Chinese Medicine
Vol. 3 No. 1 (2012) , Article ID: 18125 , 7 pages DOI:10.4236/cm.2012.31011
In Vitro Antiprotozoal and Cytotoxic Activity of the Aqueous Extract, the 80% Methanol Extract and Its Fractions from the Seeds of Brucea sumatrana Roxb. (Simaroubaceae) Growing in Democratic Republic of Congo
1Faculty of Pharmaceutical Sciences, University of Kinshasa, Kinshasa XI, Democratic Republic of Congo
2Laboratory of Pharmacognosy and Pharmaceutical Analysis, Department of Pharmaceutical Sciences, University of Antwerp, Antwerp, Belgium
3Laboratory of Microbiology, Parasitology and Hygiene (LMPH), Faculty of Pharmaceutical, Biomedical and Veterinary Sciences, University of Antwerp, Antwerp, Belgium
Email: kanyanga.cimanga@ua.ac.be
Received July 13, 2011; revised July 30, 2011; accepted August 22, 2011
Keywords: Brucea sumatrana; Simaroubaceae; Seeds; Antiprotozoal Activity; Cytotoxicity
ABSTRACT
The in vitro antiprotozoal and cytotoxic activity of the aqueous extract, the 80% methanol extract, and its different soluble fractions and subfractions from Brucea sumatrana seeds were assessed against two Trypanosoma (T. cruzi and T. brucei brucei), Leishmania infantum and chloroquine and pyrimethanine-resistant K1 strain of P. falciparum and against MRC-5 cell-lines respectively. Results indicated that the 80% methanol extract showed a cytotoxic effect against MRC-5 cell lines with CC50 value of 0.54 µg/ml. It however exhibited pronounced and non selective activity against T. cruzi (IC50 = 1.52 µg/ml, SI = 0.03) and L. infantum (IC50 = 2.41 µg/ml, SI = 0.22). It however displayed pronounced and selective effect against T. brucei brucei (IC50 < 0.25, SI > 2.16) and chloroquine and pyrimethamine-resistant K1 strain of P. falciparum (IC50 < 0.25 µg/ml, SI > 2.16). All soluble fractions and subfractions from the partition of the 80% methanol extract were found to exhibit an antiprotozoal activity with IC50 values ranging from <0.25 to 30 µg/ml. The most active was the alkaline aqueous soluble fraction exhibiting pronounced antiprotozoal activity against T. cruzi, T. b. brucei, L. infantum and chloroquine and pyrimethamine-resistant K1 strain of P. falciparum with IC50 values of 0.33, <0.25, 0.25, <0.25 µg/ml respectively resulting in high selective index values of 61.36, > 81, 81 and >81 respectively. The chloroform soluble fraction rich in alkaloid was cytotoxic against MRC-5 cell lines (CC50 = 27.09 µg/ml) and showed good activity against T. b. brucei (IC50 = 8.36 and SI = 3.24) and moderate activity against T. cruzi, L. infantum and chloroquine-pyrimethane-resistant K1 strain of P. falciparum (20 < IC50 < 30 µg/ml). Although the aqueous extract (decoction) and the total alkaloids extract showed a cytotoxic effect against MRC-5 cell lines (CC50 = 1.55 and 0.43 µg/ml respectively), they however displayed pronounced antiprotozoal activity against T. cruzi, T. b. brucei and chloroquine and pyrimethamine-resistant K1 strain of P. falciparum with IC50 values ranging from < 0.25 to 0.6 µg/ml with only a selective action against chloroquine and pyrimethamine-resistant K1 strain of P. falciparum (SI = >6.2 and >1.72 respectively). These extracts however showed good and low activity respectively against L. infantum (IC50 = 24.05 and 6.82 µg/ml respectively).
1. Introduction
The plant Brucea sumatrana Roxb., synonyms: Brucea javanica (L.) Merr., B. amarissima Desv. Ex Gomes, Gonus amarissimus Lour or Loussa amarissima O. Ktzeis a shrub belonging to the family Simaroubaceae. It is widely distributed throught Asian Pacific regions including China, Malaysia, Thailand, and Indonesia [1-4]. In these regions, the seeds (fruits) are used for the treatment of malaria, amoebic dysentery, cancer and as insecticide [5-7]. The plant is also found in some African countries and its seeds are used to treat various diseases among which fever including malaria, parasitosis, colonic diseases, headache, nematode diseases, helminthiasis and protozoal diseases [8,9].
The seeds from the Asian species have been previously investigated for its various biological activities such as antimalarial [10-16], cytotoxic and antileukemic [5,17-23], antiprotozoal against amoeba [24,25], Toxoplama gondii, Giardia intestinal [25], Trypanosoma brucei brucei and Leishmania donovani [14], Blastocystis hominis [26], Trypanosoma evansi [27] and Babesia gibsoni [3,28], and anti-inflammatory [29] activities. Different chemical constituents including quassinoids [11,12,17,19,30,31], alkaloids from cell suspension cultures of B. javanica seeds [5, 32] and lignans [20] had been reported to be present in the seeds and recognized to be responsible for different biological activities according to the case.
