Journal of Diabetes Mellitus
Vol.2 No.3(2012), Article ID:22200,8 pages DOI:10.4236/jdm.2012.23053
Sitagliptin improves vascular endothelial function in Japanese type 2 diabetes patients without cardiovascular disease*
![]()
Department of Internal Medicine (Divisions of Cardiology, Hepatology, Geriatrics, and Integrated Medicine), Nippon Medical School, Tokyo, Japan; #Corresponding Author: kentaro@nms.ac.jp
Received 31 May 2012; revised 30 June 2012; accepted 10 July 2012
Keywords: Sitagliptin; Endothelial Function; Flow-Mediated Dilation; Type 2 Diabetes Mellitus; Adiponectin
ABSTRACT
We evaluated the effect of sitagliptin on vascular endothelial function in Japanese type 2 diabetes patients without cardiovascular disease. Subjects included 24 Japanese type 2 diabetes patients without cardiovascular disease. This study was a prospective, open-label, randomized clinical trial. We divided the study subjects into 2 groups: subjects who received sitagliptin 50 mg daily (sitagliptin group, n = 12) and subjects who did not receive sitagliptin (control group, n = 12). Brachial artery flow-mediated dilation (FMD) was measured after overnight fasting. Sitagliptin administration was initiated at 1 month after enrollment in study (baseline). FMD and level of biochemical variables in the sitagliptin and control groups were measured at baseline and 3 months from baseline (3 months). We evaluated the effect of sitagliptin on vascular endothelial function by measuring FMD. FMD at 3 months was significantly higher in the sitagliptin group than in the control group (5.36% ± 2.18% vs 3.41% ± 2.29%, P = 0.040), while FMD at baseline was not significantly different between the 2 groups. In addition, FMD of the sitagliptin group at 3 months was significantly higher than that at baseline (5.36% ± 2.18% vs 3.67% ± 2.30%, P = 0.004), while no significant differences were observed in the FMD of the control group during the study period. The change in the adiponectin from baseline to 3 months was significantly higher in the sitagliptin group than that in the control group (0.82 ± 2.18 μg/mL vs 0.01 ± 0.55 μg/mL, P = 0.039). Sitagliptin improves vascular endothelial function of the brachial artery in Japanese type 2 diabetes patients without cardiovascular disease. Furthermore, elevation of adiponectin may induce reduction of endothelial dysfunction in type 2 diabetes patients treated with sitagliptin.
1. INTRODUCTION
Atherosclerosis progression plays an important role in increasing the risk of cardiovascular disease in diabetes patients [1,2]. Therefore, it is important to prevent development and progression of cardiovascular disease in diabetes patients. Ultrasound techniques are useful and noninvasive methods for evaluating atherosclerosis progression. Flow-mediated dilation (FMD) of the brachial artery measured using ultrasonography is an established method for evaluating vascular endothelial function [3]. FMD is a predictor of cardiovascular disease [4,5]. Furthermore, FMD is related to microvascular complications in diabetes patients [6,7]. Many studies reported that oral hypoglycemic agents for diabetes improve endothelial dysfunction [8-11]. However, whether vascular endothelial dysfunction in diabetes patients is improved by administration of an oral dipeptidyl peptidase-4 (DPP-4) inhibitor is unknown. DPP-4 inhibitor shows a hypoglycemic effect by elevating the plasma levels of incretin hormone in type 2 diabetes [12]. The incretin hormone, particularly glucagon-like peptide-1 (GLP-1), has an extraglycemic effect in humans [13]. GLP-1 improves brachial artery endothelial dysfunction in type 2 diabetes patients [14]. These findings indicate that the DPP-4 inhibitor may have an effect of vascular protection in addition to improving glycemic control in diabetes and non-diabetes subjects.
The aim of our study was to evaluate the effect of the DPP-4 inhibitor sitagliptin on vascular endothelial function in type 2 diabetes patients without cardiovascular disease by measuring FMD.
2. MATERIALS AND METHODS
2.1. Study Subjects
We recruited 24 type 2 diabetes patients (12 men and 12 women) without cardiovascular disease with ages ranging from 49 to 78 years (mean age 68.0 ± 8.5 years) who were treated with diet only, sulfonylurea only, metformin only, or sulfonylureas plus metformin in our division. We excluded patients receiving an α-glucosidase inhibitor, thiazolidinedione, insulin injection, GLP-1 receptor analogues (exenatide and liraglutide) injection, hormone therapy, and immunosuppressive therapy. In addition, patients treated for chronic disease such as malignant diseases, collagen diseases, and acute diseases were excluded.
