Journal of Cancer Therapy
Vol.2 No.4(2011), Article ID:7793,8 pages DOI:10.4236/jct.2011.24069
Synthesis and Biological Evaluation of Novel Homopiperazine Derivatives as Anticancer Agents
![]()
1Department of Studies in Chemistry, University of Mysore, Mysore, India; 2Department of Biochemistry, Indian Institute of Science, Bangalore, India.
Email: rangappaks@chemistry.uni-mysore.ac.in, rangappaks@gmail.com
Received June 29th, 2011; revised August 2nd, 2011; accepted August 15th, 2011.
Keywords: Cancer Therapy, Cytotoxicity, 1,4-Diazepane, Isocyanates, Isothiocyanates
ABSTRACT
In search of new anticancer agents, a series of novel 1-benzhydryl-4-(substituted phenylcarboxamide/carbothioamide)- 1,4-diazepane derivatives were designed, synthesized and characterized using 1H NMR, LCMS and elemental analysis. These molecules were evaluated for their anti-cancer activity by trypan blue exclusion and 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay on B-cell leukemic cell line, Reh. Carboxamide moiety containing derivatives showed good activity compared to the corresponding carbothioamide derivatives. In particular, 4-benzhydrylN-(3-chlorophenyl)-1,4-diazepane-1-carboxamide showed good activity with IC50 value of 18 µM.
1. Introduction
Cancer remains the leading cause of death in the World and as a result there is a pressing need for novel and effective treatments. One of the characteristic of cancer cells, that differs from their normal counterparts in a number of biochemical processes, particularly during the control of cell growth and division. Despite major breakthroughs in many areas of modern medicine over the past 100 years, the successful treatment of cancer remains a significant challenge at the start of the 21st century. Because it is difficult to discover novel agents that selectively kill tumor cells or inhibit their proliferation without the general toxicity, the use of traditional cancer chemotherapy is still very limited. In the field of chemotherapeutic drugs, the search for new, more active, more selective and less toxic compounds is still very intense, and new promising anticancer approaches are being tested [1,2]. Currently, combined anticancer therapies or multi-acting drugs are clinically preferred to traditional cytotoxic treatment, with the aim of overcoming resistance and toxicity drawbacks. These events often prevent successful treatment and are responsible for reduced survival times [3,4]. In the past 50 years, the mass screening of either synthetic derivatives or natural products has led to the discovery of the currently utilized anticancer drugs.
Homopiperazine or 1,4-diazepane ring system has demonstrated considerable utility in drug design, with derivatives demonstrating a wide range of biological activities. In recent years a variety of 1,4-diazepines were reported for inhibition of platelet aggregation [5], peptidoglycan synthesis inhibition [6], 5-HT antagonists [7,8], H3 receptor antagonists [9], as peptidomimetic scaffolds [10], biological tools [11], protein kinase inhibitors [12], matrix metalloproteinase inhibitors [13] (MMPs), and anti-HIV agents [14]. The DNA strand breaking activity was also reported [15] for diaryl diazepine. In concert with this, the development of new synthetic approaches to the 1,4-diazepine ring system and their further elaboration have provided access to a broad range of functionalized derivatives that have contributed to advances in understanding the underlying principles of structure and reactivity. In continuation of our efforts to get new chemotherapeutic agents [16-18], we herein report the synthesis of homopiperazine derivatives and their antiproliferative activity.
2. Methods
2.1. Chemistry
1H NMR spectra were recorded on Shimadzu AMX 400- Bruker, 400 MHz spectrometer using DMSO as a solvent and TMS as internal standard (chemical shift in d ppm). Spin multiplets are given as s (singlet), d (doublet), t (triplet) and m (multiplet). Mass and purity were recorded on an LCeMSD-Trap-XCT. Elemental (CHNS) analyses were obtained on Vario EL III Elementar. Silica gel column chromatography was performed using Merck 7734 silica gel (60 - 120 mesh) and Merck made TLC plates.
2.2. Synthesis of 1-benzhydryl-4-(substituted phenylcarboxamide/carbothioamide)-1,4- diazepane Derivatives 6(a-e) and 7(a-f)
1-Benzhydryl-1,4-diazepane derivatives 6(a-e) were synthesised by the method summarized in Scheme 1. Initially the compound 2, benzhydrol was synthesised by reduction of benzophenone 1 using sodium borohydride and achieved 90% yield. Compound 2 was subsequently treated with thionyl chloride to give benzhydryl chloride 3, which was directly treated with homopiperazine 4 and anhydrous potassium carbonate using dimethyl formamide as a solvent at 80˚C to give the target key intermediates 1-benzhydryl-1,4-diazepane 5. Nucleophilic substitution reaction of compound 5 with different aryl isocyanates and isothiocyanates yielded the target compounds 6(a-e) and 7(a-f).
2.2.1. Synthesis of Benzhydrol (2)
A solution of benzophenone (20.0 g, 109 mmol) in methanol was taken and cooled to 0˚C - 5˚C. Sodium borohydride (8.28 g, 219.5 mmol) was added to the solution and stirred for 5 hr at room temperature. Upon completion, the solvent was removed under reduced pressure and residue was taken in water and extracted with ethyl acetate. Finally water wash was given to the organic layer and dried with anhydrous sodium sulphate. The solvent was evaporated to get benzhydrol.

