Journal of Water Resource and Protection
Vol.07 No.16(2015), Article ID:61539,10 pages
10.4236/jwarp.2015.716113
pH Control during the Struvite Precipitation Process of Wastewaters
Diyan Radev1, Gergana Peeva2, Valentin Nenov2
1Department of Chemical Engineering, Burgas Asen Zlatarov University, Burgas, Bulgaria
2Department of Water Treatment, Burgas Asen Zlatarov University, Burgas, Bulgaria

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 29 September 2015; accepted 24 November 2015; published 27 November 2015

ABSTRACT
The high concentration of phosphorus and nitrogen in wastewater and sludge could be lowered to a certain level by struvite (MgNH4PO4∙6H2O) crystallization. One of the main factors for struvite formation is the solution pH. It can be adjusted by non-reagent carbon (CO2) dioxide stripping through the process of aeration. The intensity of the mass transfer between the air and the supernatant of dewatering sludge obtained from wastewater treatment plant is characterized by the volumetric liquid-side mass transfer coefficient, which can be estimated theoretically. It is found that the rate of pH increase depends strongly on the sparging area of the air distribution system while the air flow rate does not influence considerably the Dissolved Oxygen (DO) level which governs the CO2 stripping process. The theoretical calculated values of the volumetric mass transfer coefficient have been compared with those obtained experimentally. Based on the data obtained, relationships of pH/kLa (mass transfer coefficient) were developed. These correlations serve as a tool for prediction of pH during the struvite precipitation process.
Keywords:
Wastewater Treatment, pH, Carbon Dioxide Stripping, Mass Transfer

1. Introduction
Nitrogen and phosphorus are beneficial nutrients to many ecosystems in small amounts. In excessive, however, they cause a type of pollution called eutrophication (process causing reduction of oxygen concentration in water bodies due to significant growth of algae). The wastewaters are characterized by a high level of ammonia and phosphorus contents [1] , therefore the effluents must be treated before discharging into water bodies [2] [3] . Conventional nitrogen removal from wastewater is carried out by biological nitrification and denitrification [4] , or other methods as ion exchange [5] , microwave irradiation [6] and struvite precipitation [7] . Phosphorus can be removed from wastewaters by incorporation of phosphate into Total suspended solids (TSS) and the subsequent removal from these solids. Alternative method for P and N recovery is struvite precipitation. The product could be used as a slow release fertilizer [8] [9] . This is the reason why struvite precipitation is to be widely investigated. Struvite (MgNH4PO4・6H2O, magnesium ammonium phosphate hexahydrate―MAP) usually precipitates as a white orthorhombic crystals in a molar ratio Mg:MH4:PO4 = 1:1:1. MAP precipitation is a function of pH and molar ratios among ammonia, magnesium ions and phosphorus [10] . The solubility of the product can be defined by Ksp (solubility product constant), and struvite formation occurs when the concentration product exceeds struvite’s solubility product (supersaturation). The constant can be described by following equation [11] :
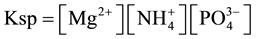
Controlled MAP precipitation has been reported in treatment of digested sludge as source of phosphorus and ammonia. pH is a crucial variable that needs to be controlled in order to maximize the product production. Many authors have reported that the range of pH for MAP precipitation is from 8 to 11 [12] . According to the diagram showing concentration of species depending on pH (Figure 1), the optimal value of pH is about 9.6 [13] .
Chemically pH can be controlled by using alkaline solutions as sodium hydroxide. High phosphorus and ammonia removal level were achieved at increased pH [14] [15] . NaOH is generally used for pH adjustment, but its addition rapidly increases pH value. Also the addition of NaOH will sharply increase the saturations of other magnesium precipitations, i.e. bobierrite and magnesite [16] . Alternative of addition of NaOH is the CO2 stripping process. Willams (1999) has used CO2 stripping for pH elevation [17] . Advantage is that pH increases slowly, which gives the optimum conditions for struvite crystal growth and crystallization process [18] . CO2 stripping is non-reagent process for increasing of pH by dissolved carbon dioxide release using aeration of liquid [19] . It is a technique where dissolved CO2 produced from an aerobic digestion process is removed from wastewater resulting in reduction of the total carbonate carbon concentration. The high concentration of CO2 in wastewater is due to the relatively low specific exchange rates which do not allow the removal of substantial quantities of CO2. Therefore, an effective CO2 control requires aeration for stripping process. When atmospheric air is placed in contact with wastewater, there is a tendency for a dissolved gas to come to equilibrium at saturation: undersaturated gas such as oxygen is transferred from the air to the wastewater, and supersaturated gas such as CO2 is transferred to the air [20] .