2.2. Study Design
The study protocol was approved by the Ethical Committee of Nippon Medical School and carried out in accordance with the principles of the Declaration of Helsinki. This study was a prospective, open-label, randomized clinical trial. This study design is shown in Figure 1. All subjects provided written informed consent before participation, and the study was approved by the local institutional review board. The baseline of the study was defined as the time at 1 month after entry into this study. Clinical characteristics of study subjects were investigated at baseline. We randomized study subjects at baseline into 2 groups using the envelope method as follows: subjects who received sitagliptin 50 mg daily (sitagliptin group, n = 12) and subjects who did not receive sitagliptin (control group, n = 12). Subjects in the sitagliptin group began receiving sitagliptin 50 mg daily in addition to treatment for diabetes at entry from baseline to 3 months from baseline (3 months), and subjects in the control group were maintained on diabetes treatment during the study period. For both groups, treatment for diabetes, excluding sitagliptin, and that for other diseases, including hypertension or dyslipidemia, were not altered during the study period. We followed the study subjects for 3 months. FMD and the levels of biochemical variables were investigated at baseline and at 3 months.
2.3. Clinical Characteristics of Study Subjects
We investigated baseline clinical characteristics of study subjects, including gender; age; body mass index (BMI); duration of diabetes; diabetes complications (retinopathy, nephropathy, and neuropathy); cardiovascular disease, smoking habit; hypertension; antihypertensive medication; statin use; treatment of diabetes; systolic and diastolic blood pressure. We measured the levels of the biochemical variables, including low-densitylipoprotein (LDL)-cholesterol, high-density-lipoprotein (HDL)-cholesterol, triglyceride, uric acid, plasma creatinine,
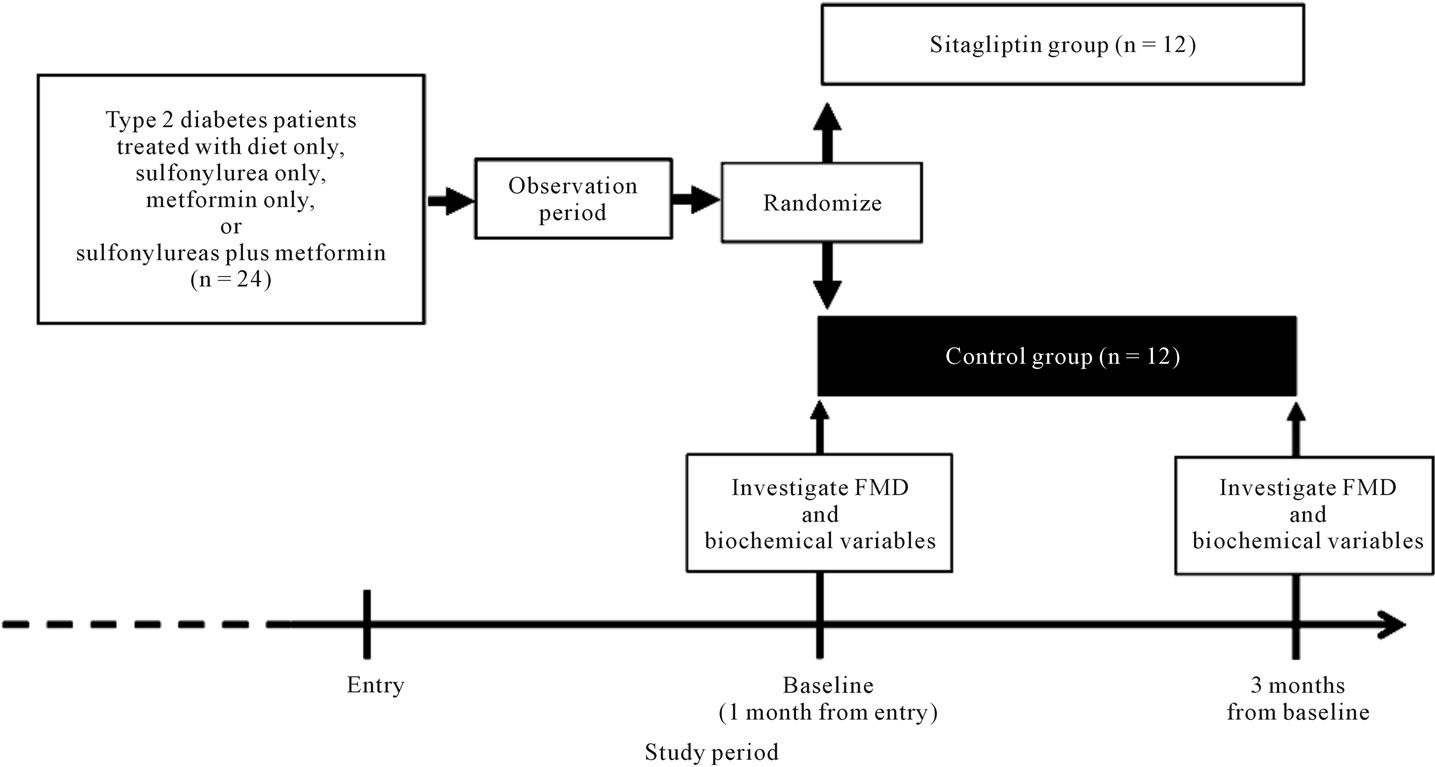
Figure 1. Study design.