To our knowledge, no plant part of Brucea sumatrana growing in African countries was not yet phytochemically and biologically investigated. Thus, the present study deals with the assessment of the in vitro antiprotozoal activity of crude extracts, fractions and subfractions from the seeds of Brucea sumatrana growing in Democratic Republic of Congo (DR Congo) against Trypanosoma cruzi, T. brucei brucei, Leishamania infantum and chloroquine and pyrimethamine-resistant K1 strain of P. falciparum. Their putative cytotoxic effect against MRC-5 cell lines growth was also evaluated.
2. Materials and Methods
2.1. Plant Materiel
Seeds of Brucea sumatrana Roxb. (Simaroubaceae) were collected in November 2007 in the district Mayombe’s Bas-Congo in DR Congo (Table 1). The plant was identified by Mr. Ngoma of the Institut de Recherche en Sciences de la Santé (I.R.S.S.) of Kinshasa, DR Congo. A voucher specimen (NM 2112007) of the plant was deposited in the herbarium of this institute. Seeds were dried at room temperature and reduced to powder.
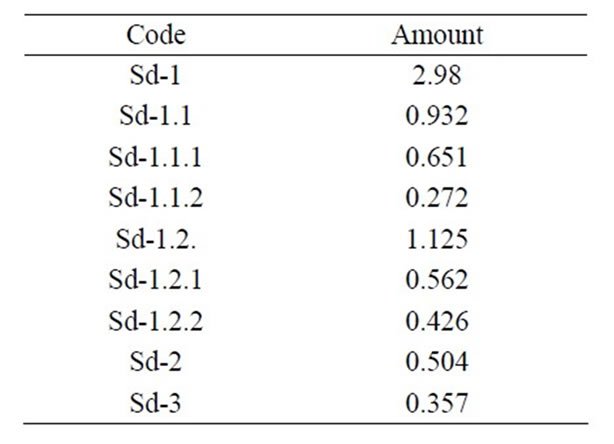
Table 1. Amount (g) of samples from Brucea sumatrana seeds.
2.2. Preparation of Extracts, Fractions and Subfractions
150 g of powdered seeds were submitted to soxhlet extraction with 80% methanol (500 ml) for 2 h. The extractive solvent was filtererd and evaporated in vacuo yieding a dried extract denoted as Sd-1. An amount of Sd-1 (1.5 g) was fractionated according to the procedure previously described [33] (Figure 1). The obtained fractions and subfractions were treated as described above yielding corresponding dried extracts denoted as Sd-1.1 to Sd-1.2.2. On the other hand, 20 g of the plant material were mixed with 100 ml distilled water and boiled for 10 min. After cooling and filtration, the filtrate was evaporated in vacuo yielding dried extract denoted as Sd-2. Another batch of 50 g of plant material was used for the extraction of the total alkaloid extract by the classical acid/base procedure using chloroform as the extractive solvent [34] and denoted as Sd-3.
2.3. Phytochemical Screening
Chemical tests were carried out on the aqueous and 80% methanol extracts from Brucea sumatrana seeds, the fractions and subfractions from the partition of the 80% methanol extract using standard procedures [34]. Saponins were identified by using the frothing test. Alkaloids were detected using Draggendorf’s and Mayer’s reagents resulting in the formation of an orange or yellow-white precipitate respectively as positive test. The presence of flavonoids was determined using aluminium chloride 5% in methanolic solution and Shinoda’s reagent (HCl + magnesium turnins) after heating for 10 min giving yellow and purple colour respectively as positive test. The test for anthraquinones was performed by adding ammonia solution (NH4OH 10% or NaOH 10%) producing red to red-purple colour as positive test. Steroids and terpenes were screened by adding Lieberman-Buchardat’s reagent (acetic anhydride/conc. H2SO4) followed by heating for 10 min until to the appearance of purplishblue colour or other colours as positive test. Tannins

Figure 1. Fractionation of the crude extract [33].
were identified with gelatin and FeCl3 5%. Moreover, the presence of alkaloids, flavonoids, steroids, terpenoids and anthraquinones was confirmed by TLC (thin layer chromatography) analysis performed on silicagel 60F254 plates Merck (thickness layer 0.25 mm) using CHCl3/ Methanol/NH3 25%: 8:2:0.5 and Ethyl acetate/iso-Propanol/NH3 25%: 16:3:1 as mobile phases, and Draggendorf’s reagent for alkaloids; n-Butanol/Acetic acid/Water: 4:1:5 (top layer) as mobile phase and Neu’s reagent (1% diphenylboric ethanolamine acid in methanol) for flavonoids; Ethylace-tate/Methanol/Water: 8:1:1 as a mobile phase and magnesium acetate 5% in methanol, and NaOH 10% or NH4OH 10% as reagents for anthraquinones; Chloroforme/Methanol: 9:1 and n-Hexane/Dichloromethane: 1:9 as mobile phases and LiebermanBuchardat’s reagent and Vanilline 1%/H2SO4 5% in methanol for steroids and terpenoids. Coumarins were detected under UV (366 nm) thanks to their blue fluorescence which becomes intense after spraying KOH 10%. Anthocyanins were detected after heating 0.01 g of each extract dissolved in 10 ml distilled water with 2 M HCl for 5 min at 100˚C producing a red colour which can be extracted into amyl alcohol or by adding 2 M NaOH dropwise giving a blue colour which change to green and becomes slowly fade.
