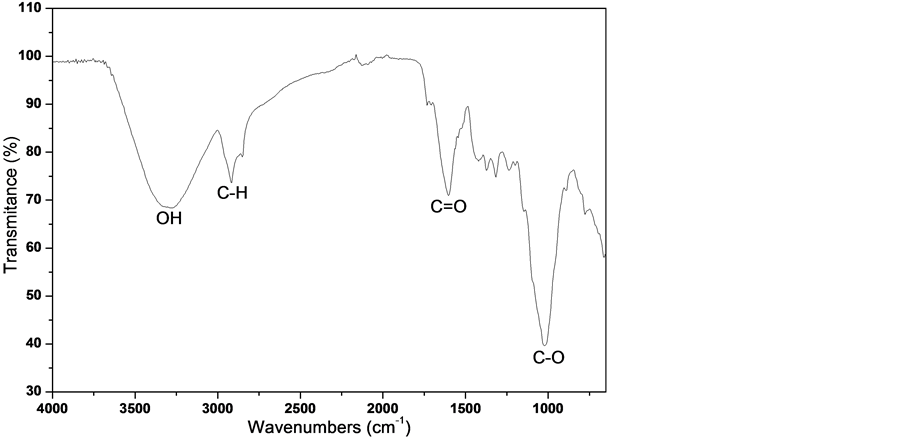Journal of Environmental Protection
Vol.07 No.12(2016), Article ID:72211,10 pages
10.4236/jep.2016.712147
Banana Peel for Acetylsalicylic Acid Retention
Araceli Veronica F. N. Ribeiro1, André Romero da Silva2, Tiago Pereira da Cunha1, Rowenna Tonani L. dos Santos1, Jairo Pinto de Oliveira3, Evaldo Vitor Pereira3, Marcus Vinicius V. J. Licinio3, Madson de Godoi Pereira4, Arnaud Victor dos Santos4, Joselito Nardy Ribeiro3*
1Instituto Federal do Espirito Santo, Campus de Vila Velha, Brazil
2Instituto Federal do Espírito Santo, Campus de Aracruz, Brazil
3Centro de Ciências da Saúde, Universidade Federal do Espírito Santo, Vitória, Brazil
4Departamento de Ciências Exatas e da Terra, Universidade do Estado da Bahia, Salvador, Brazil

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 21, 2016; Accepted: November 20, 2016; Published: November 23, 2016
ABSTRACT
A method for adsorption involving banana peel (BP) was studied to remove the pollutant acetylsalicylic acid (ASA) in aqueous medium. The results show that bioadsorbent has satisfactory maximum adsorption capacity (2.29 mg/g) for removing this analgesic and anti-inflammatory drug in aqueous solution (pH 7.0) using the Langmuir mathematical model. The tested concentrations of this pollutant were higher than the levels commonly found in the aquatic environment. This and other results suggest the BP as an alternative to ASA removal in water contaminated with pharmaceuticals pollutants.
Keywords:
Adsorption, Banana Peel, Acetylsalicylic Acid

1. Introduction
These pharmaceuticals pollutants are consistently detected in different aquatic environmental samples [1] [2] . The concentrations are usually detected in the order of µg∙L−1 and ng∙L−1 [3] . The main sources of contamination are the hospitals [4] and domestic sewage effluents [1] . These drugs and their metabolites may contaminate groundwater, rivers, lakes, and wastewater treatment plants [5] [6] . Montagner and Jardim [7] detected the presence of drugs pollutants in the
2. Material and Methods
2.1. Materials
The bioadsorbent banana peel (BP) was obtained from the banana silver fruit in fairs fruit of
2.2. Methods
2.2.1. Adsorbent Preparation and Physical Chemistry Analysis
The adsorbent preparations and physical chemistry analysis of scanning electron microscopy and spectroscopy infrared were performed as previously described by our laboratory in [13] . The BP was washed with hydrochloric acid
2.2.2. Zeta Potential
The zeta potential of the particulate material was measured using a particle size analyzer from Microtrac Model Zetatrac NPA152. Typically, aqueous suspension (2 ml) of mashed BP, pretreated at different pH, were added to a Teflon cuvette containing a pair of palladium electrodes for measuring the zeta potential which is determined via electrophoretic mobility of the particles at room temperature (25˚C on average).