fasting plasma glucose (FPG), HbA1c, plasma 1,5-anhydroglucitol (1,5-AG), and adiponectin, after overnight fasting at baseline and at 3 months. Plasma glucose level was measured using the glucose oxidase method. HbA1c level (Japan Diabetes Society, JDS) (%) was measured using high-performance liquid chromatography (HPLC, JDS Lot3). In addition, HbA1c level (JDS) was transformed into A1c (National Glycohemoglobin Standardization Program, NGSP) as follows: A1c (NGSP) (%) = HbA1c (JDS) + 0.4 [15]. Plasma level of 1,5-AG was measured using an HPLC method. The levels of HDLcholesterol, LDL-cholesterol, and triglycerides were measured using an enzymatic method. Adiponectin was measured using latex particle-enhanced turbidimetric immunoassay [16].
2.4. Measurement of FMD
FMD of the brachial artery of study subjects was measured according to a previously reported method [17]. FMD measurement on the right brachial artery was evaluated using Aand B-mode ultrasonography using a linear-array 10-MHz transducer (UNEXEF18G; UNEX Corporation, Nagoya, Japan). For the hyperemia scan, vessel diameter was measured continuously for 180 s after cuff deflation. Interobserver reproducibility of FMD measurement using UNEXEF18G was calculated with a correlation coefficient of r = 0.961 (P < 0.001) between the 2 observers, while their intraobserver variability for measurements varied by 2.4%. Subjects were instructed to abstain from smoking and caffeine consumption for at least 8 h and to lie down for 15 min before the start of the study. We measured the systolic blood pressure of the study subjects before the measurement of FMD. FMD measurement was performed according to a report by the International Brachial Artery Reactivity Task Force [18]. The baseline diameter of the brachial artery was defined as the mean diameter of that artery 5 cm proximal to the elbow joint at 4 consecutive diastolic times on an electrocardiogram before hyperemia. After determination of this baseline diameter, forearm hyperemia was induced by inflating the sphygmomanometric cuff at systolic blood pressure plus 50 mmHg before FMD measurement and continued for 5 min in the forearm. After cuff deflation, the maximum diameter after hyperemia was measured. The rate of change in diameter (%) from the baseline diameter and maximum diameter after hyperemia was recorded as the FMD. If the rate of diameter change was less than 0%, this rate was defined as 0%. During the study period, FMD was measured at baseline and at 3 months.
2.5. Statistical Analysis
The Mann-Whitney U test and chi-squire test were used to compare differences in baseline clinical characteristics, FMD, and levels of biochemical variables between the sitagliptin and control groups. Wilcoxon test was used to compare changes in FMD and the levels of biochemical variables during the study period. Data were presented as means ± standard deviation (SD), and n (%). Statistical significance was defined as P < 0.05. All analysis was performed using SPSS for Windows Ver. 12.0J (SPSS Japan Inc.).
3. RESULTS
The baseline clinical characteristics of study subjects are shown in Table 1. No significant difference was observed in the clinical characteristics between the sitagliptin and control groups. Furthermore, no significant difference was observed in the treatment of diabetes and prevalence of diabetes complications between the 2 groups. The FMD and the levels of biochemical variables, including glucose and lipid metabolism variables, and adiponectin of the study subjects at baseline and 3 months are shown in Table 2, and comparisons of changes in FMD from baseline to 3 months between the sitagliptin and control groups is shown in Figure 2. FMD at 3 months was significantly higher in the sitagliptin group than in the control group (5.36% ± 2.18% vs 3.41% ± 2.29%, P = 0.040), while no significant difference was observed in the FMD at baseline between the sitagliptin and control groups. In addition, FMD of the sitagliptin group at 3 months was significantly higher than that at baseline (5.36% ± 2.18% vs 3.67% ± 2.30%, P = 0.004), while no significant differences were observed between the FMD of the control group at baseline and at 3 months. Furthermore, the change in the FMD from baseline to 3 months was significantly higher in the sitagliptin group than in the control group (1.69% ± 1.76% vs 0.54% ± 1.52%, P = 0.009).