2.4. Assay for in Vitro Antitrypanosomal and Antilesmanial Activity
All samples were tested against Trypanosoma brucei brucei, Trypanosoma cruzi and Leishmania infantum bloodstream forms from axenic cultures in HMI-18 medium obtained from Prof. L. Maes of the Laboratory of Microbiology, Parasitology and Hygiene, Faculty of Pharmaceutical Sciences, Biomedical and Veterinary Sciences of the University of Antwerp, Belgium. Assays were performed in 96 well tissue plates, each containing 10 µl aqueous extract dilutions ranging from 100 to 0.01 µg/ml together with 190 µl of the parasite suspension (5 × 104 parasites/ml) in Hirumi (HMI) medium supplemented with 10% foetal calf serum and a solution of 5000 units penicillin/ml and 5000 µg streptomycin/ml, final concentration 2% in medium (2% P/S solution). All plates were incubated for 4 days in humidified atmosphere at 37˚C in 5% CO2. Two hours before the end of the incubation, 10 µl of Alamar BlueÒ solution were added. Fluorescence was measured after 4 hours of incubation with the Alamar BlueÒ in a fluorescence plate reader at 530 nm excitation and 590 nm emission wavelengths. The IC50 values were calculated by linear interpolation selecting values above and below the 50% mark. Melarsoprol, Benznidazol and Miltefosine were used as reference products against T. b. brucei, T. cruzi and L. infantum respectively [35].
2.5. Assay for in Vitro Antiplasmodial Activity
Extracts, fractions and subfractions were tested against the chloroquine and pyrimethanine-resistant K1strain of Plasmodium falciparum obtained from Prof L. Maes of the laboratory of Microbiology, Parasitology and Hygiene, Faculty of Pharmaceutical Sciences, Biomedical and Veterinary Sciences of the University of Antwerp, Belgium. Samples were tested against chloroquine and pyrimethamine-resistant K1 strain of Plasmodium falciparum maintained in continuous log phase growth in RPMI-1640 medium supplemented with 2% P/S solution, 0.37 mM hypoxanthine, 25 mM HEPES, 25 mM NaHCO3 and 10% O+ human serum together with 4% human O+ erythrocytes according to the method previously described [36]. All cultures and assays were conducted at 37˚C under micro-aerophilic atmosphere (4% CO2, 3% O2 and 93% N2). The in vitro antimalarial activity was assessed using an adaptation of the procedure previously described as the lactate dehydrogenase assay [37]. Twenty milligrams of each extract were dissolved in 1 ml DMSO and serially diluted tenfold with culture medium before being added to asynchronous parasite cultures. Assays were performed in 96-well tissue culture plates, each well containing 10 µl of the test sample dilutions together with 190 µl of the parasite inoculum (1% parasitaemia, 2% haematocrit). After 72 hours of incubation at 37˚C, plates were stored at –20˚C until further processing. After thawing, 20 µl of haemolysed parasite suspension from each well was transferred into another plate together with 100 µl MalstatTM reagent and 10 µl of a 1/1 mixture of PES (phenazine ethosulfate, 2 mg/ml) and NBT (nitro blue tetrazolium grade III, 0.1 mg/ml). The plates were kept in the dark for 2 hours and change in colour was measured spectrophotometrically at 655 nm. The results were expressed as percentage reduction in parasitaemia compared to control wells. The concentration causing 50% inhibition of parasite growth (IC50) was calculated from the drug concentration—response curves. Chloroquine diphosphate was used as an antiplasmodial reference drug. Each assay was done in triplicate [35].
2.6. Cytotoxicity Assay
2.6.1. Cell Cultures
Cell lines MRC-5 (human lung fibroblast) were cultured in MEM medium, supplemented with 20 mM L-glutamine, 16.5 mM NaHCO3, 5% foetal calf serum and 2% P/S solution. All cultures were kept at 37˚C and 5% CO2.
2.6.2. Testing
Assays were performed in sterile 96-well tissue culture plates, each well containing 10 µl of each sample dilutions together with 190 µl of cell suspension (2.5 × 104 cells/ml). After 7 days incubation, cell proliferation/viability was assessed after addition of MTT (Sigma) (50 µl of a 1/ 2.5 solution per well). After 4 hours of incubation at 37˚C, the % absorbance reduction at 540 nm for the treated cultures and untreated control cultures were obtained and compared, and CC50 values (50% cytotoxic concentration) were determined [35].
