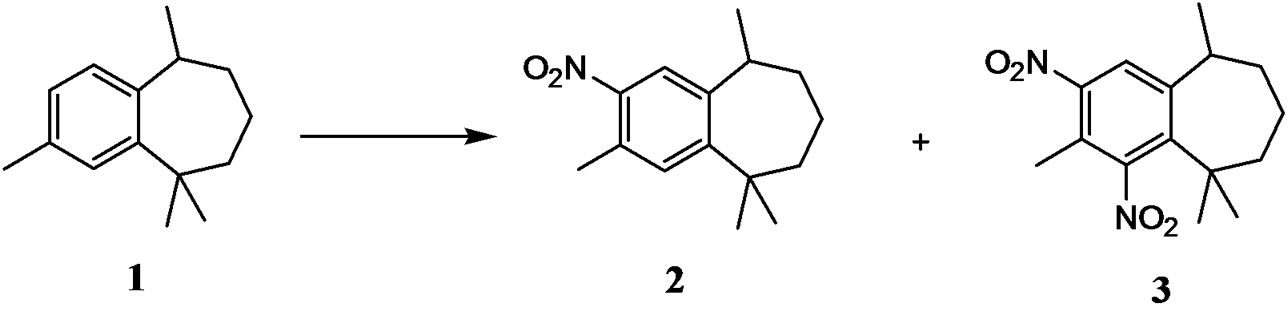
Green and Sustainable Chemistry
Vol.1 No.3(2011), Article ID:7078,5 pages DOI:10.4236/gsc.2011.13018
Combination of Transition Metals-(2,4-Pentanedionate) and P2O5 as a Regioselective Catalytic System for Nitric Acid Nitration of Ar-Himachalene
Equipe de Chimie de Coordination et Catalyse, Université Caddi Ayad, Faculté des Sciences-Semlalia, Marrakech, Morocco
E-mail: *aitali@ucam.ac.ma
Received March 1, 2011; revised March 28, 2011; accepted April 7, 2011
Keywords: Nitration, Sesquiterpene, Ar-Himachalene, M(acac)n, P2O5
Abstract
A practical system of metals-(2,4-pentanedionate) (M(acac)n (M = Fe, Zn, Co and V)) and P2O5 in the presence of HNO3 catalyzes regioselective nitration of ar-himachalene 1 to mono-nitro-ar-himachalene 2 in moderate to high yields. Under mild conditions, the selectivity of nitration of 1 to 2 is excellent compared to the classical method using the nitro-sulphuric mixture HNO3/H2SO4, both monoand di-nitro-ar-himachalene 2 and 3 are obtained. The reaction offers a very good method for the preparation of nitro-aromatic compounds and provides a useful entry to new functionalized terpenic products.
1. Introduction
The use of renewable feedstock, which may substitute those derived from petroleum, as well as the employment of low or non-toxic substances are currently great challenges in the chemical and pharmaceutical industries. This new trend is known as green chemistry [1,2]. Nitration of ar-himachalene 1, biodegradable natural sesquiterpene hydrocarbon (obtainable from renewable sources), using an environmentally friendly catalytic method may be useful source of important valuable new green precursors for the manufacture of products with high added value. Nitro aromatic compounds are themselves used as explosives and act as key substrates for the preparation of useful materials such as dyes, pharmaceuticals, per fumes and plastics [3-6]. Extensive and welldocumented reviews in this context have been published by Ingold [7], Olah and co-workers [3,8], Schofield and co-workers [9,10], and Ione [11]. The classical nitration method usually requires the use of an excess of nitric acid and assistance of strong acids such as concentrated sulfuric acid. Recent research has been focused on the development of an environmentally friendly nitration process which can avoid excess of acids to minimize waste and offer high regioselectivity. Many catalytic systems have been employed, most of them heterogeneous; such as metal nitrates of iron(III) or copper(II) supported on montmorillonite clay [12-15] have been shown to be efficient nitrating agents. Other examples involve the use of lanthanide (III), zirconium(IV) or hafnium(IV) triflate salts as catalysts of nitric acid nitration [16]. Iron (III)2,4pentanedionate [17,18] and zirconium(IV) 2,4- pentanedionate [19] have also been used as catalysts in the presence of dinitrogen pentoxide.
Within this framework, we report here an environmentally friendly one-pot catalytic procedure for the nitric acid nitration of ar-himachalene using M(acac)n /P2O5 (M = Fe, Al, Cr, Zn, Co and V) as catalytic system. This procedure would be a versatile method for aromatic nitration.