No significant difference was observed in the glucose metabolism variables between the 2 groups during the study period. However, A1c levels in the sitagliptin group at 3 months were significantly lower than those at baseline (7.51% ± 1.75 % vs 7.92% ± 1.16 %, P = 0.034), and 1,5-AG levels in the sitagliptin group at 3 months were significantly higher than those at baseline (55.03 ± 42.01 μmol/L vs 37.31 ± 24.30 μmol/L, P = 0.015), while no significant difference was observed in the levels of FPG in the sitagliptin group during the study period. In contrast, no significant difference was observed in the glucose metabolism variables in the control group during the study period. Also, no significant difference was observed in the levels of other biochemical variables between the 2 groups during study period. However, the change in the adiponectin from baseline to 3 months was significantly higher in the sitagliptin group than that in
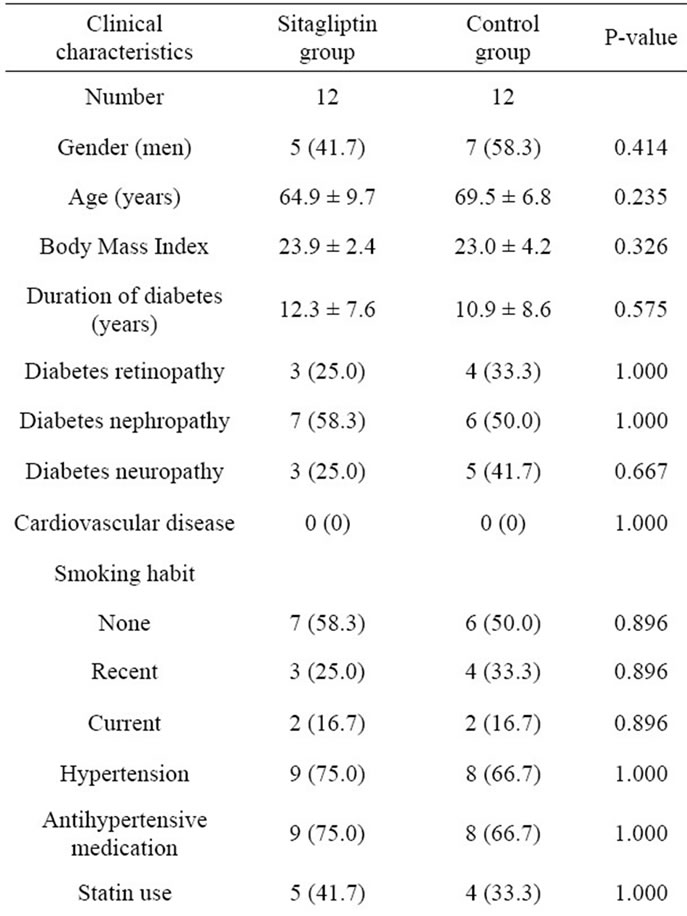
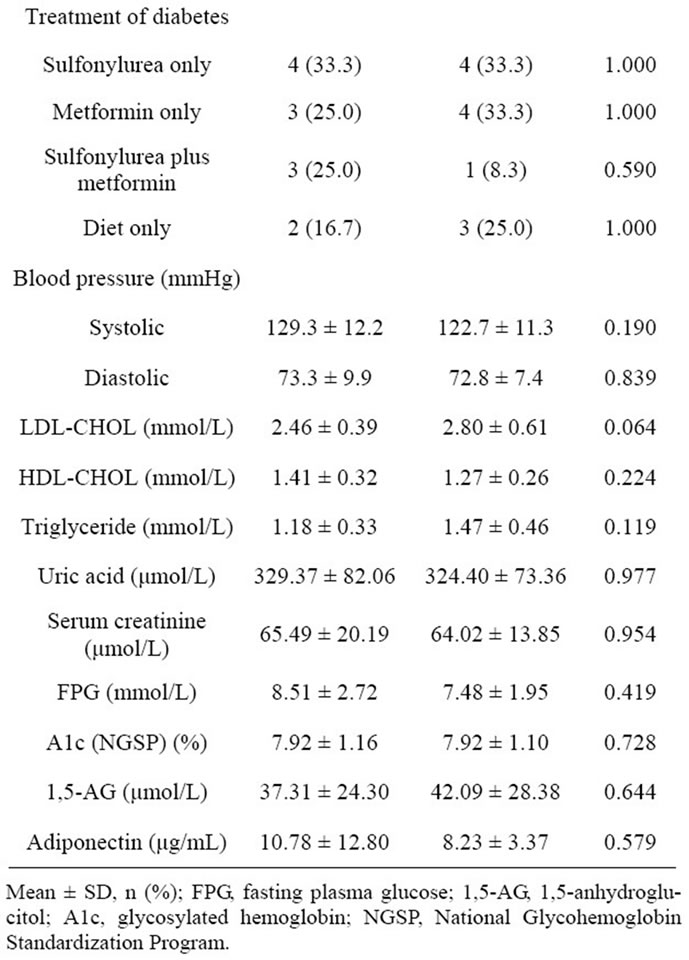
Table 1. Baseline clinical characteristics of study subjects.