3. Results and Discussion
The aqueous extract (decoction), the 80% methanol extract and its different soluble fractions and subfractions were screened for their potential antiprotozoal activity against Trypanosoma cruzi, T. brucei brucei, Leishmania infantum and chloroquine and pyrimethamine-resistant K1 strain of P. falciparum as well as their cytotoxic effect against MRC-5 cell lines. The following criteria were used to appreciate more the level of their activity: IC50 £ 5 µg/ml: pronounced activity, 5 < IC50 £ 20: good activity, 20 < IC50 £ 30: moderate activity, 30 < IC50 £ 60: low activity, IC50 > 64 µg/ml: inactive. A sample is considered as cytotoxic when CC50 < 32 µg/ml [34]. For the samples tested, the cytotoxicity to MRC-5 cell lines and activity have been compared using the selectivity index (SI) ration (SI = CC50 MRC-5 cells/IC50 protozoa). A value >1 is considered to be selective against the test protozoa and a value <1 is considered as selective against MRC-5 cell lines [14,38].
Results in Table 2 show that the 80% methanol extract (Sd-1) possessed a cytotoxic effect against MRC-5 cell lines growth with a CC50 value of 0.54 µg/ml. It however exhibited pronounced antiprotozoal activity against Trypanosoma cruzi, T. b. brucei, Leishmania infantum and chloroquine and pyrimethamine-resistant K1strain of Plasmodium falciparum with IC50 values of 1.52, <0.25, 2.41 and <0.25 µg/ml respectively. In evaluating the selectivity index (SI) of this extract (ratio CC50/IC50), it was observed that the resulting values are <1 indicating its selective action against MRC-5 cell lines than against T. cruzi and L. infantum, and confirmed that the observed activity against these two protozoa is due to the cytotoxic effect of the extract since the extract had high selective action against MRC-5 cell lines than the test parasites [14,38]. Its activity against T. b. brucei and chloroquine and pyrimethamine-resistant K1 strain of P. falciparum was selective since the SI value was >1 (Table 3) [14,38]. In a previous study, a methanol extract from the fruits of B. javanica collected in Thailand showed a cytotoxic effect against KB cells (CC50 = 9.20 ± 0.23 µg/ml) and exhibited an activity against Leishmania donovani and Trypanosoma brucei brucei trypamastigotes with IC50 values of 246.68 ± 1.20 and 500.00 ± 2.60 µg/ml respectively. This activity was related to its cytotoxic effect since the selectivity index values were 0.037 and 0.018 respectively [14].
In the present investigation, all soluble fractions and subfractions from the partition of the 80% methanol extract seeds (Sd-1) exhibited antiprotozoal activity at different extents. Briefly, the chloroform soluble fraction (Sd-1.1) from Sd-1 was devoid of cytotoxic effect against MRC-5 cell lines, and showed low and selective activity against T. cruzi and T. b. brucei with IC50 values 30 < IC50 < 60 µg/ml and was inactive against L. infantum (IC50 > 64 µg/ml) (Table 2). It however displayed pronounced activity against chloroquine and pyrimethamine-resistant K1 strain of P. falciparum with IC50 value of 2.25 µg/ml. In assessing its selectivity index, the resulting value was high (>28.24) indicating its high selective effect against this parasite. It had also good selective effect against T. cruzi and T. b. brucei since the selectivity index value were appreciable (Table 3). The petroleum ether (Sd-1.1.1) rich in lipids and waxes and
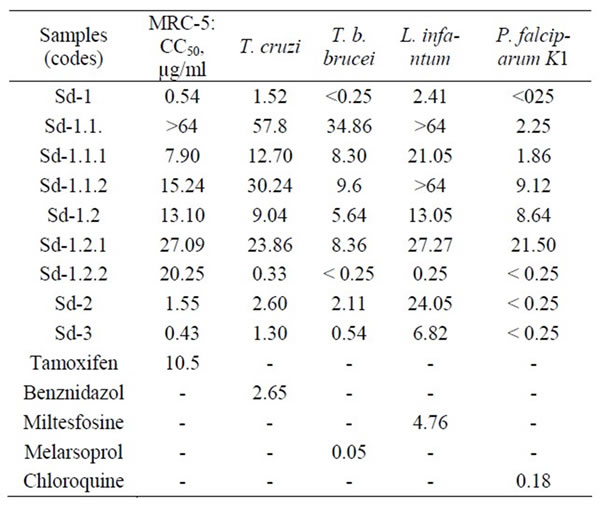
Table 2. Antiprotozoal activity of samples from Brucea sumatrana seeds (IC50, µg/ml).