Reagents and conditions: i). NaBH4, Methanol, r.t., 5 hr. ii). Thionyl chloride, MDC, 0˚C - 5˚C, 4 hr. iii). K2CO3, DMF, 80˚C, 8 hr. iv). Aryl isocyanates or aryl isothiocyanates, MDC, TEA, r.t., 5 - 6 hr.
2.2.2. Synthesis of Benzhydryl Chloride (3)
A solution of benzhydrol (16.0 g, 86.8 mmol) in dry dichloromethane was taken and cooled to 0˚C - 5˚C. Thionyl chloride (30.7 g, 258 mmol) was added to the solution and stirred for 4 - 5 hr at 0˚C - 10˚C. Upon completion, the solvent was removed under reduced pressure and residue was taken in dichloromethane for the removal of excess thionyl chloride to get benzhydryl chloride.
2.2.3. Synthesis of 1-benzhydryl-1,4-diazepane (5)
To a solution of homopiperazine 4 (5.0 g, 49.9 mmol) in dimethyl formamide, anhydrous potassium carbonate (20.7 g, 149.7 mmol) was added followed by the addition benzhydryl chloride (9.1 g, 44.9 mmol) and the reaction mixture was heated to 80˚C for 8 hr. Completion of the reaction was monitored by TLC. After completion, the solvent was removed under reduced pressure and residue was taken in water and extracted with ethyl acetate. Finally, water wash was given to the organic layer and dried with anhydrous sodium sulphate. The solvent was evaporated to get crude product, which was purified by column chromatography over silica gel (60 - 120 mesh) using chloroform: methanol (9:1) as an eluent.
2.2.4. General Procedure for Synthesis of 1- benzhydryl-4-(substituted phenylcarboxamide/ carbothioamide)-1,4-diazepane Derivatives 6(a-e) and 7(a-f)
A solution of 1-benzhydryl-1,4-diazepane (5) (1.0 eq) in dry dichloromethane was mixed. Triethylamine (3.0 eq) was added to the reaction mixture and stirred for 10 min, and then different aryl isocyanates/aryl isothiocyanates (1.0 eq) were added. The reaction mixture was stirred for 5 - 6 hr at room temperature, and monitored by TLC. Upon completion, the solvent was removed under reduced pressure and residue was taken in water and extracted with ethyl acetate. The organic layer was washed with 10% ammonium chloride solution and finally water wash was given to the organic layer and dried with anhydrous sodium sulphate. The solvent was evaporated to get crude product, which was purified by column chromatography over silica gel (60 - 120 mesh) using hexane: ethyl acetate (8:2) as an eluent.
2.2.4.1. Synthesis of 4-benzhydryl-N-(3-chlorophenyl)-1, 4-diazepane-1-carboxamide (6a)
The product 6a was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 3-chlorophenyl isocyanate (0.29 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSOd6, 400 MHz) d: 8.76 (s, 1H, -NH), 8.52 (t, 1H, Ar-H), 8.29 (m, 1H, Ar-H), 8.16 (m, 1H, Ar-H), 7.92 (s, 1H, ArH), 7.42 (d, 4H, Ar-H), 7.29 (t, 4H, Ar-H), 7.13 (m, 2H, Ar-H), 4.73 (s, 1H, -CH-), 2.67 (d, 4H, -CH2-), 2.41 (d, 4H, -CH2-), 1.65 (m, 2H, -CH2-). MS (ESI, + ion): m/z = 420.2. Elemental Analysis: Found: C, 71.61; H, 6.29; N, 9.89; Calculated for C25H26ClN3O: C, 71.50; H, 6.24; N, 10.01.
2.2.4.2. Synthesis of 4-benzhydryl-N-(4-fluorophenyl)-1, 4-diazepane-1-carboxamide (6b)
The product 6b was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 4-flurophenyl isocyanate (0.26 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described.
1H NMR (DMSO-d6, 400 MHz) d: 8.65 (s, 1H, -NH), 7.92 (d, 2H, Ar-H), 7.83 (d, 2H, Ar-H), 7.48 (t, 4H, ArH), 7.31 (m, 4H, Ar-H), 7.18 (m, 4H, Ar-H), 4.74 (s, 1H, -CH-), 2.93 (m, 4H, -CH2-), 2.78 (d, 4H, -CH2-), 1.52 - 1.63 (m, 2H, -CH2-). MS (ESI, + ion): m/z = 404.2. Elemental Analysis: Found: C, 74.49; H, 6.58; N, 10.32; Calculated for C25H26FN3O: C, 74.42; H, 6.49; N, 10.41.
2.2.4.3. Synthesis of 4-benzhydryl-N-(2,4-dichloro phenyl)-1,4-diazepane-1-carboxamide (6c)
The product 6c was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 2,4-dichlorophenyl isocyanate (0.35 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSOd6, 400 MHz) d: 8.72 (s, 1H, -NH), 7.98 (d, 1H, Ar-H), 7.90 (d, 1H, Ar-H), 7.75 (t, 2H, Ar-H), 7.41 (m, 4H, ArH), 7.29 (m, 4H, Ar-H), 7.17 (m, 4H, Ar-H), 4.86 (s, 1H, -CH-), 2.65 (t, 4H, -CH2-), 2.53 - 2.58 (m, 4H, -CH2-), 1.62 (m, 2H, -CH2-). MS (ESI, + ion): m/z = 454.9. Elemental Analysis: Found: C, 65.98; H, 5.59; N, 9.22; Calculated for C25H25Cl2N3O: C, 66.08; H, 5.55; N, 9.25.
