [4].
5. Conclusion
The clinical and radiological presentation of bone metastasis remain classic. Pain is the main revealing symptom. Conventional X-rays remain in our developing countries a reliable and inexpensive means of diagnosis of bone metastasis, despite the risk of delayed diagnosis. Prostate cancer and breast cancer are mainly responsible for bone metastasis in our series. Evolution is most often negative with average survival of 25 months, statistically correlated with the presence or absence of bone metastasis.
REFERENCES
- K. K. Li, E. Sinclair, J. Pope, et al., “A Multidisciplinary Bone Metastases Clinic at Toronto Sunnybrook Regional Cancer Centre. A Review of the Experience from 1999 to 2005,” Journal of Pain Research, Vol. 1, No. 1, 2008, pp. 43-48.
- M. S. Virk and J. R. Lieberman, “Tumor Metastasis to Bone,” Arthritis Research and Therapy, Vol. 9, No. S1, 2007, p. 10.
- R. Bileckot, R. C. Miakoundoba and J. B. Nkoua Mbon, “Two Brazzaville Series of Bone Metastases,” Carcinol Prat Afrique, Vol. 8, No. 1, 2008, pp. 57-60.
- M. Ly, A. Ly, M. Rodrigues, et al., “Cancer in Africa, a New Health Challenge. Examples of Mali and Mali Onco Association,” Bull Cancer, Vol. 97, No. 8, 2010, p. 965.
- C. Gombe-Mbalawa and G. Ibara, “Report Functioning Cancer Registry of Brazzaville from January 1st to December 31st 2010,” Cancer Registry of Brazzaville, 2011.
- L. Belaksir, N. Seknaji, M. Touimy, S. Janani, et al., “Bone Metastases: Experience of Rheumatology CHU Ibn Rushd at Casa,” Revue Du Rhumatisme, Vol. 77, No. S3, 2010, pp. A131-A325.
- N. Seknaji, M. Touimy, L. Belksir, et al., “Contribution of Bone Biopsy in Search of Primary Cancer of Bone Metastases,” Revue Du Rhumatisme, Vol. 77, No. S3, 2010, pp. A131-A325.
- M. Vandecandelaere, R. M. Filipo, B. Cortet, L. Catanzariti, B. Duquesnoy and B. Delcambre, “Bone Metastases Revealing: A Comparative Study in 30-Years Intervals,” Revue Du Rhumatisme, Vol. 71, No. 5, 2004, pp. 390-396. http://dx.doi.org/10.1016/S1169-8330(03)00304-1
- P. Houze, R. Ranaivosoar, A. C. Prost, et al., “Bone Metastases of Prostate Cancer: Contribution of Specific Determination of Bone Alkaline Phosphatase,” ImmunoAnalyse & Biologie Specialisee, Vol. 10, 1995, pp. 27-33.
- C. Gombe-Mbalawa, D. Diouf, J. B. Nkoua Mbon, et al., “Arrival of Cancer Patients in Advanced Stages: Attempted Identification of Responsibility,” Bull Cancer, Vol. 100, No. 2, 2013, pp. 167-171.
- K. Kogahashi, H. Satoh, H. Ishihakawa, et al., “Bone Metastasis Revealing Primary Tumor. Comparison of Two Series Separated by 30 Years,” Joint Bone Spine, Vol. 71, No. 3, 2004, pp. 224-229. http://dx.doi.org/10.1016/S1297-319X(03)00123-4
- M. H. Vieillard, “Hypercalcemia of Malignancy,” Reflections Rheumatology, Vol. 115, No. 3, 2009, pp. 14-16.
- D. Secarecia, “The Cancer-Related Hypercalcemia,” Canadian Family Physician, Vol. 56, No. 1, 2010, pp. 90- 92.
- H. N. Rakoto-Ratsimba, J. C. Razafinahandry, J. A. B. Razafindrabe, F. R. Raherimandimby and P. Ratokobe, “Paratesticular rhabdomyosarcoma. Case Report,” Medecine Afrique Noire, Vol. 49, No. 2, 2002, pp. 83-86.
- S. Nesa, Y. Lefebvre, J. L. Montfort, F. X. Wese and P. Van Cangh, “Paratesticular Rhabdomyosarcoma a Case Report,” Acta Urologica Belgica, Vol. 62, No. 3, 1994, pp. 37-42.
- J. Nsonde-Malanda, E. Makosso, J. F. Peko and C. Gombe-Mbalawa, “Bone Metastases of Endometrial Adenocarcinoma. A Case Report with Review of the Literature,” Medecine Afrique Noire, Vol. 54, No. 6, 2007, pp. 313- 316.
- P. N. Scutellari, G. Addonisio, R. Righi and M. Giganti, “Diagnostic Imaging of Bone Metastasis,” Radiological Medicine, Vol. 100, No. 6, 2000, pp. 429-435.
- D. Vilain, A. Hameg and C. Tainturier, “Update on Bone Scintigraphy in Urological Cancers in Adults,” Advances in Urology, Vol. S7, 2008, pp. 202-207.
- C. Proust, J. Proust and A. Maubon, “Imaging Bone Metastases. Nuclear Medicine,” Functional and Metabolic Imaging, Vol. 30, No. 3, 2006, pp. 149-154.
- L. Belaksir, M. Touimy, S. Janani, et al., “Revealing Bone Metastasis of Prostate Cancer,” Revue Du Rhumatisme, Vol. 77, No. S3, 2010, pp. A131-A325.
NOTES
*Corresponding author.