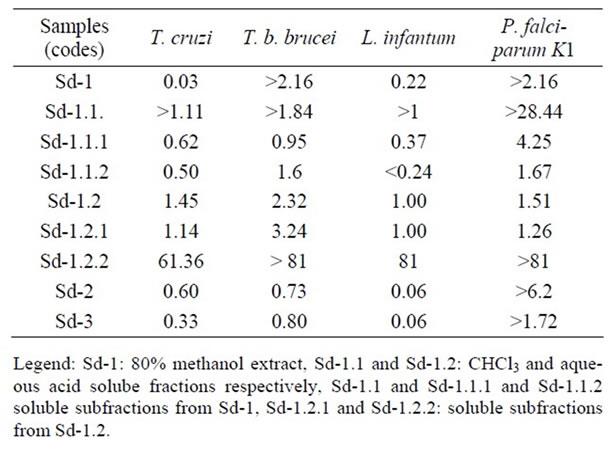
Table 3. Selective index (SI) values of samples from Brucea sumatrana seeds.
the 80% methanol (Sd-1.1.2) soluble subfractions rich in steroids and terpenes from Sd-1.1 had the same antiprotozoal spectrum. They possessed a cytotoxic effect against MRC-5 cell lines (7 < CC50 < 16 µg/ml). Sd-1.1.1 showed good activity against T. b. brucei and T.cruzi (IC50 = 8.30 and 7.90 µg/ml respectively) and moderate activity against L. infantum (IC50 = 21.05 µg/ml). Regarding its selective index values against these protozoa, it was concluded that the observed activity was due to its cytotoxity since the SI were <1. In contrast, this subfraction, exhibited pronounced antiplasmodial activity against the multidrug-resistant K1 strain of P. falciparum with IC50 value of 1.86 µg/ml and with appreciable selectivity index (Table 3). Sd-1.1.2 displayed good activity against T. b. brucei and chloroquine and pyrimethamineresistant K1 strain of P. falciparum with IC50 values of 9.6 and 9.12 µg/ml with appreciable selectivity index (Table 3). It showed low activity against T. cruzi and was inactive against L. infantum (Table 2). The acid aqueous soluble fraction (Sd-1.2), its chloroform (Sd- 1.2.1) and alkaline-aqueous phase (Sd-1.2.2) soluble fraction rich in alkaloids and phenolic compounds respectively, had also the same antiprotozoal spectrum. These samples were however cytotoxic against MRC-5 cell lines since their CC50 values are less than 32 µg/ml (Table 2) [34]. Sd-1.2 showed good activity against T. cruzi, T. b. brucei and chloroquine and pyrimethamineresistant K1 strain of P. falciparum with IC50 value of 9.04, 5.64 and 8.64 µg/ml respectively with selective effect (SI > 1), and showed good activity against L. infantum (Table 2). Sd-1.2.1 exhibited good and selective activity against T. b. brucei with IC50 value of 8.36 µg/ml and SI value of 3.24, moderate and selective activity against T. cruzi, L.infantum and chloroquine and pyrimethamine-resistant K1 strain of P. falciparum (20 < IC50 < 28 µg/ml, SI > 1). Interestingly, Sd-1.2.2 displayed pronounced activity against T. cruzi, T. b. brucei, L. infantum and multidrug-resistant K1 strain of P. falciparum with IC50 < 1 µg/ml (Table 2). In evaluating its selectivity index values, the resulting values were high values of 61.36 against T. cruzi, 81 against L. infantum and >81 against T. b. brucei and multidrug-resistant K1 strain of P. falciparum indicating its high selecive action against these protozoa. This finding suggested that the active constituents may be phenolic compounds.
The aqueous extract (decoction, Sd-2) and the total alkaloids extract (Sd-3) also showed the same wide range of antiprotozoal spectrum. Although they showed a cytotoxic effect against MRC-5 cell lines (CC50 = 1.55 and 0.43 µg/ml respectively), they exhibited pronounced activity against T. cruzi, T. b. brucei and chloroquine and pyrimethamine-resistant K1 strain with IC50 values in the range of 1 to 3 µg/ml (Table 2). Except for chloroquine and pyrimethamine-resistant K1 strain for which the activity of these fractions was selective (1 < SI < 7), it was however seen that their effect against the remaining protozoa was correlated to their cytotoxic effect and was not selective (SI < 1). In contrast, the aqueous extract (macerate) from the seeds of B. javanica collected in Thailand was found to be inactive against L. donovani and T. b. brucei (IC50 > 500 µg/ml) [14]. The difference in activity of this aqueous extract specially against T. brucei brucei as reported by these authors compared to our results can be only attributed in part to the type of preparation (macerate versus decoction).
In comparison of the IC50 values recorded for all samples to selected parasites in the present study, it was observed that most samples had smaller IC50 values against T. T. b. brucei (<0.25 to 8.36 µg/ml), T. cruzi (0.43 to 1.55 µg/ml) and chloroquine-resistant and pyrimethamine-resistant K1 strain of P. falciparum (<0.25 to 2.25 µg/ml) indicating that these three parasites are more sensitive than L. infantum (0.25 to >64 µg/ml). These samples had in addition appreciable selective action against sensitive parasites (Table 2).
