Advances in Biological Chemistry
Vol. 2 No. 2 (2012) , Article ID: 18979 , 5 pages DOI:10.4236/abc.2012.22021
A study of the levels of urinary microalbumin in non-diabetic normotensive obese individuals
![]()
Department of Biochemistry, M.S. Ramaiah Medical College, Bangalore, India
Email: hasit_lad@yahoo.co.in
Received 13 February 2012; revised 15 March 2012; accepted 25 March 2012
Keywords: non-diabetic normotensive obesity; microalbuminuria; cardiovascular disease risk
ABSTRACT
Diabetes mellitus, hypertension and obesity are associated with endothelial dysfunction. Microalbuminuria is an early sign of endothelial dysfunction. The occurrence of microalbuminuria in long standing diabetes mellitus and hypertension is well established. This study intends to find the occurrence of microalbuminuria in non-diabetic normotensive obese individuals. Objectives: To estimate urinary albumin creatinine ratio (UACR) in non-diabetic normotensive obese individuals. Design and Methods: 41 nondiabetic normotensive obese adults with Body Mass Index wang##Bracket# 23 kg/m2 were taken as cases and 41 age and sex matched healthy non-obese adults with Body Mass Index < 23 kg/m2 were taken as controls. Anthropometric measurements (Body Mass Index and Waist circumference) and biochemical estimations (fasting blood glucose, lipid profile & spot urinary albumin creatinine ratio) were carried out. Results: Urinary albumin creatinine ratio was lesser than the established microalbuminuric range of 30 - 300 mg/g, in both cases and controls irrespective of the values obtained for lipid profile and anthropometric indices. Conclusion: Microalbuminuria may not be present in obese patients without diabetes and hypertension.
1. INTRODUCTION
Obesity is a chronic metabolic disorder that often results from increase in energy intake or decrease in energy expenditure or a combination of both [1]. According to the World Health Organization (WHO) in 2005, there were about 1.6 billion overweight individuals worldwide [2]. A major concern with obesity is cardio metabolic risk frequently associated with atherosclerotic cardiovascular disease or diabetes mellitus. Obesity brings about alteration in adipose tissue metabolism and is associated with release of hormones and peptides that contribute to the complications of obesity including metabolic syndrome, type 2 DM and cardiovascular disease [1].
Obesity, especially abdominal (visceral), measured as Waist Circumference, is associated with chronic low grade inflammation characterized by cytokine production and increased acute phase reactants. The adipose tissue in obesity is infiltrated by macrophages as well, which are an important source of inflammation [3]. Various inflammatory mediators released from adipose tissue play an important role in endothelial dysfunction by direct or indirect actions on the vascular endothelium [3]. Urinary albumin excretion especially in the microalbuminuric range (30 - 300 mg/g creatinine) may represent an early sign of widespread derangement of endothelial function inducing an atherosclerotic process [4]. In hypertension and diabetes increased rate of albumin excretion is associated with high risk of cardiovascular morbidity and mortality. Slightly elevated urinary albumin excretion rate in microalbuminuric range has been reported to predict increased cardiovascular morbidity and mortality even in nondiabetic subjects [5]. In patients with metabolic syndrome which includes insulin resistance, diabetes mellitus, hypertension, dyslipidemia and obesity, microalbuminuria is considered as a marker of endothelial dysfunction [6]. This study was undertaken to observe whether or not microalbuminuria is present in obese individuals without DM and hypertension.
2. MATERIALS AND METHODS
The study was conducted on patients attending the outpatient health check-up clinic at M S Ramaiah Memorial Hospital, Bangalore, India. The study was conducted after taking the required Ethical clearance from the ethical review board and all patients who signed the informed consent participated in the study. Two groups of non-diabetic normotensive adult individuals in the age group of 20 - 60 yrs were selected. Group I as controls comprised of 41 non-obese adults with BMI < 23 kg/m2 and Group II had 41 obese adults as cases with BMI > 23 kg/m2 as per WHO standards for Asians [7]. Inclusion criteria: Individuals clinically diagnosed as obese with BMI > 23 Kg/m2, age group of 20 - 60 years and of both the sexes.
Exclusion criteria were as follows:
1) Subjects with any co-morbid conditions like diabetes mellitus, hypertension, obesity due to any endocrinal etiology, familial hyperlipidemia, impaired fasting glucose, urinary tract infection, chronic liver and kidney diseases, and malignancy; 2) Patients with positive clinical history of stroke, transient ischemic attacks, angina, myocardial infarction and intermittent claudication; 3) Patients who are smokers; or on drugs like corticosteroids and oral contraceptive pills.