The gas―liquid equilibrium influences the transfer of CO2 between air and wastewater. The carbonate species, 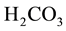 ,
,  , and
, and , are involved in instantaneous equilibrium among each other, as shown in Figure 1. In summary, dissolved CO2 exists in the wastewater as part of the carbonate acid―base system. Therefore, the concentration of total dissolved CO2 can be altered by change of the total amount of carbonate carbon in the solution. The intensity of this process is characterized by the volumetric liquid-side mass transfer
, are involved in instantaneous equilibrium among each other, as shown in Figure 1. In summary, dissolved CO2 exists in the wastewater as part of the carbonate acid―base system. Therefore, the concentration of total dissolved CO2 can be altered by change of the total amount of carbonate carbon in the solution. The intensity of this process is characterized by the volumetric liquid-side mass transfer
Figure 1. Concentration of species with pH.
coefficient, . If we estimate the volumetric liquid-side mass transfer coefficient, then we can predict pH solution by the following equation [21] :
. If we estimate the volumetric liquid-side mass transfer coefficient, then we can predict pH solution by the following equation [21] :

where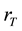 ,
, 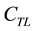 ,
, 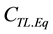 ,
, 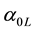 and
and  are the CO2 transfer reaction rate, total carbonates concentration in the liquid phase, equilibrium concentration of total carbonates in the liquid phase, H2CO3 fraction of the total carbonates in the liquid phase and equilibrium concentration of H2CO3 fraction of the total carbonates in the liquid phase, respectively.
are the CO2 transfer reaction rate, total carbonates concentration in the liquid phase, equilibrium concentration of total carbonates in the liquid phase, H2CO3 fraction of the total carbonates in the liquid phase and equilibrium concentration of H2CO3 fraction of the total carbonates in the liquid phase, respectively.
In gas-liquid reactors, mass transfer from the gas phase to the liquid phase is a key parameter of the process. The 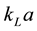 value in gas-liquid contacting equipment has mostly been determined by the oxygen physical absorption or desorption technique (classical method). In the actual large-scale aeration system, the application of the existing method for
value in gas-liquid contacting equipment has mostly been determined by the oxygen physical absorption or desorption technique (classical method). In the actual large-scale aeration system, the application of the existing method for 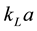 determination can be limited by various factors such as absorption rate from air, complicated operating conditions, measuring equipment quality and cost, and also operator skills. Therefore, a simple theoretical way to predict the volumetric liquid-side mass transfer coefficient would be appreciated. These values can be predicted theoretically if we know how to estimate the liquid-side mass transfer coefficient
determination can be limited by various factors such as absorption rate from air, complicated operating conditions, measuring equipment quality and cost, and also operator skills. Therefore, a simple theoretical way to predict the volumetric liquid-side mass transfer coefficient would be appreciated. These values can be predicted theoretically if we know how to estimate the liquid-side mass transfer coefficient 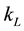 and the specific interfacial area, separately [22] -[25] . In order to improve the accuracy of the theoretical model for calculating the volumetric mass transfer coefficient for pH adjustment using air stripping, experimental data were needed for comparison.
and the specific interfacial area, separately [22] -[25] . In order to improve the accuracy of the theoretical model for calculating the volumetric mass transfer coefficient for pH adjustment using air stripping, experimental data were needed for comparison.
2. Materials and Methods
The digested sludge was taken from one of the digesters of wastewater treatment plant (WWTP) (Pomorie, Bulgaria). The sludge contains 9310 mg P04-P/kg solids and dry matter of 26 g/l as TSS. Principle scheme of WWTP-Pomorie and sampling point (digested sludge after digester) is shown in Figure 2. Al2(SO4)3 is dosed before aeration tank.