With respect to the level of activity showed by respecttive reference products against these selected protozoa, it was however seen that the samples Sd-1, Sd-2 and Sd-3 showed higher cytotoxic effect (19.4, 6.8 and 24.4-times more cytotoxic) than tamoxifen, against MRC-5 cell lines growth, Sd-1, Sd-1.2.2 and Sd-3 exhibited higher activity (1.7, 8 and 2-times more cytotoxic) than benznidazol against T. cruzi growth, while the activity of Sd-2 was comparable to that of this reference product against this parasite. Sd-1 and Sd-1.2.2 displayed higher activity (2 and 19-times more cytotoxic) than miltesfosine against L. infantum growth. The activity of Sd-1, Sd-1.2.2, Sd-2 and Sd-3 was comparable to that of chloroquine against chloroquine and pyrimethamine-resistant K1strain of P. falciparum. On the other hand, it is well known that compounds responsible for the antiplasmodial activity of B. sumatrana seeds growing in Asian regions are quassinoids. These compounds were also recognized be responsible for the antiprotozoal activity against Trypanosoma evansi infecting animals [27], but compounds active against other Trypanosoma and Leishmania specie from this plant part are still unknown. This finding demonstrates that these samples need extensive phytochemical investigations leading to the isolation and structural elucidation of active principles which would be considered as lead compounds.
Phytochemical screening conducted on the aqueous and 80% methanol extract from B. sumatrana seeds in the present study by TLC using different mobile phases and chemical reagents reported in the literature [34], revealed the presence of steroids and/or terpenes, alkaloids and phenolic compounds as major constituents, which can account for the observed biological activity.
4. Conclusion
This is the first report of the in vitro antiprotozoal activity of extracts, fractions and subfractions from Brucea sumatrana seeds growing in DR Congo, an African country, on a large number of protozoa species. In addition, this study shows that the aqueous extract, the 80% methanol extract and its different soluble fractions and subfractions possessed potential and interesting antiprotozoal activity against the selected protozoa according to the case. Most samples were found to exhibit pronounced or good activeity which might be contributed in reducing infection. Based on this study, fractions and subfractions with high activity will be further phytochemically studied to isolate and identify active constituents.
REFERENCES
- M. O. Hasbi, “Pharmacognostic Study of Brucea amarissima Merr. from Gowa (South Sulawesi, Indonesia), Isolation and Identification of quassinoid Compound by Thin Layer Chromatography,” Research Report, Vol. 1, No. 1, 1979, pp. 6-7.
- W. Tang and G. Eisenbrand, “Chinese Drugs of Plant Origin. Chemistry, Pharmacology, and Use Traditional and Modern Medicine,” Springer-Verlag, New York, 1992.
- Subeki, H. Matssura, K. Tabahashi, K. Nabeta, M. Yamasaki, Y. Maede and K. Katakura, “Screening of Indonesian Medicinal Plant Extracts for Antibabesial Activity and Isolation of New Quassinoids from Brucea javanica,” Journal of Natural Products, Vol. 70, No. 10, 2007, pp. 1654-1657. doi:10.1021/np070236h
- A. NoorShahida, T. W. Wong and C. Y. Choo, “Hypoglycemic Effect of quassinoids from Brucea javanica (L.) Merr. (Simaroubaceae) Seeds,” Journal of Ethnopharmacology, Vol. 124, No. 3, 2009, pp. 586-591. doi:10.1016/j.jep.2009.04.058
- M. M. Anderson, M. J. O’Neill, J. D. Phillipson and D. C. Warhurst, “In Vitro Cytotoxic of a Series of Quassinoids from Brucea javanica Fruits against KB Cells,” Planta Medica, Vol. 157, No. 1, 1991, pp. 62-64. doi:10.1055/s-2006-960020
- C. Karin, S. B. Liu, S. L. Yang,. M. F. Roberts and J. D. Phillipson, “Production of Canthin-6-One Alkaloids by Cell Suspension Cultures of Brucea javanica (L.) Merr,” Plant Cell Reports, Vol. 9, No. 5, 1990, pp. 261-263.
- http://www.henriettesherbal.com
- J. M. Watt and M. C. Breyer-Brandwijk, “Medicinal and Poisonous Plants of Southern and Eastern Africa,” Livingstone, Edinburg, 1962.
- B. Ngoma and M. Bitengeli, “Therapeutic Application of Brucea sumatrana Roxb. (Simaroubaceae),” Al-Birunya, Vol. 9, No. 1, 1993, pp. 101-108.
- K. Pavanand, W. Nutakul, T. Dechatiwongse, K. Yoshihira, K. Yongvanitchit, J. P. Scovill, L. L. Flippen-Anderson, R. Gilardi, C. George, P. Kanchanapee and H. K. Webster, “In Vitro Antimalarial Activity of Brucea Javanica against Multi-Drug Resistant Plasmodium falciparum,” Planta Medica, Vol. 52, No. 1, 1986, pp. 108- 111. doi:10.1055/s-2007-969092
- M. J. O’Neill, D. H. Bray, P. Boardman, J. D. Phillipson, D. C. Warhurst, W. Peters and M. Suffers, “Plants as Sources of Antimalarial Drugs: In Vitro Antimalarial Activities of Some Quassinoids,” Antimicrobial Agents Chemotherapy, Vol. 30, No. 1, 1986, pp. 101-104.