2.2.4.4. Synthesis of 4-benzhydryl-N-(3- methoxyphenyl)-1,4-diazepane-1-carboxamide (6d)
The product 6d was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 3-methoxyphenyl isocyanate (0.28 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSOd6, 400 MHz) d: 8.69 (s, 1H, -NH), 8.48 (t, 1H, Ar-H), 8.27 (m, 1H, Ar-H), 8.11 (m, 1H, Ar-H), 7.9 (s, 1H, ArH), 7.4-7.48 (d, 4H, Ar-H), 7.27 (t, 4H, Ar-H), 7.14 (m, 2H, Ar-H), 4.72 (s, 1H, -CH-), 2.62 (d, 4H, -CH2-), 2.44 (d, 4H, -CH2-), 1.64 (m, 2H, -CH2-).
MS (ESI, +ion): m/z = 416.3. Elemental Analysis: Found: C, 75.19; H, 6.95; N, 10.19; Calculated for C26H29N3O2: C, 75.15; H, 7.03; N, 10.11.
2.2.4.5. Synthesis of 4-benzhydryl-N-(4-methoxyphenyl)-1,4-diazepane-1-carboxamide (6e)
The product 6e was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 4-methoxyphenyl isocyanate (0.28 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSOd6, 400 MHz) d: 8.84 (s, 1H, -NH), 7.57 (d, 2H, Ar-H), 7.45 (t, 4H, Ar-H), 7.32 (m, 2H, Ar-H), 7.21-7.3 (t, 4H, Ar-H), 7.16 (t, 2H, Ar-H), 4.72 (s, 1H, -CH-), 2.6-2.69 (t, 4H, -CH2-), 2.53 (m, 4H, -CH2-), 2.21 (s, 3H, Ar-CH3), 1.52 - 1.6 (m, 2H, -CH2-).
MS (ESI, +ion): m/z = 416.2. Elemental Analysis: Found: C, 75.17; H, 6.95; N, 10.01; Calculated for C26H29N3O2: C, 75.15; H, 7.03; N, 10.11.
2.2.4.6. Synthesis of 4-benzhydryl-N-(2-chlorophenyl)-1,4-diazepane-1-carbothioamide (7a)
The product 7a was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 2-chlorophenyl isothiocyanate (0.32 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSO-d6, 400 MHz) d: 8.61 (s, 1H, -NH), 8.15 (d, 1H, Ar-H), 7.92 (m, 1H, Ar-H), 7.85 (m, 1H, Ar-H), 7.72 (t, 1H, Ar-H), 7.42 (m, 4H, Ar-H), 7.22 (d, 4H, Ar-H), 7.13 (m, 2H, Ar-H), 4.83(s, 1H, -CH-), 2.72 (d, 4H, -CH2-), 2.31 (d, 4H, -CH2-), 1.74 (m, 2H, -CH2-). MS (ESI, +ion): m/z = 436.2. Elemental Analysis: Found: C, 68.94; H, 5.89; N, 9.49; S, 7.27; Calculated for C25H26ClN3S: C, 68.87; H, 6.01; N, 9.64; S, 7.35.
2.2.4.7. Synthesis of 4-benzhydryl-N-(2-fluorophenyl)-1, 4-diazepane-1-carbothioamide (7b)
The product 7b was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 2-flurophenyl isothiocyanate (0.29 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSO-d6, 400 MHz) d: 8.66 (s, 1H, -NH), 8.19 (d, 1H, Ar-H), 7.94 (m, 1H, Ar-H), 7.82 (m, 1H, Ar-H), 7.74 (t, 1H, Ar-H), 7.45 (m, 4H, Ar-H), 7.27 (d, 4H, Ar-H), 7.16 (m, 2H, Ar-H), 4.87(s, 1H, -CH-), 2.76 (d, 4H, -CH2-), 2.34 (d, 4H, -CH2-), 1.75 (m, 2H, -CH2-). MS (ESI, +ion): m/z = 420.2. Elemental Analysis: Found: C, 71.66; H, 6.27; N, 10.16; S, 7.77; Calculated for C25H26FN3S: C, 71.57; H, 6.25; N, 10.02; S, 7.64.
2.2.4.8. Synthesis of 4-benzhydryl-N-(4-fluorophenyl)-1, 4-diazepane-1-carbothioamide (7c)
The product 7c was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 4-flurophenyl isothiocyanate (0.29 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSO-d6, 400 MHz) d: 8.59 (s, 1H, -NH), 7.92 (d, 2H, Ar-H), 7.83 (d, 2H, Ar-H), 7.48 (t, 4H, Ar-H), 7.31 (m, 4H, Ar-H), 7.18 (m, 4H, Ar-H), 4.74 (s, 1H, -CH-), 2.93 (m, 4H, -CH2-), 2.78 (d, 4H, -CH2-), 1.52-1.63 (m, 2H, -CH2-). MS (ESI, + ion): m/z = 420.2. Elemental Analysis: Found: C, 71.68; H, 6.22; N, 9.91; S, 7.70; Calculated for C25H26FN3S: C, 71.57; H, 6.25; N, 10.02; S, 7.64.