The sludge was centrifuged in lab scale and the obtained supernatant was aerated in an up-flow reactor aiming pH elevation. Analytical measurement for chemical oxygen demand (COD, mgO2/L), NH4-N (mg/L) and PO4 (mg/L) were determined by Spectrophotometer (HACH LANGE DR 3900) using cuvette test (Cuvette test-
Figure 2. (a) Scheme of WWTP Pomorie and sampling point of digested sludge; (b) Sludge treatment in lab scale.
range 5 - 90 mg/L PO4; range 100 - 2000 mgO2/L COD; range 2.5 - 60 mg/L NH4-N) and the obtained data is listed in Table 1. The samples were tested for magnesium and calcium content by the Ethylenediaminetetraacetic acid (EDTA) complexometric method [26] . The preliminary obtained information related to the concentrations of ammonia and phosphorous (o-PO4) shows that the liquid phase following the dewatering of sludge from conventional WWTP contains significant concentration of these constituents for MAP precipitation. The concentrations of the dissolved oxygen and solution pH were measured by Multi-Parameter Meter (HQ40d Portable).
Plastic cylindrical column with diameter of 0.05 m was used as reactor for the CO2 stripping process (Figure 3). Plastic perforated plate was installed at the bottom of the reactor and served as a gas sparger. Supernatant of 1000 ml was placed in the reactor. Air compressor was run and air flow was passing through the plastic perforated plate and the supernatant was aerated. The first series of experiments were carried out at different air volumetric flow rates (0.067 m/s; 0.134 m/s; 0.201 m/s) using large specific area yield by plate with number of orifices 132 and a diameter of each orifice 2 mm. For the second series of experiments was used a plate with a single orifice and airflow rate of 0.067 m/s. Dissolved oxygen and pH level were measured in both sets of experiments. Dissolved oxygen and pH level were measured in both sets of experiments aiming determination influence of organic matter on pH elevation. For this purpose the supernatant was diluted 2, 3 and 4 times using distilled water and COD levels in the diluted samples were 650 mgO2/L, 400 mgO2/L, and 320 mgO2/L, respectively. The measurement of oxygen concentration was needed for the experimental calculation of the volumetric mass transfer coefficient,
where:


Table 1. Values of ammonia (mg/L), phosphate (mg/L), COD (mgO2/L), calcium ions (mg/L) and magnesium ions (mg/L) in the supernatant.
Figure 3. Experimental setup.
For the theoretical prediction of the volumetric liquid-side mass transfer coefficient




The 
3. Results and Discussion
3.1. Influence of Different Air Flow Rates
pH elevation and oxygen concentrations at different air flow rates were observed (Figure 4 and Figure 5). The results show evidently that within the volumetric air rate applied the effect of pH increase follows a similar mode
Figure 4. pH elevation by air stripping column applying different volumetric air rates.
Figure 5. Concentration of oxygen apllying different volumetric air rates.
of change. More rapidly oxygen saturation of the supernatant within the first 5 minutes of aeration was observed. pH increases from 7.5 to 8.4 were achieved by air stripping within 20 minutes, while pH values were increased up to 9 after 250 minutes of aeration. Actually, pH values from 8.3 to 8.5 are enough for MAP precipitation by CO2 stripping process. But many studies are showing that the optimal pH is in the range of 9 to 9.5 [33] [34] . The targeted pH of 9 was achieved after 4 hours of aeration. The slow change of pH after the 20th minute of aeration is an advantage of the CO2 stripping process because it restricts the rapid increase of solution saturation. As such conditions the struvite crystallization process predominates [16] .
3.2. Influence of Different Sparging Area
Although the curves follow similar mode of change (Figure 6 and Figure 7), the rate of pH elevation and oxygen concentration is higher at larger developed sparging area compared to the rate of pH elevation and oxy- gen concentration using gas sparger with single orifice. When gas sparger with 1 orifice was used for aretation, pH was increased to 8.4 within 120 minuttes. For the same period of aeration but different gas sparger with 132 orifices pH achieved value of 8.8. The result is demonstrating that larger developed surface area lead in rapidly pH increasing because of faster oxygen saturation of solution and carbon dioxide stripping.
3.3. Influence of Organic Contain
The dependence of pH elevation on organic contain was determined (Figure 8). pH values of 8.7 and 9 (starting of pH = 8.1) were achieved within 250 minutes of aeration at COD = 650 mgO2/L, COD = 400 mgO2/L and COD = 320 mgO2/L, respectively. The experiment indicated that higher organic contain in the supernatant
Figure 6. pH elevation applying different gas spargers.