- M. J. O’Neill, D. H. Bray, P. Boardman, L. I. Chan and J. D. Phillipson, “Plants as Sources of Antimalarial Drugs, Parts 4: Activity of Brucea javanica Fruits against Chloroquine-Resistant and Pyrimethamine-Resistant Plasmodium falciparum in Vitro and against Plasmodium berghei in Vivo,” Journal of Natural Products, Vol. 50, No. 1, 1987, pp. 41-48. doi:10.1021/np50049a007
- H. S. Kim, Y. Shibata, N. Ko, N. Ikemoto, Y. Ishizuka, N. Murakami, M. Sugimoto, M. Kobayashi and Y. Wataya, “Potent in Vivo Antimalarial Activity of 3,15-Di-O-acetylbruceolide against Plasmodium berghei Infection in Mice,” Parasitology International, Vol. 48, No. 3, 2000, pp. 271-274. doi:10.1016/S1383-5769(99)00023-9
- M. D. R. Camacho, J. D. Phillipson, S. L. Croft, P. N. Solis, S. J. Marshall and S. A. Ghazanfar, “Screening of Plant Extracts for Antiprotozoal and Cytotoxic Activities,” Journal of Ethnopharmacology, Vol. 89, No. 2-3, 2003, pp. 185-191. doi:10.1016/S0378-8741(03)00269-1
- J. Nguyen-Pouplin, H. Tan, H. Tran, T. A. Phan, C. Dolecek, J. Farrar, T. H. Tran, P. Caron, B. Bodo and P. Grellier, “Antimalarial and Cytotoxic Activities of Ethnopharmacologically Selected Medicinal Plants from South Vietnam,” Journal of Ethnopharmacology, Vol. 109, No. 3, 2007, pp. 417-427. doi:10.1016/j.jep.2006.08.011
- N. Sriwilaijaroen, S. Kondo, P. Nanthasri, S. Auparakkitanon, Y. Suzuki and P. Vilairat, “Antiplasmodial Effects of Brucea javanica (L.) Merr. and Eurycoma longifolia Jack and Their Combination with Chloroquine and Quinine on Plasmodium falciparum in Culture,” Tropical Medicine and Health, Vol. 38, No. 1, 2010, pp. 61-68. doi:10.2149/tmh.2009-11
- K. H. Lee, Y. Imakura, Y. Sumida, R. Y. Wu, I. H. Hall and H. C. Huang, “Antitumor Agents. 33. Isolation and Structural Elucidation of Bruceoside A and B, Novel Antileukemic Quassinoid Glycosides, and Brucein D and E from Brucea javanica,” Journal of Organic Chemistry, Vol. 47, No. 13, 1979, pp. 2180-2185. doi:10.1021/jo01327a031
- K. H. Lee. N. Hayashi, M. Okano and M. Juichi, “Antitumor Agents. 65. Brusatol and Cleomiscosin-A, Antileukemic Principles from Brucea javanica,” Journal of Natural Products, Vol. 47, No. 3, 1984, pp. 550-551. doi:10.1021/np50033a030
- T. Sakaki, S. Yoshimura, T. Tsuyuki, T. Takahashi, T. Honda and P. Yadanzioside, “A New Antileukemic Quassinoid Glycoside from Brucea javanica (L.) Merr. with the 3-O-(b-D-glucopyranosyl)-bruceantin Structure,” Chemical and Pharmaceutical Bulletin, Vol. 34, No. 10, 1986, pp. 4447-4450.
- L. Luyengi, N. Suh, H. H. S. Fong, J. M. Pezzuto and A. D. Kinghorn, “A Lignan and Four Terpenoids from Brucea javanica That Induce Differentiation with Cultured HL-60 Promyelocytic Leukemia Cells,” Phytochemistry, Vol. 43, No. 2, 1996, pp. 409-412. doi:10.1016/0031-9422(96)00258-0
- I. M. Kim, S. Takashima, Y. Hitotsuyanagi, T. Hasuda and K. Takeya, “New Quassinoids Javanicolides C and D and Javanicosides B-F from Seeds of Brucea javanica,” Journal of Natural Products, Vol. 67, No. 1, 2004, pp. 863-868. doi:10.1021/np030484n
- Y. Hitotsuyanagi, I. H. Kim, T. Hasuda, Y. Yamauchi and K. Takeya, “A Structure-Activity Relationship Study of Brusatol, an Antitumor Quassinoid,” Tetrahedron, Vol. 62, No. 20, 2006, pp. 4262-4271. doi:10.1016/j.tet.2006.01.083
- L. Pan, Y. W. Chin, H. B. Chai, T. N. Ninh, D. D. Soejarto and A. D. Kinghorn, “Bioactivity-Guide Isolation of Cytotoxic Constituents of Brucea javanica Collected in Vietnam,” Bioorganic and Medicinal Chemistry, Vol. 17, No. 6, 2009, pp. 2219-2224. doi:10.1016/j.bmc.2008.10.076
- C. W. Wright, M. J. O’Neill, J. D. Phillipson and D. C. Warhurst, “Use of Microdilution to Assess in Vitro Antiamoebic Activities of Brucea javanica Fruits, Simaruba amara, Stem, and a Number of Quassinoids,” Antimicrobial Agents Chemotherapy, Vol. 32, No. 11, 1988, pp. 1725-1729.