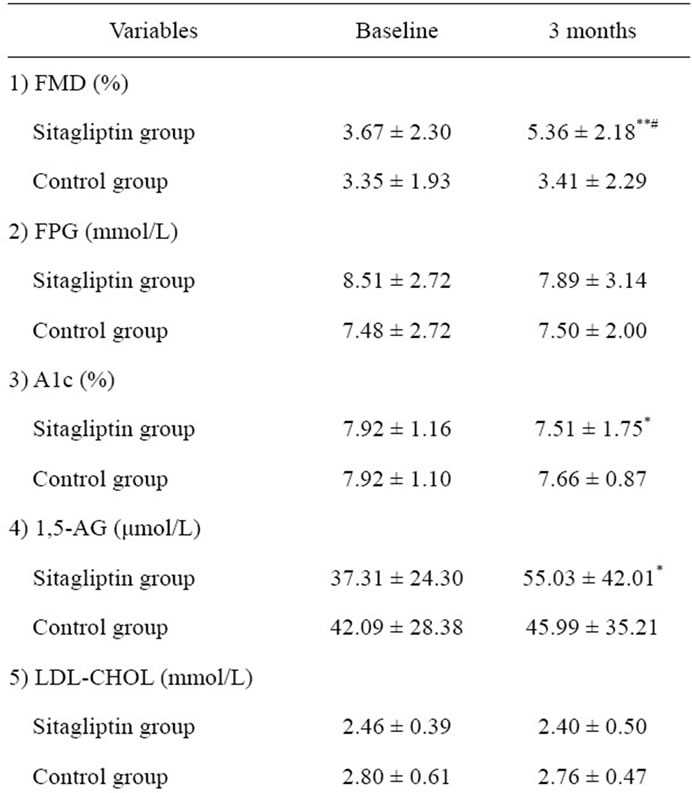
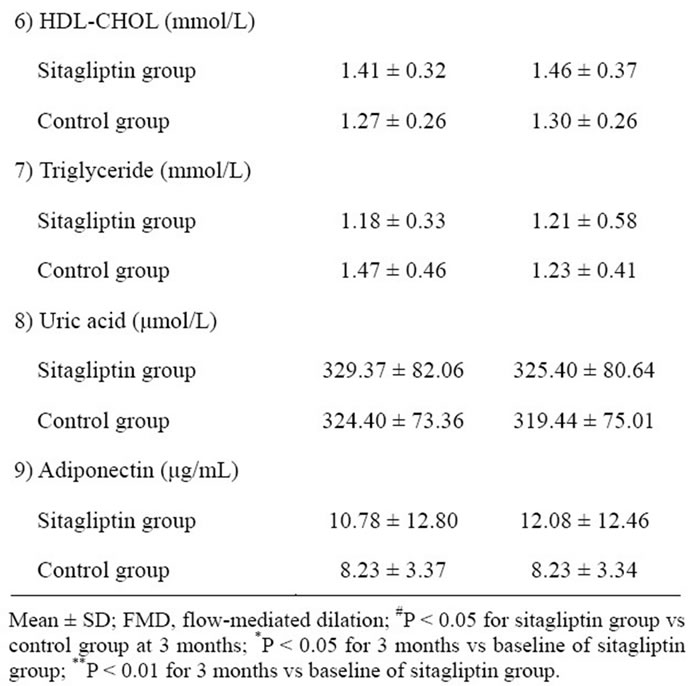
Table 2. Comparison of flow-mediated dilation and biochemical variables of study subjects at baseline and at 3 months.
the control group (0.82 ± 2.18 μg/mL vs 0.01 ± 0.55 μg/mL, P = 0.039) (Figure 3).