Figure 7. Concentration of oxygen applying different gas spargers.
Figure 8. pH elevation applying different organic contain.
resulting in higher increase of pH value.
3.4. Mass Transfer Coefficients
The predicted of experimental data are plotted in Figure 9. The figure shows that there is relative agreement between the predicted and the experimental coefficients. The data obtained show that the experimental data are within the range of −25% +25% of the theoretical trend (the central continuous line).
The theoretical and experimentally obtained mass transfer coefficients are also compared at different air volumetric rates (w, m/s) (Figure 10). These results show at higher air volumetric rates the experimental coefficients are close to the theoretical values.
Aiming to find relationships between pH, w [m/s] and KLa [s−1], a logarithmic function was applied. Actually, three functions were followed, namely pH = f(w), Kla = f(w) and pH = f(kLa) at constant time of aeration. We choose time of aeration two minutes. The pH/w and kLa/w functions are presented in Figure 11. Evidently, at higher air velocity kLa and pH are increased. However, at the values of air velocity higher than 0.2 m/s the influence of air velocity is negligible. The direct relation between pH and kLa is given in Figure 12. For the three curves shown in Figure 11 and Figure 12 the correlation coefficients over 0.98 (Figure 11 and Figure 12). Such relationships could serve as a prediction tool for pH in struvite precipitation reactor.
For the specific case of struvite precipitation of supernatant of digested sludge (parameters: [

KLa = 0.0142.ln(W) + 0.0571
pH = 0.2431.ln(W) + 8.4214
pH = 0.451.ln(KLa) + 9.5426
These functions describe the interactions between the pH, w (m/s) and kLa.
4. Conclusions
CO2 stripping was applied as an alternative method for pH elevation of supernatant taken from digested sludge. It was found that the rate of increase of pH depended strongly on the sparging area of the air distribution system while the air flow rate did not influence considerably DO level which governed the CO2 stripping process. Based on the data obtained, relationships of pH/kLa (mass transfer coefficient) were developed. These correlations served as a tool for prediction of pH during the struvite precipitation process.
Relationships among pH, w [m/s] and KLa [s−1] were determined for the specific case of struvite precipitation using supernatant of digested sludge.
Acknowledgements
The support of the Project MIS ETC 2614, Scientific Network for Earthquakes, Landslide, and Flood hazard
Figure 9. Comparison between the theoretical and the experimental volumetric liquid-side mass transfer coefficients (◆: experimental data).
Figure 10. Volumetric liquid-side mass transfer coefficient as a func- tion of gas velocity.
Figure 11. Relation between pH and w (m/s), and Kla and w (m/s).
Figure 12. Relation between pH and Kla.
prevention, funded under JOP “Black Sea Basin 2007-2013”.
Cite this paper
DiyanRadev,GerganaPeeva,ValentinNenov, (2015) pH Control during the Struvite Precipitation Process of Wastewaters. Journal of Water Resource and Protection,07,1399-1408. doi: 10.4236/jwarp.2015.716113
References
- 1. Deng, L.W., Zheng, P. and Chen, Z.A. (2006) Anaerobic Digestion and Post-Treatment of Swine Wastewater Using IC-SBR Process with Bypass of Raw Wastewater. Process Biochemistry, 41, 965-969.
http://dx.doi.org/10.1016/j.procbio.2005.10.022 - 2. Khan, F.A. and Ansari, A.A. (2005) Eutrophication: An Ecological Vision. The Botanical Review, 71, 449-482.
http://dx.doi.org/10.1663/0006-8101(2005)071[0449:EAEV]2.0.CO;2 - 3. Lee, S.I., Weon, S.Y., Lee, C.W. and Koopman, B. (2003) Removal of Nitrogen and Phosphate from Wastewater by Addition of Bittern. Chemosphere, 51, 265-271.
http://dx.doi.org/10.1016/S0045-6535(02)00807-X - 4. Welander, U., Henrysson, T. and Welander, T. (1998) Biological Nitrogen Removal from Municipal Landfill Leachate in a Pilot Scale Suspended Carrier Biofilm Process. Water Research, 32, 1564-1570.