- C. W. Wright, M. M. Anderson, D. Allen, J. D. Phillipson, G. C. Kirby, D. C. Warhurst and H. R. Chang, “Quassinoid Exhibit Greater Selectivity against Plasmodium falciparum than againt Entamoeba histolytica, Giardia inetstinalis or Toxoplasma gondii in Vitro,” Journal of Eukariotic Microbiology, Vol. 40, No. 3, 1993, pp. 244- 246. doi:10.1111/j.1550-7408.1993.tb04910.x
- N. Sawangjaroen and K. Sawangjaroen, “The Effect of Extracts from Anti-Diarrheic Thai Medicinal Plants on the in Vitro Growth of the Intestinal Protozoa Parasite Blastocytis hominis,” Journal of Ethnopharmacology, Vol. 98, No. 1-2, 2005, pp. 67-72. doi:10.1016/j.jep.2004.12.024
- B. S. Bawm, H. Matsuura, A. Elkhateeb, K. Nabeta, Subeki, N. Nonaka, Y. Oku and K. Katakura, “In Vitro Antitrypanosamal Activities of Quassinoid Compounds from the Fruits of a Medicinal Plant, Brucea javanica,” Veterinary Parasitology, Vol. 158, No. 4, 2008, pp. 288- 294. doi:10.1016/j.vetpar.2008.09.021
- A. Elkhateeb, M. Yamasaki, M. Maede, K. Katakura, K. Nabeta and H. Matsuura, “Anti-Babesial Quassinoids from the Fruits of Brucea javanica,” Natural Product Communications, Vol. 3, No. 1, 2008, pp. 1-4.
- I. H. Hall, K. H. Lee, Y. Imakura, M. Okano and A. Johnson, “Anti-inflammatory Agents. III. Structure-Activity Relationships of Brusatol and Related Quanssinoids,” Journal of Pharmaceutical Science, Vol. 72, No. 11, 1983, pp. 1282-1284. doi:10.1002/jps.2600721111
- S. Yoshimura, T. Sakaki, M. Ishibashi, T. Tsuyuki, T. Takahashi, K. Matsushita and T. Honda, “Structures of Yadanziolides A, B, and C, New Bitter Principles from Brucea javanica,” Chemical and Pharmaceutical Bulletin, Vol. 32, No. 11, 1984, pp. 4698-4700. doi:10.1248/cpb.32.4698
- S. Yoshimura, T. Sakaki, M. Ishibashi, T. Tsuyuki, T. Takahashi and T. Honda, “Constituents of Seeds of Brucea javanica. Structures of New Bitter Principles, Yandanziolides A, B, C, Yandaziosides F, I, J, and L,” Bulletin of the Chemical Society of Japan, Vol. 58, No. 9, 1985, pp. 2673-2679. doi:10.1246/bcsj.58.2673
- M. E. Wagih, G. Alam, S. Wiryodagdo and K. Attia, “Improved Production of the Indole Alkaloid Canthin-One from Cell Suspension Culture of Brucea javanica (L.) Merr.,” India Journal of Science and Technology, Vol. 1, No. 1, 2008, pp. 1-6.
- L. A. Mitcher, Y. H. Park, D. Clark and G. W. Clark III, “Antimicrobial Agents from Higher Plants. An Investigation of Hunnemannia fumariaefolia pseudoalcoholates of Sanguinarine and Chelerythrine,” Journal of Natatural Products, Vol. 41, No. 1, 1978, pp. 145-150.
- J. B. Harborne, “Phytochemical Methods. A Guide to Modern Techniques of Plants Analysis,” Chapman & Hall, London, 1998.
- K. Kuypers, P. Cos, E. Ortega-Barria, D. Vanden Berghe and L. Maes, “Bioassays for Some Parasitic Protozoa, Screening Concepts and Standard in Vitro and in Vivo Laboratory Model,” In: M. P. Gupta, S. S. Handa and K. Vanish, Eds., Biological Screening of Plant Constituents, International Centre for Science and High Technology, Trieste, 2006, pp. 7-18.
- W. Trager and J. Jensen, “Human Malaria Parasites in Continuous Culture,” Science, Vol. 193, No. 4254, 1976, pp. 673-675. doi:10.1126/science.781840
- M. T. Makler, J. M. Ries, J. A. Williams, J. E. Bancroft, R. C. Piper, B. L. Gibbins and D. J. Hinrichs, “Parasite Lactate Dehydrogenase as an Assay for Plasmodium falciparum Drug Sensitivity,” American Journal of Tropical Medicine and Hygiene, Vol. 48, No. 6, 1993, pp. 739- 741.
- L. G. Tona, K. G. Mesia, T. H. Nanga, K. R. Cimanga, S. Apers, P. Cos, L. Maes, L. Pieters and A. J. Vleitinck, “In Vitro Antiprotozoal and Cytotoxic Activities of Plant Extracts from Democratic Republic of Congo,” Recent Research Development in Plant Science, Vol. 4, No. 1, 2007, pp. 41-60.