4. DISCUSSION
We showed that sitagliptin improves FMD of the brachial artery in Japanese type 2 diabetes patients without cardiovascular disease. To our knowledge, this is the first study evaluating the effect of improvement in endothelial
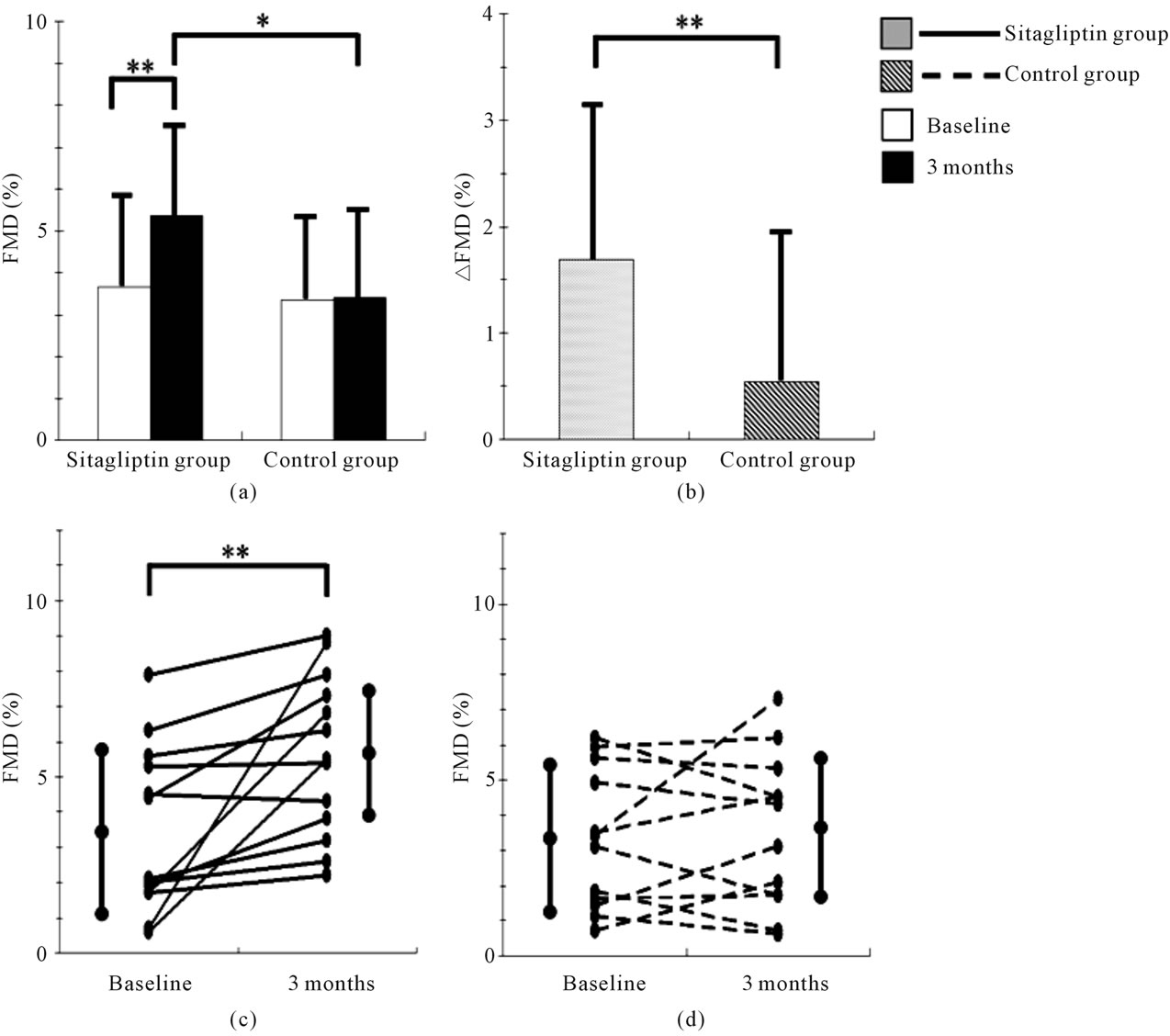
Figure 2. Comparisons of changes in flow-mediated dilation (FMD). (a) Comparisons of mean FMD values in the sitagliptin and control groups between baseline and 3 months; (b) Comparison of the mean change in FMD from baseline to 3 months between the sitagliptin and the control group; (c) Changes in FMD of all subjects of the sitagliptin group from baseline to 3 months; (d) Changes in FMD of all subjects of the control group from baseline to 3 months. ∆FMD = (FMD at 3 months) − (FMD at baseline). *P < 0.05, **P < 0.01.
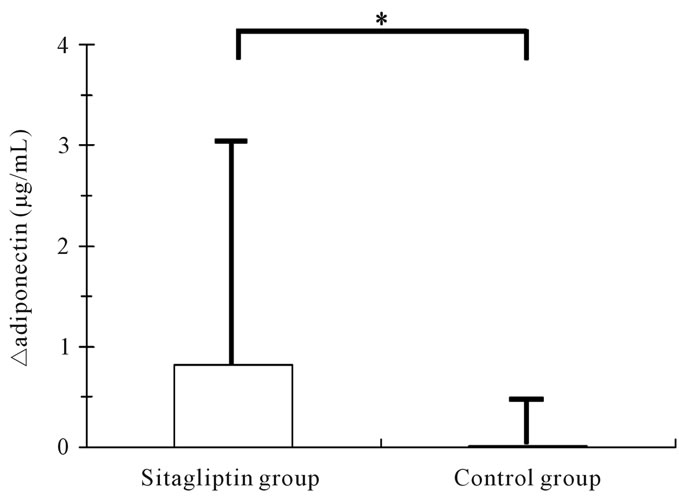
Figure 3. Comparisons of changes in adiponectin from baseline to 3 months. ΔAdiponectin = (adiponectin at 3 months) – (adiponectin at baseline). *P < 0.05.
function in type 2 diabetes patients by administration of DPP-4 inhibitors.