2. Results and Discussion
Ar-himachalene 1, sesquiterpene male-produced pheromone component of the flea beetle Aphthona flava [20, 21], was isolated with only 0.5% from essential oil of Atlantic Cedar trees (Cedrus atlantica). The essential oil is mainly composed of sesquiterpene hydrocarbons a-himachalene, b-himachalene, and g-himachalene which together can make up almost 70% of the composition [2223]. Several papers describe the synthesis of ar-hymachalene 1 in multistep starting from citronellal or by conversion of ar-turmerone [24,25]. In our laboratory, we have developed a more practical process for the synthesis of ar-hymachalene 1 in good yield by dehydrogenation of the mixture of a-, band g-himachalene [26].
In preliminary studies, we have investigated the nitration of ar-hymachalene with classical method using nitric acid and sulfuric acid as the nitrating mixture at room temperature in free solvent condition. The reaction lead to a mixture of mononitro-ar-himachalene 2 (10% - 85%) and dinitro-ar-himachalene 3 (13% - 80%) with good conversion (75% - 98%) (Table 1 and Scheme 1).
We have also shown that molar ratio of HNO3:H2SO4: ar-hymachalene has a high effect on the selectivity of the nitration. Thus, with a large excess of HNO3 and H2SO4, 90 % of ar-himachalene 1 was converted in to 11 % of mononitro 2 and 89 % of dinitro 3 (Table 1, entry 1). On the other hand, a decrease of the amount of HNO3 and H2SO4 act favourably to an increase of selectivity on mononitro 2. The low selectivity and the problem regarding the waste disposal make this process uneconomic and hazardous in view of environment. That is why the research in avoiding the use of sulfuric acid in the nitration of aromatics is of great interest to minimize the pollution and promote the greater yield of valuable isomer.
Contributing to this widespread area, we have investigated an environmentally friendly and efficient catalytic system for nitration of ar-hymachalene 1 using metals (2,4-pentanedionate)/P2O5 (metal = Fe, Zn, Al, Co, Cr and V) as catalytic system in the presence of nitric acid.
The results are summarized in Table 2. Nitration of arhymachalene 1 was carried out in a solution containing 0.1 mmol of M(acac)n, 14.82 mmol of P2O5 and 267 mmol of nitric acid at room temperature. We note here that mononitro-ar-himachalene 2 was the unique obtained product; no dior tri-nitrated products were observed. In the absence of M(acac)n, ar-hymachalene 1 was not converted (Entry 6). While Al(acac)3 or Cr(acac)3 was not effective (Table 2, entries 8, 9), Fe(acac)3, Zn(acac), Co(acac)3 and V(acac)2 were highly effective and selective (Table 2, entries 10-13) catalysts. Especially, when Fe(acac)3 was used as the catalyst, the nitration of ar-hymachalene 1 proceeded selectively at room temperature and 93% of mononitro-ar-himachalene 2 was obtained (Table 2, entry 10). On the other hand, in the absence of P2O5, nitric acid nitration with Fe(acac)3 did not proceed (Table 2, entry 7).
It is presumed that a highly reactive nitrating agent is generated in situ by the reaction of P2O5 and nitric acid in the presence of M(acac)n as catalyst. Effectively, it has been reported that N2O5 can be prepared from nitric acid in the presence of P2O5 [27]. Therefore, N2O5 is highly corrosive and liberates toxic nitrogen oxides. Generating it in situ with its decomposition by organometalic catalysts can resolve this problem.
Several reaction conditions were examined; the obtained results are summarized in Table 3. This table shows that the yields of the product depend on the nature of the reaction medium. In nitromethane or acetonitrile, the yields were relatively modest with an increase the reaction time (Entries 16, 17).