http://dx.doi.org/10.1016/S0043-1354(97)00351-5 - 5. Liu, Y.H., Kwag, J.H., Kim, J.H. and Ra, C.S. (2011) Recovery of Nitrogen and Phosphorus by Struvite Crystallization from Swine Wastewater. Desalination, 277, 364-369.
http://dx.doi.org/10.1016/j.desal.2011.04.056 - 6. Cho, J.H., Lee, J.E. and Ra, C.S. (2009) Microwave Irradiation as a Way to Reutilize the Recovered Struvite Slurry and to Enhance System Performance. Journal of Animal Science and Technology (Korea), 51, 337-342.
http://dx.doi.org/10.5187/JAST.2009.51.4.337 - 7. Rahman, M.M., Liu, Y.H., Kwag, J.H. and Ra, C.S. (2011) Recovery of Struvite from Animal Wastewater and Its Nutrient Leaching Loss in Soil. Journal of Hazardous Materials, 186, 2026-2030.
http://dx.doi.org/10.1016/j.jhazmat.2010.12.103 - 8. Münch, E.V. and Barr, E. (2001) Controlled Struvite Crystallisation for Removing Phosphorus from Anaerobic Digester Sidestreams. Water Research, 35, 151-159.
http://dx.doi.org/10.1016/S0043-1354(00)00236-0 - 9. Laridi, R., Auclair, J.C. and Benmoussa, H. (2005) Laboratory and Pilot-Scale Phosphate and Ammonium Removal by Controlled Struvite Precipitation Following Coagulation and Flocculation of Swine Wastewater. Environmental Technology, 26, 525-536.
http://dx.doi.org/10.1080/09593332608618533 - 10. Abbona, F. and Boistelle, R. (1979) Growth Morphology and Crystal Habit of Struvite Crystals (MgNH4PO4·6H2O). Journal of Crystal Growth, 46, 339-354.
http://dx.doi.org/10.1016/0022-0248(79)90082-4 - 11. Ohlinger, K.N., Young, T.M., Schroeder, E.D. (1999) Kinetics Effects on Preferential Struvite Accumulation in Waste-water. Journal of Environmental Engineering, 125, 730-737.
http://dx.doi.org/10.1061/(ASCE)0733-9372(1999)125:8(730) - 12. Hao, X.D., Wang, C.C., Lan, L. and Loosdrecht, M.C.M.V. (2008) Struvite Formation, Analytical Methods and Effects of pH and Ca2+. Water Science and Technology, 58, 1687-1692.
- 13. El Diwani, G., El Rafie, Sh., El Ibiari, N.N. and El-Aila, H.I. (2007) Recovery of Ammonia Nitrogen from Industrial Wastewater Treatment as Struvite Slow Releasing Fertilizer. Desalination, 214, 200-214.
http://dx.doi.org/10.1016/j.desal.2006.08.019 - 14. Stratful, I., Scrimshaw, M. and Lester, J. (2001) Conditions Influencing the Precipitation of Magnesium Ammonium Phosphate. Water Research, 35, 4191-4199.
http://dx.doi.org/10.1016/S0043-1354(01)00143-9 - 15. Battistoni, P., de Angelis, A., Pavan, P., Prisciandaro, M. and Cecchi, F. (2001) Phosphorus Removal from a Real Anaerobic Supernatant by Struvite Crystallization. Water Research, 35, 2161-2178.
http://dx.doi.org/10.1016/S0043-1354(00)00498-X - 16. Zeng, L. and Li, X. (2006) Nutrient Removal from Anaerobically Digested Cattle Manure by Struvite Precipitation. Journal of Environmental Engineering and Science, 5, 285-294.
http://dx.doi.org/10.1139/s05-027 - 17. Williams, S. (1999) Struvite Precipitation in the Sludge Stream at Slough Wastewater Treatment Plant and Opportunities for Phosphorus Recovery. Environmental Technology, 20, 743-747.
http://dx.doi.org/10.1080/09593332008616869 - 18. Song, Y.H., Qiu, G.L., Yuan, P., Cui, X.Y., Peng, J.F., Zeng, P., Duan, L., Xiang, L.C. and Qian, F. (2011) Nutrients Removal and Recovery from Anaerobically Digested Swine Wastewater by Struvite Crystallization without Chemical Additions. Journal of Hazardous Materials, 190, 140-149.