2.2.3. Evaluation of pH
The solutions containing 250 ml of 100 mg∙l−1 ASA and
2.2.4. Evaluation of Stir Time
At this stage we evaluated the influence of contact time between ASA and BP in the adsorptive process. Therefore, solutions containing 250 ml of 100 mg∙l−1 ASA (pH 7.0) were magnetically stirred (800 rpm) at different times (from 0 to 25 minutes) in contact with
2.2.5. Evaluation of Mass
The solutions containing 250 ml of 100 mg∙l−1 ASA (pH 7.0) were magnetically stirred (800 rpm at 15 minutes) at different mass values of triturated BP (from 0.5 to
2.2.6. Obtaining the Maximum Adsorption Capacity
At this stage we evaluated the maximum adsorption capacity (MAC) of BP for ASA at 25˚C ± 1˚C. Adsorption isotherms were built for estimating the MAC value. This value represents the amount of ASA which can be adsorbed by
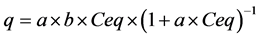 (1)
(1)
where q represents the quantity of ASA (mg) adsorbed in triturated BP (g) (mg∙g−1), a represents the constant related to adsorption energy (l∙mg−1), b is the maximum ASA adsorption capacity of the BP (mg∙g−1), and Ceq is the equilibrium ASA concentration (mg∙l−1). The linearizing of this equation permits the obtaining of Equation (2):
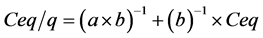 (2)
(2)
which permits calculation of MAC value.
3. Results and Discussion
3.1. Scanning Electron Microscopy and Spectroscopy Infrared Analysis
The scanning electron microscopy revealed that the BP adsorbent an irregular morphology characterized by the presence of concavities of different sizes (Figure 1).
Furthermore, the data obtained through the spectroscopy infrared analysis (Figure 2)
Figure 1. Scanning electron microscopy of banana peel with magnifications of 1000x.
Figure 2. Spectroscopy infrared analysis of banana peel.
of BP revealed the presence of important chemicals groups, for interaction with ASA structure (Figure 3). The following groups were found on BP: O-H group (3500 - 3400 cm−1) probably from the alcohols, C-H sp3 carbon in approximately 2920 cm−1, C=O in approximately at 1666 cm−1 and, finally the presence of the band at 1000 cm−1 may indicate the presence of the carbon group C-O [19] . The presence of these chemical groups is mainly due to the occurrence of biomolecules such as cellulose, hemicellulose and lignin [9] .
Finally, the superficial morphology and presence these chemical groups in BP suggests that this natural adsorbent has important characteristics for interaction with ASA.
3.2. Zeta Potential
The zeta potential analysis of triturated BP, present in aqueous suspension (2 ml) at different pH, revealed that surface charge of this natural adsorbent becomes more negative in increase of pH (Figure 4). Probably this increase of negative charge occurred because of deprotonation of O-H, -COOH, and other chemical groups from various biomolecules of BP constituents. Duran and Flores [20] observed this same behavior when performing the zeta potential analysis of vermicompost natural adsorbent.
3.3. Evaluation of pH
The adsorption tests at different pH of ASA solutions showed that adsorption percentage
Figure 3. Acetylsalicylic Acid structure.
Figure 4. Zeta potential analysis of triturated BP at different pH.
of ASA decreases by increasing the pH of the aqueous solution (Figure 5). Possibly, this result is due to the increase of negative charges of the adsorbate (pka 3.50) (Figure 6) and adsorbent at higher pH, causing repulsion between ASA and BP. This result is in agreement with Figure 5 that showed an increase in the negative surface charge BP at higher pH than 3.0. Duran and Flores [20] observed that vermicompost adsorbent acquires a negative charge at high pH which makes an efficient adsorbent for the removal of the positive lead (Pb2+) adsorbate in an aqueous medium. Despite the pH 3.0 is considered to be more efficient in removing the ASA from aqueous medium, it was decided to use the pH 7.0 due to its use in water treatment plants in Brazil.
3.4. Evaluation of Stir Time
Figure 7 showed that the satisfactory percentage adsorption of the drug occurred at 15 minutes of stirring and after this time observed a decrease in the drug adsorption. Probably, this decrease is related to the desorption of ASA from the BP, due to the system’s constant stirring and weak interactions between the drug and the banana peel [10] .
3.5. Evaluation of Mass
Figure 8 shows the influence of the BP adsorbent mass on percentage of ASA adsorption. The better retention of the drug occurred with use of 1.5 g of adsorbent. The
Figure 5. Influence of pH in the percentage adsorption of ASA by BP.
Figure 6. Protonated (a) and unprotonated (b) ASA structure.
Figure 7. Influence of the stir time in the percentage of ASA adsorption by BP.
Figure 8. Influence of the banana peel mass in the percentage of ASA adsorption.
quantity of adsorbent to be used is very important; not only to have a good retention of the pollutant, but also to project the area required to stock the adsorbent resulting from the treatment [10] .
3.6. Maximum Adsorption Capacity (MAC)
To determine the MAC of BP adsorbent to ASA, adsorption isotherm (Figure 9) was obtained from different ASA concentrations. Then the isotherm was linearized (Figure 10) according to the Langmuir Mathematical Model [18] . The MAC value 2.29 mg/g
Figure 9. Isotherm adsorption.