2.2.4.9. Synthesis of 4-benzhydryl-N-(3-methoxyphenyl)-1,4-diazepane-1-carbothioamide (7d)
The product 7d was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 3-methoxyphenyl isocyanate (0.28 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSOd6, 400 MHz) d: 8.61 (s, 1H, -NH), 7.59 (d, 2H, Ar-H), 7.45 (t, 4H, Ar-H), 7.32 (m, 2H, Ar-H), 7.21-7.3 (t, 4H, Ar-H), 7.16 (t, 2H, Ar-H), 4.72 (s, 1H, -CH-), 2.6-2.69 (t, 4H, -CH2-), 2.53 (m, 4H, -CH2-), 2.21 (s, 3H, -OCH3), 1.52 - 1.6 (m, 2H, -CH2-). MS (ESI, +ion): m/z = 432.1. Elemental Analysis: Found: C, 72.29; H, 6.69; N, 9.81; S, 7.55; Calculated for C26H29N3OS: C, 72.35; H, 6.77; N, 9.74; S, 7.43.
2.2.4.10. Synthesis of 4-benzhydryl-N-(4-methoxyphenyl)-1,4-diazepane-1-carbothioamide (7e)
The product 7e was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), 4-methoxyphenyl isothiocyanate (0.28 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSO-d6, 400 MHz) d: 8.62 (s, 1H, -NH), 7.57 (d, 2H, Ar-H), 7.45 (t, 4H, Ar-H), 7.32 (m, 2H, Ar-H), 7.27 (t, 4H, Ar-H), 7.16 (t, 2H, Ar-H), 4.72 (s, 1H, -CH-), 2.67 (t, 4H, -CH2-), 2.53 (m, 4H, -CH2-), 2.21 (s, 3H, -OCH3), 1.52 - 1.6 (m, 2H, -CH2-). MS (ESI, +ion): m/z = 432.2. Elemental Analysis: Found: C, 72.39; H, 6.82; N, 9.75; S, 7.33; Calculated for C26H29N3OS: C, 72.35; H, 6.77; N, 9.74; S, 7.43.
2.2.4.11. Synthesis of 4-benzhydryl-N-phenyl-1,4- diazepane-1-carbothioamide (7f)
The product 7f was obtained by reaction of 1-benzhydryl-1,4-diazepane (5) (0.50 g, 1.88 mmol), phenyl isothiocyanate (0.25 g, 1.88 mmol) and triethylamine (0.57 g, 5.64 mmol) in dichloromethane using the general experimental procedure as described. 1H NMR (DMSOd6, 400 MHz) d: 8.60 (s, 1H, -NH), 7.75 (d, 2H, Ar-H), 7.52 (m, 6H, Ar-H), 7.45(m, 5H, Ar-H), 7.21 (t, 2H, ArH), 4.74 (s, 1H, -CH-), 2.93 (d, 4H, -CH2-), 2.65 (d, 4H, -CH2-), 1.53 (m, 2H, -CH2-). MS (ESI, +ion): m/z = 402.2. Elemental Analysis: Found: C, 74.61; H, 6.89; N, 10.29; S, 8.07; Calculated for C25H27N3S: C, 74.77; H, 6.78; N, 10.46; S, 7.98.
2.3. Biology
2.3.1. Cell Lines and Culture Conditions
B-cell leukemia cell line (Reh), a kind gift from Dr. Michael R. Lieber, USA, was used for the present study. The cells were cultured in RPMI 1640 (SERA LAB, UK) containing 10% FBS (GIBCO BRL, USA), 100 U of Penicillin G/ml and 100 μg of streptomycin/ml (Sigma– Aldrich, USA) at 37˚C in a humidified atmosphere containing 5% CO2.
2.3.2. Trypan Blue Assay
The cytotoxicity induced by 6(a-e) and 7(a-f) were tested on Reh cells by using trypan blue dye exclusion assay [19,20]. Reh cells were seeded at a density of 1 × 105 cells/ml, grown for 24 h and the compounds were added at a concentration of 10, 50, 100 and 250 μM. DMSO treated Reh cells were used as a vehicle control. Following treatment with the compounds, the cells were collected at an interval of 24 h and resuspended in 0.4% Trypan blue (Sigma-Aldrich, USA). The number of viable cells was counted using haemocytometer. The IC50 value (50% inhibition of cell growth) was estimated following 48 and 72 h of treatment with the respective compounds. Each experiment was repeated a minimum of 3 times and the values obtained were plotted as a graph.