http://dx.doi.org/10.1016/j.jhazmat.2011.03.015 - 19. Ohlinger, K.N., Young, T.M. and Schroeder, E.D. (1998) Predicting Struvite Formation in Digestion. Water Research, 32, 3607-3614.
http://dx.doi.org/10.1016/S0043-1354(98)00123-7 - 20. Summerfelt, S.T., Vinci, B.J. and Piedrahita, R.H. (2000) Oxygenation and Carbon Dioxide Control in Water Reuse Systems. Aquacultural Engineering, 22, 87-108.
http://dx.doi.org/10.1016/S0144-8609(00)00034-0 - 21. Shanableh, A. (2009) Carbon Dioxide Transfer with Chemical Equilibrium Reactions: An Alternative Mathematical Approach. American Journal of Engineering and Applied Sciences, 2, 726-734.
- 22. Nedelchev, S. and Jordan, U. (2006) A New Correction Factor for Theoretical Prediction of Mass Transfer Coefficients in Bubble Columns. Journal of Chemical Engineering of Japan, 39, 1237-1242.
http://dx.doi.org/10.1252/jcej.39.1237 - 23. Ivanov, Zh., Stefanov, Zh. and Bogdanov, B. (2011) Gas-Side Mass Transfer Coefficient of Laboratory Column Equipped with One Sieve Tray. Proceedings: Chemical Technologies, 50, 51-55.
- 24. Fan, L.S. and Tsuchiya, K. (1990) Butterworth-Heinemann Series in Chemical Engineering, Stoneham, U.S.A.
- 25. Terasaka, K., Inoue, Y., Kakizaki, M. and Niwa, M. (2004) Simultaneous Measurement of 3-Dimensional Shape and Behavior of Single Bubble in Liquid Using Laser Sensors. Journal of Chemical Engineering of Japan, 37, 921-926.
http://dx.doi.org/10.1252/jcej.37.921 - 26. Budesinsky, B.W. (1975) A Simultaneous EDTA-Metric Determination of Calcium and Magnesium with Antipyrylazo III and Thymolphthalexon. Microchemical Journal, 20, 17-21.
http://dx.doi.org/10.1016/0026-265X(75)90107-1 - 27. Higbie, R. (1935) The Rate of Absorption of a Pure Gas into a Still Liquid during Short Periods of Exposure. Transactions of the AIChE, 31, 365-389.
- 28. Mendelson, H.D. (1967) The Prediction of Bubble Terminal Velocities from Wave Theory. AIChE Journal, 13, 250-253.
http://dx.doi.org/10.1002/aic.690130213 - 29. Georgiev, D. and Vlaev, S.D. (2012) Bioprocess Improvement by Design-Modified Bioreactor Flow Properties. Biotechnology & Biotechnological Equipment, 26, 3182-3186.
http://dx.doi.org/10.5504/BBEQ.2012.0031 - 30. Miller, D.N. (1974) Scale-Up of Agitated Vessels Gas-Liquid Mass Transfer. AIChE Journal, 20, 445-453.
http://dx.doi.org/10.1002/aic.690200303 - 31. Painmanakul, P., Loubiere, K., Hebrard, G., Mietton-Peuchot, M. and Roustan, M. (1999) Dynamics of Gas-Liquid Flows in Bubble Column Reactors. Chemical Engineering Science, 54, 5237-5243.
http://dx.doi.org/10.1016/S0009-2509(99)00245-6 - 32. Wilkinson, P.M. and Haringa, H. (1994) Mass Transfer and Bubble Size in a Bubble Column under Pressure. Chemical Engineering Science, 49, 1417-1427.
http://dx.doi.org/10.1016/0009-2509(93)E0022-5 - 33. Fattah, K.P., Mavinic, D.S., Koch, F.A. and Jacob, C. (2008) Determining the Feasibility of Phosphorus Recovery as Struvite from Filter Press Centrate in a Secondary Wastewater Treatment Plant. Journal of Environmental Science and Health Part A, 43, 756-764.
- 34. Uysala, A., Yilmazel, Y.D. and Demirer, G.N. (2010) The Determination of Fertilizer Quality of the Formed Struvite from Effluent of a Sewage Sludge Anaerobic Digester. Journal of Hazardous Materials, 181, 248-254.
http://dx.doi.org/10.1016/j.jhazmat.2010.05.004


