Endothelium-dependent arterial dilation is mediated by the release of endothelium-derived nitric oxide [18]. Endothelial dysfunction in diabetes is caused by increased levels of oxidative stress induced by abnormalities in glucose metabolism [19]. Endothelial dysfunction is improved by administration of oral hypoglycemic agents [8-11]. These agents improve the abnormalities in glucose metabolism and thus reduce oxidative stress. DPP-4 inhibitor shows a hypoglycemic effect by increasing the plasma level of incretin hormone in type 2 diabetes patients, as previously described [12]. Furthermore, DPP-4 inhibitors suppress endogenous glucose production and enhance insulin secretion [20]. Therefore, sitagliptin may too have the same effect as that of other oral hypoglycemic agents in improving endothelial dysfunction by improving abnormalities in glucose metabolism and reducing oxidative stress. In addition, the levels of 1,5-AG, which are thought to reflect postprandial glucose levels, in the sitagliptin group at 3 months were significantly higher than those at baseline. This result suggests that the improvement in endothelial dysfunction in the sitagliptin group is due to a reduction of postprandial hyperglycemia and fluctuation of glucose levels. DPP-4 inhibitor reduces postprandial glucose levels until 240 min after meal injection [20]. Several studies showed that postprandial hyperglycemia was improved in type 2 diabetes patients receiving DPP-4 inhibitor monotherapy [21] and DPP-4 inhibitor plus sulfonylurea and metformin therapy [22,23]. The mean amplitude of glycemic excursion in daily glucose profiles was negatively correlated with fasting FMD in type 2 diabetes patients [24]. Acarbose and nateglinide improve postprandial hyperglycemia and endothelial dysfunction [9,25]. In addition, repetitive fluctuations in the levels of glucose or insulin enhanced adhesion of monocytes to the endothelium of the rat thoracic aorta [26]. These results suggest that improvement of fasting vascular endothelial dysfunction by sitagliptin in type 2 diabetes patients is associated with a reduction in postprandial hyperglycemia and glucose fluctuation.
Furthermore, our study demonstrated that the increase of plasma adiponectin level of sitagliptin group was significantly higher than that of control group. This result suggests that adiponectin may be possible relevant factor of reduction of endothelial dysfunction in diabetes patients treated with sitagliptin. Lim et al. demonstrated that circulating levels of adiponectin was increased significantly in sitagliptin treatment and sitagliptin had protective properties against restenosis after carotid injury and therapeutic implications for treating macrovascular complications in OLETF rats [27]. Low adiponectin level is a risk factor for the subsequent development of cardiovascular diseases [28,29]. In contrast, adiponectin has protective effect of reactive oxygen species, and stimulates AMPK activation in endothelial cells, leading to activation of eNOS in vascular endothelial cells [30]. This result suggests that elevation of adiponectin may induce reduction of endothelial dysfunction in our study subjects treated with sitagliptin.
Our study has some limitations. First, we could not assess plasma GLP-1 levels. Vildagliptin decreases reactive oxygen species-induced vascular endothelial cell senescence depending on GLP-1 level in diabetes rats [31]. The GLP-1 analogue liraglutide inhibits the effect of endothelial damage in human vein endothelial cells [32]. Furthermore, exendin-4 inhibits monocyte adhesion to endothelial cells and attenuation of atherosclerosis, and these effects are dependent on exendin-4 levels [33]. These findings suggest that higher GLP-1 levels induce a greater effect of repair of endothelial function in type 2 diabetes patients. Second, subjects with cardiovascular disease were excluded in the present study. The aim of the present study is to evaluate the effect of sitagliptin on vascular endothelial function in Japanese type 2 diabetes patients. Early and predominantly functional changes in the vessel wall can be measured by brachial artery FMD as a surrogate marker of endothelial function. However, we assume that study subjects with cardiovascular disease may show morphological changes of the brachial artery vessel wall. Therefore, FMD is considered as an insufficient surrogate marker for evaluating effect of sitagliptin on vascular function in subjects with cardiovascular disease. If we evaluate the effect of sitagliptin on atherosclerosis in subjects who have high prevalence of advanced morphological wall changes can be expected, surrogate marker for evaluating morphological vascular wall damage, such as intima-media thickness (IMT) of carotid artery, should be added. The prospective study in subjects including cardiovascular disease is needed to evaluate the effect of sitagliptin on atherosclerosis by using surrogate markers of endothelial function (FMD) and morphological wall change (such as IMT). And finally, no significant association was observed between FMD and other inflammation or oxidative stress markers (data not shown). We consider that a double-blind, crossover, placebo-controlled trial in a large number of study subjects is necessary for evaluating the results of our study.