2.3.3. MTT Assay
Effect of 6(a-e) and 7(a-f) on cell proliferation was tested by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [19,21]. Reh cells were seeded in duplicates in a 96-well plate at 1 × 105 cells/well. After 24 h of cell culture, the compounds were added at a concentration of 10, 50, 100 and 250 μM and incubated for 24, 48 and 72 h. The cells were harvested after appropriate time intervals and the MTT reagent (5 mg/ml, Sigma-Aldrich, USA) was added and incubated for 4 h. The insoluble MTT formazan products were then solubilized in a detergent containing 50% N, N-dimethylformamide (Sigma-Aldrich, USA) and 10% SDS (Amresco, USA) and incubated for 2 h. The absorbance was measured at 570 nm on a multiwell ELISA plate reader (Molecular Devices, USA) scanning spectrophotometer. Cells grown in culture media alone or in DMSO were used as controls. The data showing effect on cell proliferation of Reh cells by HPI and HPIS series compounds are expressed as percentage of inhibition. The experiment was repeated three independent times and the values obtained were plotted as a bar diagram indicating error bars.
3. Results and Discussion
In the present study we investigated the cytotoxic effect of 6(a-e) and 7(a-f) on the B-cell leukemia cell line, Reh. Cells were treated with 10, 50, 100 and 250 µM of 6(a-e) and 7(a-f) and subjected to trypan blue assay. The cells were counted at an interval of 24 h, till the cells attained a stationary phase.
Cells treated with DMSO were used as a vehicle control. Results showed that addition of 6(a-e) and 7(a-f) affected the cell viability at higher concentrations and induced cell death in a timeand dose-dependent manner (Figure 1). However, in case of most of the compounds, the cell viability was not affected at the lowest concentration (10 µM) studied (Figure 1)
Among the compounds studied, 6a was the most sensitive, showing the lowest IC50 value of approximately 18µM, at 72 h of treatment, (Table 1). Thus, the trypan blue assay results suggest that most of the compounds studied were less toxic to Reh cells.
The effect of 6(a-e) and 7(a-f) on cell proliferation

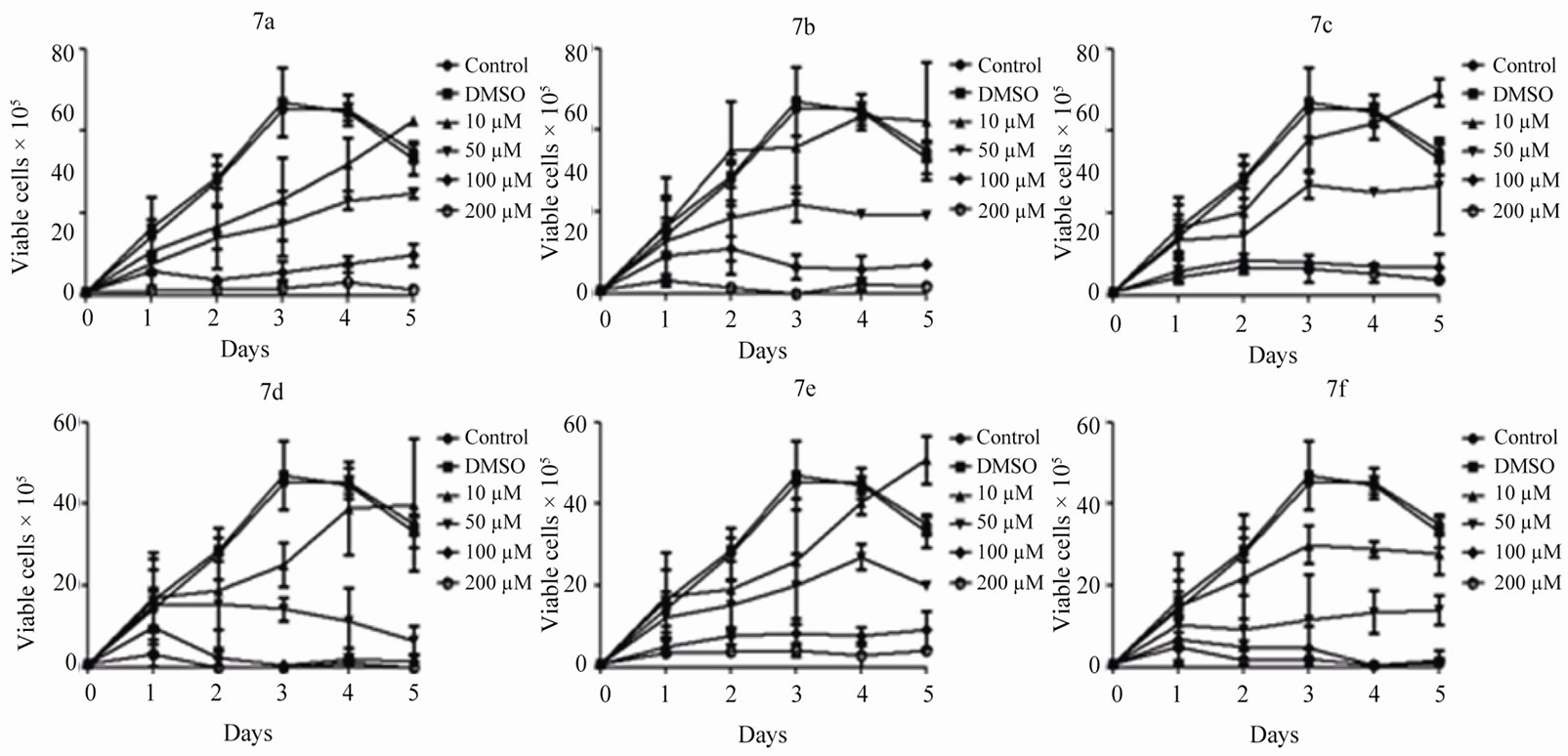
Figure 1. Cytotoxic effect of 6(a-e) and 7(a-f) on B-cell leukemic cell line, Reh. Approximately 1 × 105 cells/ml were cultured and the compounds were added after 24 h at a concentration of 10, 50, 100 and 250 µM. Cells alone and cells treated with DMSO were used as control and vehicle control, respectively. After addition of the compound, live cells were counted following staining with trypan blue at an interval of 24 h, till the cells reached stationary phase. Data obtained were plotted as a graph and error bars are indicated.