Figure 10. Isotherm linearized adsorption.
obtained for ASA adsorption by BP can be considered satisfactory, because drugs are commonly found in aquatic environmental at µg/L or ng/L [3] [4] . In previous studies [9] and [13] also found satisfactory MAC values for paracetamol and tetracycline utilizing the natural adsorbents green coconut mesocarp and sugar cane bagasse respectively. Ribeiro et al. [11] found interesting results for the removal of paracetamol using sugar cane bagasse and vegetable sponge as natural adsorbents. Finally was verified that several authors studied diverse types of natural adsorbents for retention of several chemical pollutants from water in stirring system and columns. These studies obtained satisfactory MAC values [21] .
4. Conclusion
The results suggest that BP has characteristics that qualify it as a possible natural adsorbent for water treatment containing ASA pollutant. This material showed satisfactory maximum adsorption capacity. Finally, the results encourage more detailed studies, including the retention of other pollutants and economic viability.
Acknowledgements
The authors would like to thank the Federal Institute of Espírito Santo (Vila Velha, ES, Brazil) by financial support, and Laboratory of Cellular Ultra structure Carlos Alberto Redins of Federal University of Espírito Santo (Vitória, ES, Brazil) for the use of scanning electron microscopes.
Cite this paper
Ribeiro, A.V.F.N., da Silva, A.R., da Cunha, T.P., dos Santos, R.T.L., de Oliveira, J.P., Pereira, E.V., Licinio, M.V.V.J., de Godoi Pereira, M., dos Santos, A.V. and Ribeiro, J.N. (2016) Banana Peel for Acetylsalicylic Acid Retention. Journal of Environmental Protection, 7, 1850-1859. http://dx.doi.org/10.4236/jep.2016.712147
References
- 1. Ziylan, A. and Ince, N.H. (2011) The Occurrence and Fate of Anti-Inflammatory and Analgesic Pharmaceuticals in Sewage and Fresh Water: Treatability by Conventional and Non-Conventional Process Comparative Study of MR Imaging Profile of Titanium Pedicle Screws. Journal of Hazardous Materials, 187, 24-36.
https:/doi.org/10.1016/j.jhazmat.2011.01.057 - 2. Daneshvar, A., Aboulfadl, K., Viglino, L., Broseus, R., Sauve, S., Madoux-Humery, A.S., Weyhenmeyer, G.A. and Prevost, M. (2012) Evaluating Pharmaceuticals and Caffeine as Indicators of Fecal Contamination in Drinking Water Sources of the Greater Montreal Region. Chemosphere, 88, 131-139.
https:/doi.org/10.1016/j.chemosphere.2012.03.016 - 3. Hilton, M.J. and Thomas, K.V. (2003) Determination of Selected Human Pharmaceutical Compounds in Effluent and Surface Water Samples by High-Performance Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Journal of Chromatography A, 1015, 129-141.
https:/doi.org/10.1016/S0021-9673(03)01213-5 - 4. Azuma, T., Arima, N., Tsukada, A., Hirami, S., Matsuoka, R., Moriwake, R., Ishiuchi, H., Inoyama, T., Teranishi, Y., Yamaoka, M., Mino, Y., Hayashi, T., Fugita, Y. and Masada, M. (2016) Detection of Pharmaceuticals and Phytochemicals Together with Their Metabolites in Hospital Effluents in Japan, and Their Contribution to Sewage Treatment Plant Influents. Science of the Total Environmental, 548-549, 189-197.
https:/doi.org/10.1016/j.scitotenv.2015.12.157 - 5. Fatta-Kassinos, D., Meric, S. and Nikolaou, A. (2011) Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Analytical and Bioanalytical Chemistry, 399, 251-275.
https:/doi.org/10.1007/s00216-010-4300-9 - 6. Tewari, S., Jindal, R., Kho, Y.L., Eo, S. and Choi, K. (2013) Major Pharmaceutical Residues in Wastewater Treatment Plants and Receiving Waters in Bangkok, Thailand, and Associated Ecological Risks. Chemosphere, 91, 697-704.
https:/doi.org/10.1016/j.chemosphere.2012.12.042 - 7. Montagner, C.C. and Jardim, W.F. (2011) Spatial and Seasonal Variations of Pharmaceuticals and Endocrine Disruptors in the Atibaia River, Sao Paulo State (Brazil). Journal of the Brazilian Chemical Society, 22, 1452-1462.
https:/doi.org/10.1590/S0103-50532011000800008 - 8. Zhao, Y., Geng, J., Wang, X., Gu, X. and Gao, S. (2011) Adsorption of Tetracycline onto Goethite in the Presence of Metal Cations and Humic Substances. Journal of Colloid and Interface Science, 361, 247-251.
https:/doi.org/10.1016/j.jcis.2011.05.051 - 9. Ribeiro, J.N., Ribeiro, A.V.F.N., Oliveira, J.P., Leao, R.T. and Cunha, T.P. (2016) Green Mesocarp Coconut for Treatment of Water Contaminated with Paracetamol and Tetracycline. International Journal of Scientific Research, 5, 319-323.