Anthropometric indices such as BMI (body weight in Kg/height in m2) and Waist circumference (WC) in cm were recorded in both the groups. WC was evaluated as the minimum circumference between xiphoid process and umbilicus. Cut off points for obese subjects were BMI > 23 kg/m2 and WC ≥ 80 cm for females and ≥90 cm for males as per WHO standards for Asians [7]. Around 5 ml of spot urine sample in a sterile container was collected for determination of spot urinary albumin excretion and urinary creatinine. Urinary albumin excretion was calculated as urinary albumin creatinine ratio (UACR). Blood samples after 10 - 12 hours of overnight fasting was collected for estimation of fasting blood glucose & complete lipid profile. Estimation of urinary albumin was carried out by immunoturbidimetric assay Tina-quant Albumin Gen.2 kit (Roche Diagnostics Indianapolis USA cat. No. 0449658 190) method [8] and urinary creatinine was estimated by Buffered kinetic jaffes reaction without deproteinization, Creatinine Jaffe Gen. 2 kit (Roche Diagnostics Indianapolis USA cat. No. 04810716 190) method [9]. UACR was calculated as mg of albumin/g of creatinine. Blood glucose was assayed by Hexokinase kit (Siemens Healthcare Diagnostics Ltd., Frimely, Camberley, UK cat. No. DF40) method [10]. Serum total cholesterol by enzymatic kit (Siemens Healthcare Diagnostics Ltd. Frimely, Camberley, UK cat. No. DF27) method [11], serum triglyceride by enzymatic kit (Siemens Healthcare Diagnostics Ltd. Frimely, Camberley, UK cat. No. DC 6A) method [12] and serum HDL by accelerator selective detergent kit (Siemens Healthcare Diagnostics Ltd. Frimely, Camberley, UK cat. No. DF 48B) method [13]. Serum LDL and VLDL were calculated from the estimated values of cholesterol, triglyceride and HDL, using the equation of Frieldwald et al. [14]
3. STATISTICAL ANALYSIS
Statistical analysis was performed using t-test and Pearson’s correlation in SPSS 12.0 for Windows Program.
4. RESULTS & DISCUSSION
Results are shown in tables 1-6.
BMI and WC were significantly high in cases compared to controls as shown in Table 1. Table 2 shows the urinary albumin levels in the cases and controls. The urinary albumin levels in cases showed a mean ± SD of 2.34 ± 2.56 and 1.71 ± 2.10 in controls with “p” value of 0.22, which was not significant. Both the cases and controls were divided depending on the urinary albumin levels into 3 categories viz 0 - 5, >5 up to 10 and >10 mg/g creatinine. However, urinary albumin excretion was not observed in the microalbuminuric range of ACR (Albumin Creatinine Ratio) 30 - 300 mg/g in spot urine samples of both cases and controls irrespective of the lipid profile values. In a study by valensi et al, daily urine albumin excretion was significantly higher in obese people than lean people with prevalence of microalbuminuria in non diabetic obese people [15].
No positive correlation was observed between urinary albumin levels with anthropometric indices i.e. BMI & waist circumference as shown in table 3. These findings are similar to a study made by Tijen Erdem Yesim et al., where microalbuminuria was not detected in obese women

Table 1. Anthropometric parameters of Groups I and II.
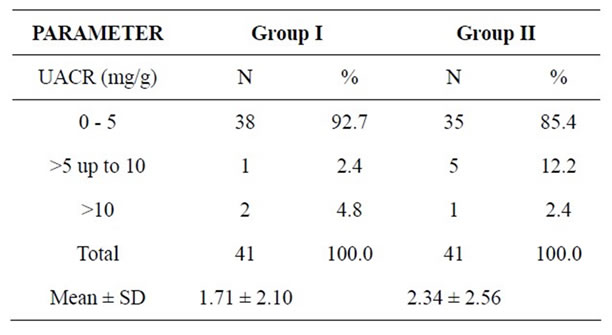
Table 2. Comparison of UACR between both the groups.
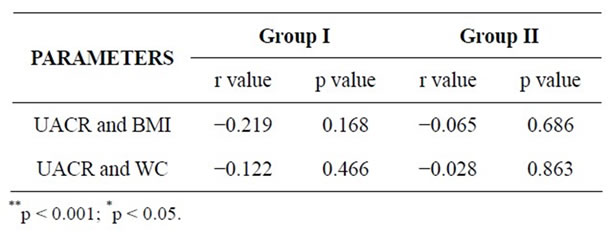
Table 3. Correlations between UACR and Anthropometric indices in both the groups.