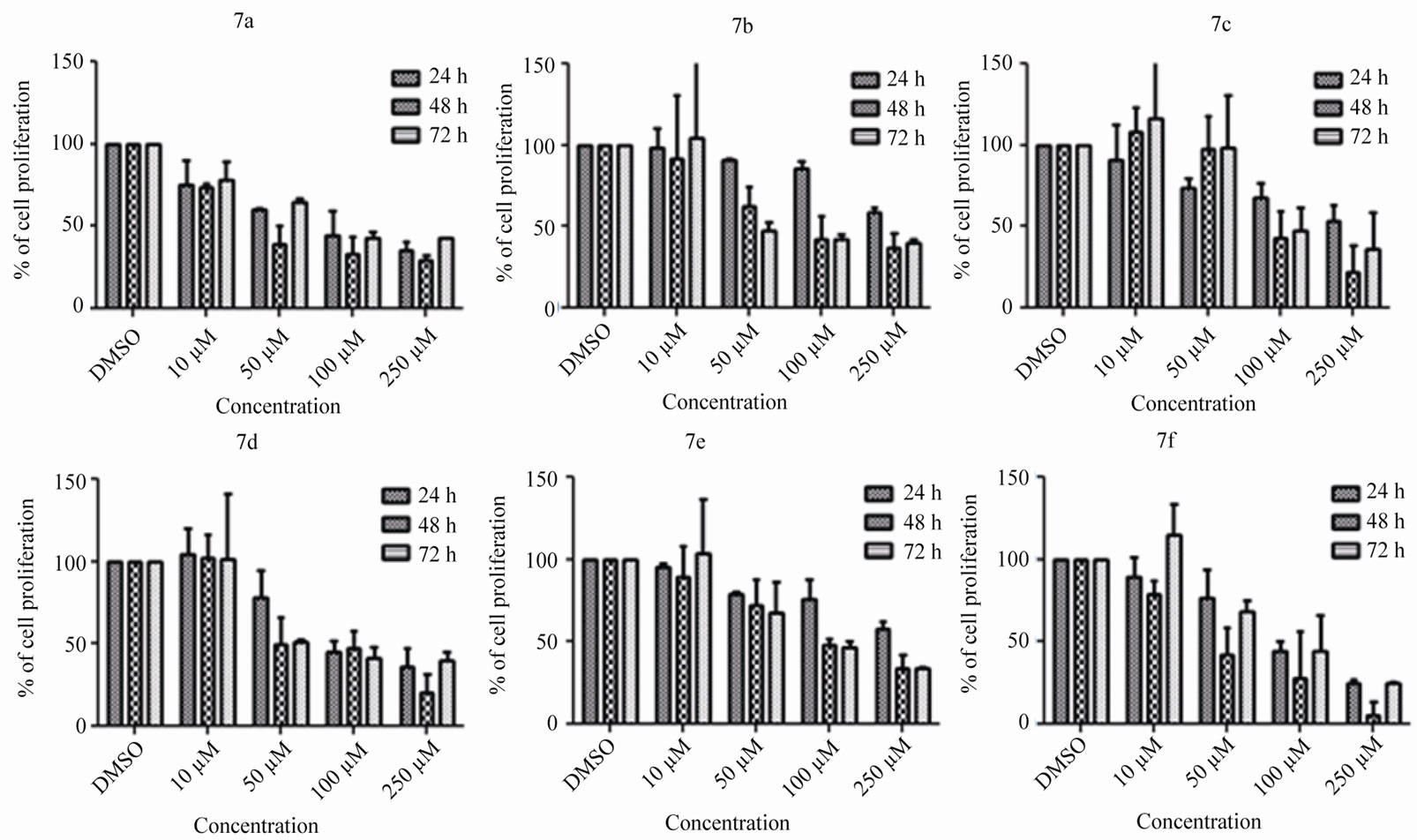
Figure 2. Determination of effect of 6(a-e) and 7(a-f) on cell proliferation by MTT assay. Reh cells grown for 24 h were treated with 6(a-e) and 7(a-f) at a concentration of 10, 50, 100 and 250 µM. The cells were collected following 24, 48 and 72 h of treatment and subjected to MTT assay as specified in methods. The proliferation of DMSO treated (vehicle control) Reh cells was considered as 100% and the relative inhibition following treatment with compounds are shown as a bar diagram. Error bars are also indicated.