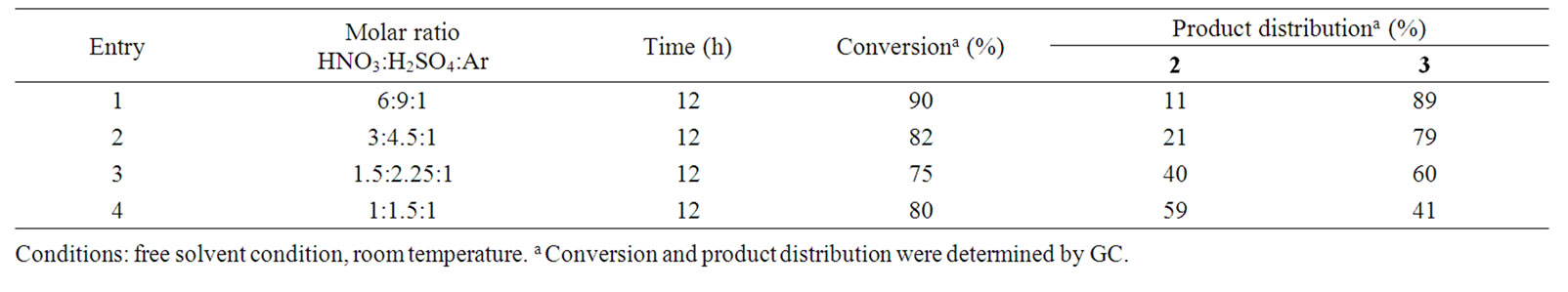
Table 1. Nitration of ar-himachalene by nitro-sulphuric mixture HNO3/H2SO4.
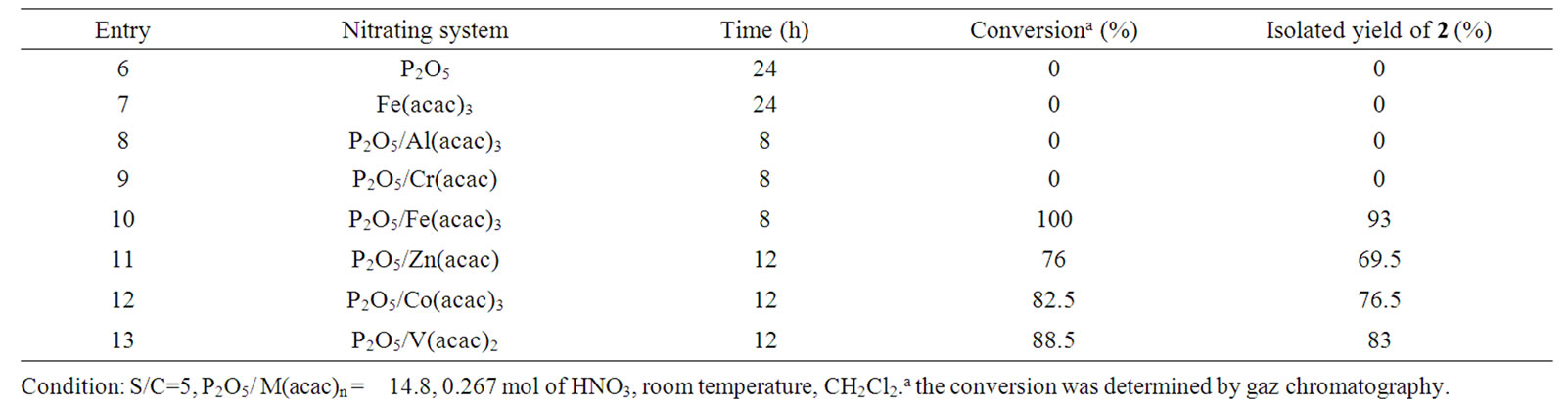
Table 2. Nitration of Ar-himachalene catalyzed by metal-2,4-pentanedionate/P2O5 (metal = Fe, Zn, Al, Co, Cr and V) in the presence of nitric acid.