- 10. Raymundo, A.S., Zanarotto, R., Belisario, M., Pereira, M.G., Ribeiro, J.N. and Ribeiro, A.V.F.N. (2010) Evaluation of Sugar-Cane Bagasse as Bioadsorbent in the Textile Wastewater Treatment Contaminated with Carcinogenic Congo Red Dye. Brazilian Archives of Biology and Technology, 53, 931-938.
https:/doi.org/10.1590/S1516-89132010000400023 - 11. Ribeiro, A.V.F.N., Belisario, M., Galazzi, R.M., Balthazar, D.C., Pereira, M.G. and Ribeiro, J.N. (2011) Evaluation of Two Bioadsorbents for Removing Paracetamol from Aqueous Media. Electronic Journal of Biotechnology, 14, 1-10.
- 12. Pereira, M.G, Neta, L.C.S, Fontes, M.P.F., Nascimento, A., Matos, T.C., Sachdev, R.L., Santos, A.V., Souza, M.O.G., Andrade, M.V., Paulo, M.M., Ribeiro, J.N. and Ribeiro, A.V.F.N. (2014) An Overview of the Environmental Applicability of Vermicompost: From Wastewater Treatment to the Development of Sensitive Analytical Methods. The Scientific World Journal, 2014, Article ID: 917348.
https:/doi.org/10.1155/2014/917348 - 13. Ribeiro, A.V.F.N., Cosmo, P.C., Pereira, M.G., Dalfior, B.M., Goncalves, G.S., Licinio, M.V.V.J., Endringer, D.C., Oliveira, J.P. and Ribeiro, J.N. (2014) Use of Sugarcane Bagasse for Adsorption of Tetracycline in Aqueous Medium. Indian Journal of Applied Research, 4, 10-14.
https:/doi.org/10.15373/2249555X/JAN2014/4 - 14. Pereira, M.G., Matos, T.C., Santos, N.C.V., Santos, A.M., Neta, L.C.S., Ribeiro, J.N., Ribeiro, A.V.F.N., Oliveira, J.P., Guimaraes, M.C.C. and Licinio, M.V.V.J. (2015) Evaluation of Vermicomposts for Decontaminating Aqueous Media Containing Metallic Ions and Synthetic Dyes. In: Bates, L., Org., Humic Substances and Natural Organic Matter, Nova Science Publishers, Hauppauge, Vol. 1, 83-114.
- 15. Ribeiro, J.N., Ribeiro, A.V.F.N., Licinio, M.V.V.J., Pereira, M.G., Dalfior, B.M., Oliveira, J.P., Cosmo, P.C., Galazzi, R.M., Pereira, E.V. and Guimaraes, M.C.C. (2014) Studies on the Removal of Paracetamol from Aqueous Medium Using Vermicompost. Analytica, 12, 64-68.
- 16. Ali, A. and Saeed, K. (2016) Phenol Removal from Aqueous Medium Using Chemically Modified Banana Peel Powder as Low-Cost Adsorbent. Desalination and Water Treatment, 57, 11242-11254.
https:/doi.org/10.1080/19443994.2015.1041057 - 17. Salim, R.M., Chowdhury, A.J.K., Rayathulhan, R., Yunus, K. and Sakar, M.Z.I. (2016) Biosorption of Pb and Cu from Aqueous Solution Using Banana Peel Powder. Desalination and Water Treatment, 57, 303-314.
- 18. Pereira, M.M., Korn, M., Santos, B.B. and Ramos, M.G. (2009) Vermicompost for Tinted Cationic Dyes Retention. Water Air and Soil Pollution, 200, 227-235.
https:/doi.org/10.1007/s11270-008-9906-6 - 19. Silverstein, R.M., Basller, G.C. and Morril, T.C. (1979) Spectrotometry Identification of Organic Compouds. 3rd Edition, John Willey & Sons, Hoboken.
- 20. Duran, A.C. and Flores, I. (2009) Evaluation of Lead(II) Immobilization by a Vermicom-post Using Adsorption Isotherms and IR Spectroscopy. Bioresource Technology, 100, 1691-1694.
https:/doi.org/10.1016/j.biortech.2008.09.013 - 21. Belisario, M., Borges, P.S., Galazzi, R.M., Del Piero, P.B., Ribeiro, A.V.F.N. and Ribeiro, J.N. (2009) The Employment of Natural Waste at Treatment of Effluents Contaminated with Polluting Medicines. InterSciencePlace, 2, 1-13.