Table 1. IC50 value of 6(a-e) and 7(a-f) was calculated based on MTT assay at 72 h in Reh cell lines.
was tested by using MTT assay. Reh cells were treated with 10, 50, 100 and 250 µM of 6(a-e) and 7(a-f) series of compounds as specified above. Cells were harvested after 24, 48 and 72 h and were subjected to MTT assay Results showed that 6(a-e) affected the cell proliferation at a concentration of 50 µM onwards (Figure 2).
When we compare the activities of these two series of compounds 6(a-e) and 7(a-f), we observed that the compounds with carboxamide functionality showed good activity compared with the corresponding carbothioamide derivatives. Compound 6b with 4-fluorophenyl carboxy group attached to homopiperazine showed good activity with IC50 value of 30 µM compared to compound 7c with 4-fluorophenyl carbothio group attached to homopiperazine with IC50 value 65 µM. In the same way, compounds 6d and 6e with 3-methoxy and 4-methoxy groups on the phenyl ring of aryl carboxy moiety sowed good activity with IC50 values of 30 µM and 42 µM respectively compared to the compounds 7d and 7e having 3- methoxy and 4-methoxy groups on the phenyl ring of aryl carbothio moiety attached to homopiperazine with IC50 values of 55 µM and 70 µM respectively. From the above observations, it is clear that compounds with aryl carboxy moiety attached to homopiperazine showed good activity compared to the corresponding aryl carbothio moiety containing compounds.
4. Conclusions
In conclusion, we synthesized a series of novel 1-benzhy-dryl-4-(substituted phenylcarboxamide/carbothioamide)-1,4-diazepane and investigated their antiproliferative activity on Reh cells. Compounds with carboxamide linkage showed good activity compared to that of compounds with carbothioamide linkage. Compound 6a showed good potency activity compared to all the compounds tested. Further derivatisation and studies to know the mechanism of action are underway.
5. Acknowledgements
The authors are grateful to UGC and CSIR for financial support to KSR. We thank Mridula Nambiar for critical reading of the manuscript. This work was supported by Lady Tata Memorial Trust international award for leukemia research (London) for SCR. KPM is supported by IISc postdoctoral fellowship, Bangalore, India.
REFERENCES
- C. Sawyers, “Targeted Cancer Therapy,” Nature, Vol. 432, 2004, pp. 294-297.
- Q. Li and W. Xu, “Novel Anticancer Targets and Drug Discovery in Post Genomic Age,” Current Medicinal Chemistry Anticancer Agents, Vol. 5, 2005, pp. 53-63.
- S. K. Mencher and L. G. Wang, “Promiscuous Drugs Compared to Selective Drugs,” BMC Clinical Pharmacology, Vol. 5, 2005, pp. 3-9.
- A. Jimeno and M. Hidalgo, “Multitargeted Therapy: Can Promiscuity Be Praised in an Era of Political Correctness?” Critical Reviews in Oncology/Hematology, Vol. 59, No. 2, 2006, pp. 150-158. doi:j.critrevonc.2006.01.005
- Y. Kawakami, H. Kitani, S. Yuasa, M. Abe, M. Moriwaki, M. Kagoshima, M. Terasawa and T. Tahara, “Structural Optimization of 4-(2-Chlorophenyl)-9-methyl-6H-thieno [3, 2-f]-[1,2,4]triazolo[4,3-a][1,4]diazepines as Antagonists for Platelet Activating Factor: Pharmacological Contribution of Substituents at the 2- and 6-Positions of a Condensed Ring System,” European Journal of Medicinal Chemistry, Vol. 31, No. 9, 1996, pp. 683-692. doi:0223-5234(96)85877-6
- K. Spencer, N. Santosh and L. Resnick, “Synthesis of the Liposidomycin Diazepanone,” Tetrahedron Letters, Vol. 33, No. 38, 1992, pp. 5485-5486. doi:S0040-4039(00)61123-1
- S. Kato, H. Harada, and T. Morie, “Efficient Synthesis of (6R)-6-Amino-1-methyl-4-(3-methylbenzyl)hexahydro-1H-1,4-diazepine from Methyl (2R)- and (2S)-1-Benzyloxy-carbonylaziridine-2-carboxylates,” Journal of the Chemical Society, Perkin Transactions, Vol. 1, 1997, pp. 3219-3225. doi:10.1039/a703661b
- Y. Hirokawa, I. Fujiwara, K. Suzuki, H. Harada, T. Yoshikawa, N. Yoshida, and S. Kato, “Synthesis and Structure-Affinity Relationships of Novel N-(1-Ethyl-4- methylhexahdro-1,4-diazepiN-6-yl)pyridine-3-carboxamides with Potent Serotonin 5-HT3 and Dopamine D2 Receptor Antagonistic Activity,” Journal of Medicinal Chemistry, Vol. 46, 2003, pp. 702-715. doi:10.1021/jm020270n
- M. P. Curtis, W. Dwight, J. Pratt, M. Cowart, T. A. Esbenshade, K. M. Kruger, G. B. Fox, J. B. Pan, T. G. Pagano, A. A. Hancock, R. Faghih and Y. Bennani, “DAmino Acid Homopiperazine Amides: Discovery of A-320436, a Potent and Selective Non-Imidazole Histamine H3-Receptor Antagonist,” Archiv der Pharmazie, Vol. 337, No. 4, 2004, pp. 219-229. doi:10.1002/ardp.200300844
- C. Taillefumier, S. Thielges, et al., “Anomeric Spiroannelated 1,4-Diazepine 2,5-diones from Furano Exo-Glycals: Towards a New Class of Spironucleosides,” Tetrahedron, Vol. 60, No. 10, 2004, pp. 2213-2224.