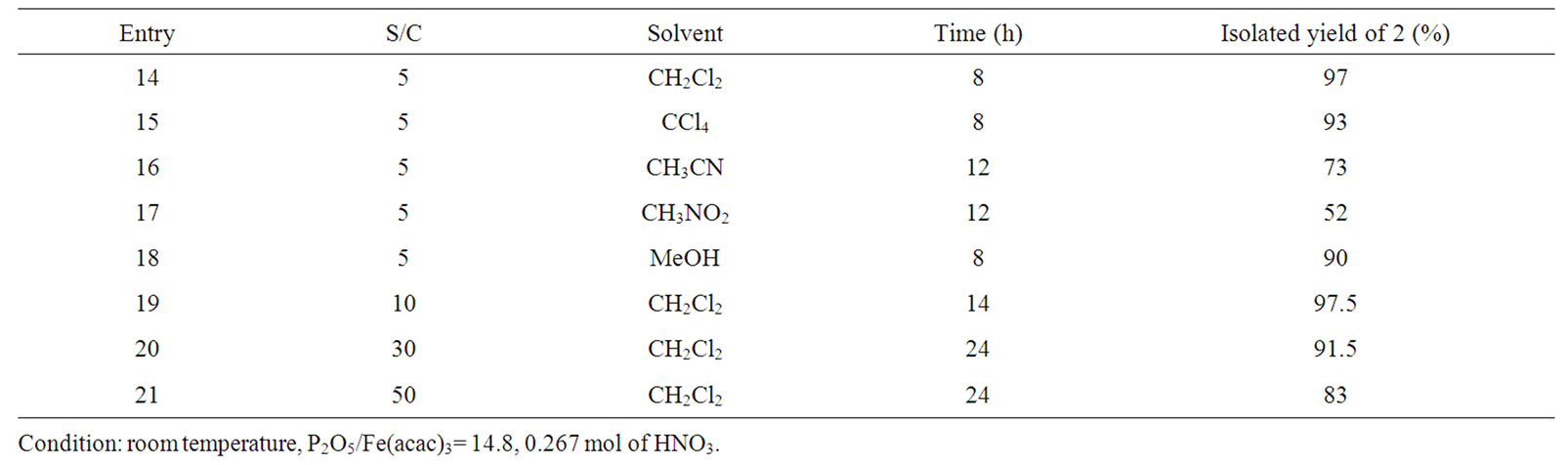
Table 3. Effect of the solvent and the substrate/catalyst ratio (S/C) in the nitration of ar-hymachalene catalysed by Fe(acac)3.
Therefore, in dichloromethane, tetrachlomethane and methanol (Entries 14, 15 and 18), the reaction mixture was homogeneous and the yields were very high (≥90%). We note here that the corresponding product was then obtained in 100% selectivity. This table also shows the effect of the amount of catalyst. With molar ratio S/C = 10 in dichloromethane after 14 h, the nitration product was obtained in 97.5% yield (Entry 19). Moreover, when we increased the molar ratio (S/C) from 30 to 50, no appreciable loss in activity was observed (Entries 20, 21).
On the other hand, using the reported procedure [27], HNO3 in the presence of P2O5/silica gel, only 51% of ar-hymachalene 1 were converted to mono-nitro 2 (68%) and di-nitro 3 (32%) after 17 h.
This study reveals that the use of Fe(acac)3, Zn(acac), Co(acac)3 and V(acac)2 in the presence of P2O5 gives excellent results in nitric acid nitration of ar-hymachalene 1.
3. Conclusions
Regioselective nitration of ar-hymachalene 1 to 3,5,5,9- Tetramethyl-2-nitro-6,7,8,9-tetrahydro-5H-benzocycloheptene 2 was achieved using series of metals-2,4- pentanedionate/P2O5 as catalysts with high conversion and selectivity. Nitric acid was used as nitrating agent without use of sulphuric acid, which makes this process environmentally benign. High yields of nitrated products were obtained with catalyst loadings of 5 - 50 mol%.
4. Experimental Section
Chemicals were purchased from the Fluka, Merck and Aldrich chemical companies and used as received. Arylhimachalene is prepared according to our previews work [26]. NMR studies were performed on a Bruker Avance 300 spectrometer in CDCl3, chemicals shifts are given in ppm relative to external TMS and coupling constant (J) in Hz. Mass spectra were recorded on a GC-MS Thermofinnigan Polaris-Q mass spectrometer.
5. Procedure of Catalytic Studies
Addition of 30 ml CH2Cl2 and Fe(acac)3 (1 mmol) to a stirred solution of P2O5 (14,8 mmol) and HNO3 (0,267 mol) (63% w/w) at 0˚C results in the formation of the nitrating complex as a light pink solid. When the solution becomes yellow, 5 mmol of substrate was added to the reaction, which is stirred at room temperature for appropriate time. The solution was then quenched with 5 ml of HCl 10%. The reaction was extracted with ethyl acetate and washed with aqueous Na2CO3 and brine. Removal of solvent and purification by column chromatography over silica gel afforded the pure nitrated compounds in near quantitative yields.