without DM and hypertension and urinary albumin excretion was similar in obese and lean women [4]. In the present study, only those cases that had familial hyperlipidemia were excluded by history taking. During the course of the study, it was observed that there were a few individuals in the controls as well as in the cases who exhibited abnormal lipid profile as shown in Table 4. Comparison of lipid profile between all cases and all controls will not be authentic in this situation. Hence, an attempt was made to observe the association of urinary albumin with lipid profile parameters after dividing the cases and controls into two groups viz. those in whom lipid profile values were abnormal and those in whom lipid profile values were well within the established reference ranges as shown in tables 5 and 6. The correlation amongst urinary albumin, serum total cholesterol (TC) and serum triglycerides (TG) was nearly perfect (r value 0.980 and 0.922 respectively), and the correlation among UAE, LDL and VLDL was very large (r value 0.822 and 0.885 respectively) in cases with abnormal lipid profile. There was no negative correlation between UAE and HDL cholesterol observed in the same group. On the other hand, controls with abnormal lipid profile showed nearly perfect correlation between UACR and TC (r value 0.969) and among UAE, TG, LDL and VLDL there

Table 4. Number of individuals in the controls and cases exhibiting abnormal lipid profile in both groups.

Table 5. Correlations between UACR and Abnormal lipid profile parameters in both the groups.
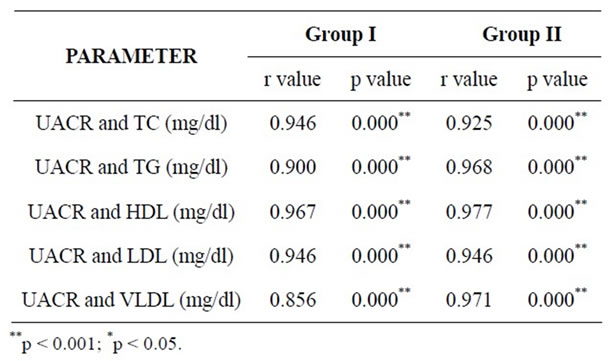
Table 6. Correlations between UACR and lipid profile parameters within established range in both the groups.
was a very large correlation (r values 0.796, 0.751 and 0.886 respectively). In cases with normal lipid profile, there was nearly perfect correlation among UAE, TC, TG, LDL and VLDL (r values 0.946, 0.925, 0.968 and 0.971 respectively) and no negative correlation with HDL. In controls with normal lipid profile correlation among UAE, TC, TG and LDL was nearly perfect (r values 0.946, 0.900 and 0.946 respectively) and very large with VLDL (r value 0.856).
In a cross sectional analysis of 1160 cases of type 1 diabetes mellitus, progressive increase in albuminuria was associated with elevations in proatherogenic LDL. In addition, this study also revealed that in patients of essential hypertension with/without DM, microalbuminuria was associated with low HDL and increased serum total cholesterol. The most consistent association between lipoprotein abnormalities and microalbuminuria is low HDL cholesterol which suggests that clearance of LDL cholesterol may be as important as low levels of HDL to avoid cellular injury. Thus, apparent association between microalbuminuria and cardiovascular disease may be related to this adverse risk factor profile [16]. Previous reports show that the presence of microalbuminuria and macroalbuminuria is associated with high risk of CVD incidence and mortality in high risk individuals like patients with hypertension and DM. Furthermore, investigators have reported that microalbuminuria predicts CVD and mortality rates in community based samples [17]. The beginning of endothelial dysfunction and there by atherosclerosis, is multi factorial. Factors such as hyperglycemia, insulin resistance, procoagulant state and adhesion molecules may also play their part in the above mentioned pathology. While these factors can be accepted as contributors for MAU in diabetic patients, in nondiabetic normotensive obese individuals collective factors may not converge and give rise to wide-spread endothelial dysfunction and microalbuminuria [18]. However, a recent community based follow up study (median follow up 6 years) by Johan Arlov et al. in 1568 nondiabetic normotensive individuals revealed that participants with urinary ACR greater than or equal to the sex specific median (≥3.9 mg/g for men, ≥7.5 mg/g for women) in a single void sample experienced nearly a threefold risk of CVD and a borderline significantly increased risk of death compared with those with urinary ACR below the median there by predicting the development of CVD with low levels of urinary albumin excretion well below the current microalbuminuric threshold. The study contemplates lowering of the established microalbuminuric range for predicting risk of CVD [19]. The present study also has reflected the fact that urinary albumin levels in nondiabetic normotensive obese individuals are much lesser than the established microalbuminuric range. This pilot study can be extended into a follow up study on a larger population observing the number of cases progressing to CVD. If the observation shows normotensive nondiabetic obese individuals progressing to cardiovascular disease, perhaps, the idea of modifying and lowering the microalbuminuric range may be reinforced. Limitations of the present study could are 1) Small study population who come to the hospital only for an annual medical check up; 2) Difficulty in follow up; 3) Urinary albumin levels were assessed only on a single void urine sample in the present study. Previous studies suggest that urinary albumin levels may exhibit considerable intra individual variations; 4) Hyperlipidemia due to other causes was not excluded.