- D. J. Lauffer and M. D. Mullican, “A Practical Synthesis of (S) 3-Tert-butoxycarbonylamino-2-oxo-2,3,4,5-tetrahydro-1,5-benzodiazepine-1-acetic Acid Methyl Ester as a Conformationally Restricted Dipeptido-Mimetic for Caspase-1 (ICE) Inhibitors,” Bioorganic and Medicinal Chemistry Letters, Vol. 12, No. 8, 2002, pp. 1225-1227.
- S. A. Lakatosh, Y. N. Luzikov and M. N. Preobrazhenskaya, “Synthesis of 6H-Pyrrolo[3’,4’:2,3][1,4]diazepino [6,7,1-hi]indole-8,10(7H,9H)-diones Using 3-Bromo-4- (indol-1-yl) Maleimide Scaffold,” Organic & Biomolecular Chemistry, Vol. 1, 2003, pp. 826-833. doi:10.1039/b211163b
- J. I. Levin, J. F. Dijoseph, L. M. Killar, A. Sung, T. Walter, M. A. Sharr, C. E. Roth, J. S. Skotnicki and J. D. Albright, “The Synthesis and Biological Activity of a Novel Series of Diazepine MMP Inhibitors,” Bioorganic and Medicinal Chemistry Letters, Vol. 8, No. 19, 1998, pp. 2657- 2662. doi:S0960-894X(98)00473-9
- Y. L. Janin, A. M. Aubertin, A. Chiaroni, C. Riche, C. Monneret, E. Bisagani and D. S. Grierson, “Imidazo (1,5-G)(1,4)diazepines, TIBO Analogues Lacking the Phenyl Ring: Synthesis and Evaluation as Anti-HIV Agents,” Tetrahedron, Vol. 52, No. 48, 1996, pp. 15157- 15170.
- N. Mibu, M. Yukawa, N. Kashige, Y. Iwase, Y. Goto, F. Miake, T. Yamaguchi, S. Ito and K. Sumoto, “Synthesis and DNA Strand Breakage Activity of Some 1,4-Diazepines,” Chemical & Pharmaceutical Bulletin, Vol. 51, No. 127, 2003, pp. 27-31. doi:10.1248/cpb.51.27
- D. S. Prasanna, C. V. Kavitha, K. Vinaya, S. R. Ranganatha, Sathees C. Raghavan and K. S. Rangappa, “Synthesis and Identification of a New Class of Antileukemic Agents Containing 2-(Arylcarboxamide)-(S)-6-amino-4,5, 6,7-tet-rahydrobenzo[d]thiazole,” European Journal of Medicinal Chemistry, Vol. 45, No. 11, 2010, pp. 5331- 5336.
- D. S. Prasanna, C. V. Kavitha, B. Raghava, K. Vinaya, S. R. Ranganatha, S. C. Raghavan and K. S. Rangappa, “Synthesis and Identification of a New Class of (S)-2,6- Diamino-4,5,6,7-tetrahydrobenzo[d]thiazole Derivatives as Potent Antileukemic Agents,” Investigational New Drugs, Vol. 28, No. 4, 2010, pp. 454-465.
- D. S. Prasanna, C. V. Kavitha, K. Vinaya, S. R. Ranganatha, B. Raghava, Y. C. Sunil Kumar, Sathees C. Raghavan, K. S. Rangappa, “Synthesis and Antileukemic Activity of 1-((S)-2-Amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)-3-(substituted phenyl)urea Derivatives,” Bulletin of the Chemical Society of Japan, Vol. 83, No. 6, 2010, pp. 689-697. doi:10.1246/bcsj.20090318
- C. V. Kavitha, M. Nambiar, C. S. Ananda Kumar, B. Choudhary, K. Muniyappa, K. S. Rangappa and S. C. Raghavan, “Novel Derivatives of Spirohydantoin Induce Growth Inhibition Followed by Apoptosis in Leukemic Cells,” Biochemical Pharmacology, Vol. 77, No. 3, 2009, pp. 348-363. doi:j.bcp.2008.10.018
- K. K. Chiruvella, V. Kari, B. Choudhary, M. Nambiar, R. G. Ghanta and S. C. Raghavan, “Methyl Angolensate, a Natural Tetranortriterpenoid Induces Intrinsic Apoptotic Pathway in Leukemic Cells,” FEBS Letters, Vol. 582, No. 29, 2008, pp. 4066-4076.
- M. S. Shahabuddin, M. Nambiar, B. Choudhary, G. M. Advirao and S. C. Raghavan, “A Novel DNA Intercalator, Butylamino-pyrimido[4’,5’:4,5] selenolo(2,3-b)quinoline, Induces Cell Cycle Arrest and Apoptosis in Leukemic Cells,” Investigational New Drugs, Vol. 28, No. 1, 2010, pp. 35-48.