3,5,5,9-Tetramethyl-2-nitro-6,7,8,9-tetrahydro-5H-benzocycloheptene 2, yellow oil, 1H NMR δ: 1,2 (3H, m, CH3), 1.24 (2H, m, CH2), 1.25- 1,35 (6H, s, CH3), 1.46 (3H, d, J 6.9, CHCH3), 1.70 - 1.85 (4H, m, CHH), 2.45 (3H, s, CH3), 3,2 (1H, m, CHCH3), 2,48 (2H, s,ArCH2), 7.71 (1H, s, ArH), 7,75 (1H, s, ArH); 13C NMR δ: 20.79, 21.09, 24.18, 30, 34.14, 34.78, 36.42, 40.29, 40.97, 122.21, 130.93, 131.48, 143.86, 147. 25, 154.03. EIMS, m/z 247.1 (M+, 100), 232.1 (68.5), 230.1 (28), 144.1 (18), 41.1 (19). HRMS, 247.0288.
2,5,9,9-Tetramethyl-1,3-dinitro-6,7,8,9-tetrahydro-5H-benzocycloheptene 3, yellow oil, 1H NMR δ: 1,2 (3H, m, CH3), 1.24 (2H, m, CH2), 1.25 - 1,35 (6H, s, CH3), 1.46 (3H, d, J 6.9, CHCH3), 1.70 - 1.85 (4H, m, CHH), 2.45 (3H, s, CH3), 3,2 (1H, m, CHCH3), 2,48 (2H, s,ArCH2), 7,8 (1H, s, ArH); 13C NMR δ: 14.34, 20.4, 22.9, 30.34, 31.63, 34.48, 35.49, 42.51, 44.61, 122.43, 123.62, 132, 143.22, 146.38, 149. EIMS, m/z 292.1 (M+, 100), 248.1 (86), 232.1 (61.5), 230.1 (32), 144 (21), 41.1 (19). HRMS, 292.1090.
6. References
[1] P. T. Anastas and J. Warner, “Green chemistry: Theory and Practice,” Oxford University Press, New York, 1998, p. 30.
[2] E. J. Lenardao, R. A. Freitag, M. J. Dabdoub, A. C. Batista and C. C. Silveira, “Green Chemistry: The 12 Principles of Green Chemistry and It Insertion in the Teach and Research Activities,” Quimica Nova, Vol. 26, No. 1, 2003, 123-129.
[3] G. A. Olah, R. Malhorta and S. C. Narang, “Nitration Methods and Mechanisms,” VCH, New York, 1989.
[4] F. F. Becker and B. K. Banik, “Polycyclic Aromatic Compounds as Anticancer Agents: Synthesis and Biological Evaluation of Some Chrysene Derivatives,” Bioorganic & Medicinal Chemistry Letters, Vol. 50, 1998, pp. 2877-2880.
[5] H. Zollinger, “Color Chemistry: Properties and Applications of Organic Dyes,” 2nd Edition, John Wiley, New York, 1991, p. 496.
[6] R. Meyer, J. Kohler and A. Homburg, “Explosives,” 5th Edition, Wiley, New York, 2002, p. 253. doi:10.1002/3527600515
[7] C. K. Ingold, “Structure and Mechanism in Organic Chemistry,” 2nd Edition, Cornell University Press, Ithaca, NY, 1969, p. 563.
[8] G. A. Olah and S. J. Kuhnin, “Friedel-Crafts and Related Reactions,” John Wiley, New York, Vol. 3, 1964, p. 1153.
[9] J. G. Hoggett, R. B. Moodie, J. R. Penton and K. Schofield, “Nitration and Aromatic Reactivity,” Cambridge University Press, London, 1971, p. 757.
[10] K. Schofield, “Aromatic Nitration”, Cambridge University Press, London, 1980, p. 368.
[11] B. Baghernejad, M. M. Heravi, H. A. Oskooie, Y. S. Beheshtiha, “Synthesis and Biological Evaluation of Some Chrysene Derivatives an Efficient and Regioselective Nitration of Phenols Using NH4NO3, KHSO4,” Gazi University Journal of Science, Vol. 22, No. 3, 2009, pp. 169- 173.
[12] Cornélis, A. Gerstmans and P. Laszlo, “Regioselective Liquid-Phase Toluene Nitration with Modified Clays as Catalysts,” Chemical Letters, Vol. 17, No. 11, 1988, pp. 1839-1842;
[13] B. Gigante, P. Laszlo, A. Cornélis and M. J. MarceloCurto, “Nitrations of Nitroaromatics over Aluminum Silicates,” European Patent No. 94/19310, 1994.