5. CONCLUSION
In obesity, which is not complicated by DM and hypertension, urinary albumin levels in the current range of microalbuminuria may not be observed. However, if proper follow up study is carried out on a larger population it may establish a lower reference range and the usefulness of microalbuminuria as a predictor of CVD risk in non-diabetic normotensive obese individuals.
6. ACKNOWLEDGEMENTS
I am greatly thankful to my respected guide Dr. Vasudha K.C. for her constant encouragement, support and help during this research work. I heartily thank Dr. N.S. Murthy, research coordinator and Prof. of community medicine MSRMC Bangalore for the statistical analysis.
![]()
![]()
REFERENCES
- Park, K. (2007) Non communicable diseases, textbook of preventive and social medicine. M/s Banarasidas Bhanot Publishers, Jabalpur, 332-336.
- World Health Organisation. (2008) Fact sheet: Obesity and overweight. http://http://www.who.int/mediacentre/factsheets/fs311/en/print.html
- Chudek, J., Adamczak, M., Nieszporek, T. and Wiêcek, A. (2006) The adipose tissue as an endocrine organ—A nephrologists perspective. Contrib Nephrol, 151, 70-90. doi:10.1159/000095320
- Tijen, E.Y., Serdal, U., Erkan, C., et al. (2007) Investigation of microalbuminuria in nondiabetic, normotensive obese women. The Japanese Society of Internal Medicine, 46, 1963-1965.
- Yudkin, J.S., Forrest, R.D. and Jackson, C.A. (1988) Microalbuminuria as predictor of vascular disease in nondiabetic subjects. The Lancet, 2, 530-533. doi:10.1016/S0140-6736(88)92657-8
- Alessia, F. and Leopoldo, R. (2005) Metabolic sydrome and endothelial dysfunction. Current Hypertension Reports, 7, 88-95.
- WHO/IASO/IOTF. (2000) The Asia-Pacific perspective: Redefining obesity and its treatment. Health Communication, Melbourne.
- (1990) Multicenter study of Tina-quant Albumin in urine and β-N-acetyl-glucosaminidase (β-NAG) in urine. Wien Klin Wochenschr, 103, 1-64.
- Bartels, H. and Bohmer, M. (1971) Micro-determination of creatinine. Clinica Chimica Acta, 32, 81-85.
- Kunst, A., Drager, B. and Ziegenhorn, J. (1983) UV methods with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergemeyer, H.Y., Ed., Methods of Enzymatic Analysis, Vol VI, Verlag Chemie, Deerfield, 163-172.
- Stadtman, T.C. (1957) [63] Preparation and assay of cholesterol and ergosterol. Methods in Enzymology, 3, 392- 394. doi:10.1016/S0076-6879(57)03403-5
- Seidel, J., et al. (1993) AACC Meeting extract 34. Clinical Chemistry, 39, 1127.
- Sugiuchi, H., Uji, Y., Okabe, H., Irie, T., et al. (1995) Direct measurement of high-density lipoprotein in cholesterol in serum with polyethylene GLYCOL-modified enzymes and sulfated α-Cyclodextrin. Clinical Chemistry, 41, 717-723.
- Friedwald, W.T., Levy, R.I., Fredrickson, D.S., et al. (1972) Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of preparative ultracen-trifuge. Clinical Chemistry, 18, 499-502.
- Valensi, P., Assayag, M., Busby, M., et al. (1996) Microalbuminuria in obese patients with or without hypertension. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 20, 20574- 20579.
- Garg, J.P. and Bakris, G.L. (2002) Microalbuminuria: Marker of vascular dysfunction, risk factor for cardiovascular disease. Vascular Medicine, 7, 35-43. doi:10.1191/1358863x02vm412ra
- Romundstad, S., Holmen, J., Kvenild, K., Hallan, H. and Ellekjaer, H. (2003) Microalbuminuria and all cause mortality in 2089 apparently healthy individuals: A 4.4-year follow-up study. The Nord-Trondelag Health Study (HUNT), Norway. American Journal of Kidney Diseases, 42, 466- 473. doi:10.1016/S0272-6386(03)00742-X
- Naidoo, D.P. (2002) The link between microalbuminuria, endothelial dysfunction and cardiovascular disease in diabetes. Cardiovascular Journal of Africa, 13, 194-199.
- Arnlov, J., Evans, J.C., Meigs, J.B., et al. (2005) Lowgrade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: The framinghan heart study. Circulation, 112, 969- 975. doi:10.1161/CIRCULATIONAHA.105.538132