[14] M. Choudary, M. Sateesh, M. Lakshmi, K. K. Rao, K. V. Ram, K. V. Raghavan and J. P. Sarma, “Selective Nitration of Aromatic Compounds by Solid Acid Catalysts,” Chemical Communications, Vol. 26, No. 1, 2000, pp. 25- 26. doi:10.1039/a908011b
[15] B. Gigantee, A. O. Prazeres, M. J. Marcelo-Curto, A. Cornelis and P. Laszlo, “Mild and Selective Nitration by Claycop,” The Journal of Organic Chemistry, Vol. 60, No. 11, 1995, pp. 3445-3447.
[16] F. J. Waller, A. G. M. Barrett, D. C. Braddock, R. M. McKinnell and D. Ramprasad, “Lanthanide(III) and Group IV Metal Triflate Catalysed Electrophilic Nitration: ‘Nitrate Capture’ and the Rôle of the Metal Centre,” Journal of the Chemical Society, Perkin Transactions 1, No. 8, 1999, pp. 867-871. doi:10.1039/a809914f
[17] R. R. Bak and A. J. Smallridge, “A Fast and Mild Method for the Nitration of Aromatic Rings,” Tetrahedron Letters, Vol. 42, No. 38, 2001, pp. 6767-6769. doi:10.1016/S0040-4039(01)01378-8
[18] H. Suzuki, S. Yonezawa, N. Nonoyama and T. Mori, “Iron(III)-Catalysed Nitration of Non-Activated and Moderately Activated Arenes with Nitrogen Dioxide-Molecular Oxygen under Neutral Conditions,” Journal of the Chemical Society, Perkin Transactions 1, No. 19, 1996, pp. 2385-2389. doi:10.1039/p19960002385
[19] J. H. Adrian, W. R. Millar and P. B. John, “Atom-Efficient Electrophilic Aromatic Nitration by Dinitrogen Pentoxide Catalysed by Zirconium(IV) 2,4-Pentanedionate,” Organic & Biomolecular Chemistry, Vol. 2, 2004, pp. 90- 92.
[20] R. J. Bartelt, A. A. Cosse, B. W. Zilkowski, D. Weisleder and F. A. Momany, “Male-Specific Sesquiterpenes from Phyllotreta and Aphthona Flea Beetles,” Journal of Chemical Ecology, Vol. 27, No. 12, 2000, pp. 2397-2423.
[21] K. Mori, “Synthesis of (R)-ar-Turmerone and Its Conversion to (R)-ar-Himachalene, a Pheromone Component of the Flea Beetle: (R)-ar-Himachalene Is Dextrorotatory in Hexane, While Levorotatory in Chloroform,” Tetrahedron: Asymmetry, Vol. 16, No. 3, 2005, pp. 685-692. doi:10.1016/j.tetasy.2004.11.077
[22] J. C. Chalchat, R. P. Garry and A. Michet, “Essential Oil in Sawdust of Cedrus Atlantica from Morocco,” JOER, Vol. 6, 1994, pp. 323-325.
[23] R. P. Adams, “Cedar Wood Oil—Analysis and Properties,” In: H. F. Linskens and J. F. Jackson, Eds., Modern Methods of Plant Analysis New Series, Vol. 12, Essential Oils and Waxes, Springer-Verlag, New York, 1991, pp. 159-173.
[24] K. Mori, “Synthesis of Optically Active Pheromones,” Tetrahedron, Vol. 45, No. 11, 1989, pp. 3233-3298. doi:10.1016/S0040-4020(01)81007-3
[25] R. C. Pandey and S. Dev, “Studies in Sesquiterpenes— XXX 1: Synthesis of ar-Himachalene and Himachalanes,” Tetrahedron, Vol. 24, No. 10, 1968, pp. 3829-3839 doi:10.1016/S0040-4020(01)92590-6
[26] B. Abouhamza, S. Allaoud and A. Karim, “2,4-Dimethyl -3-Phenyl-[2’-Methyl-3’-Chloro]-7-Chloro-8-Methyl-1,3-Diaza-2,4-Diboranaphtalene Tricarbonylchromium Complexes,” Molecule, Vol. 6, No. 6, 2001, p. M211.
[27] A.R. Hajipour and A. E. Ruoho, “Nitric Acid in the Presence of P2O5 Supported on Silica Gel—A Useful Reagent for Nitration of Aromatic Compounds under Solvent-Free Conditions,” Tetrahedron Letters, Vol. 46, No. 48, 2005, pp. 8307-8310. doi:10.1016/j.tetlet.2005.09.178