5. CONCLUSION
Sitagliptin improves endothelial dysfunction in Japanese type 2 diabetes patients without cardiovascular disease. Furthermore, the results of our study suggest that this effect was induced not only by improving glucose metabolism abnormalities but also by the extraglycemic effect of the DPP-4 inhibitor. Also, elevation of adiponectin may induce reduction of endothelial dysfunction in type 2 diabetes patients treated with sitagliptin.
![]()
![]()
REFERENCES
- Haffner, S.M., Agostino, R.D., Jr., Saad, M.F., O’Leary, D.H., Savage, P.J., Rewers, M., Selby, J., Bergman, R.N. and Mykkänen, L. (2000) Carotid artery atherosclerosis in type-2 diabetic and nondiabetic subjects with and without symptomatic coronary artery disease (The insulin resistance atherosclerosis study). American Journal of Cardiology, 85, 1395-1400. doi:10.1016/S0002-9149(00)00784-0
- Li, J., Luo, Y., Xu, Y., Yang, J., Zheng, L., Hasimu, B., Yu, J. and Hu, D. (2007) Risk factors of peripheral arterial disease and relationship between low ankle-brachial index and mortality from all-cause and cardiovascular disease in Chinese patients with type 2 diabetes. Circulation Journal, 71, 377-381. doi:10.1253/circj.71.377
- Ter Avest, E., Stalenhoef, A.F. and de Graaf, J. (2007) What is the role of non-invasive measurements of atherosclerosis in individual cardiovascular risk prediction? Clinical Science, 112, 507-516. doi:10.1042/CS20060266
- Yeboah, J., Crouse, J.R., Hsu, F.C., Burke, G.L. and Herrington, D.M. (2007) Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation, 115, 2390- 2397. doi:10.1161/CIRCULATIONAHA.106.678276
- Yeboah, J., Folsom, A.R., Burke, G.L., Johnson, C., Polak, J.F., Post, W., Lima, J.A., Crouse, J.R. and Herrington, D.M. (2009) Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation, 120, 502-509. doi:10.1161/CIRCULATIONAHA.109.864801
- Watanabe, K., Suzuki, T., Nakano, H. and Oba, K. (2007) Relationship between diabetic retinopathy and vascular endothelial function in elderly type 2 diabetic Japanese patients without cardiovascular disease. Geriatrics and Gerontology International, 7, 348-351. doi:10.1111/j.1447-0594.2007.00423.x
- Suetsugu, M., Takebayashi, K. and Aso, Y. (2007) Association between diabetic microangiopathy and vascular endothelial function evaluated by flow-mediated vasodilatation in patients with type 2 diabetes. International Journal of Clinical Practice, 61, 920-926. doi:10.1111/j.1742-1241.2006.01223.x
- Chen, L.L., Yu, F., Zeng, T.S., Liao, Y.F., Li, Y.M. and Ding, H.C. (2011) Effects of gliclazide on endothelial function in patients with newly diagnosed type 2 diabetes. European Journal of Pharmacology, 659, 296-301. doi:10.1016/j.ejphar.2011.02.044
- Shimabukuro, M., Higa, N., Chinen, I., Yamakawa, K. and Takasu, N. (2006) Effects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: A randomized crossover study. Journal of Clinical Endocrinology and Metabolism, 91, 837-842. doi:10.1210/jc.2005-1566
- Mather, K.J., Verma, S. and Anderson, T.J. (2001) Improved endothelial function with metformin in type 2 diabetes mellitus. Journal of the American College of Cardiology, 37, 1344-1350. doi:10.1016/S0735-1097(01)01129-9
- Papathanassiou, K., Naka, K.K., Kazakos, N., Kanioglou, C., Makriyiannis, D., Pappas, K., Katsouras, C.S., Liveris, K., Kolettis, T., Tsatsoulis, A. and Michalis, L.K. (2009) Pioglitazone vs glimepiride: Differential effects on vascular endothelial function in patients with type 2 diabetes. Atherosclerosis, 205, 221-226. doi:10.1016/j.atherosclerosis.2008.11.027
- Athén, B., Landin-Olsson, M., Jansson, P.A., Svensson, D. Holmes, M. and Schweizer, A. (2004) Inhibition of dipeptidyl peptidase-4 reduces glycemia, susutains levels in type 2 diabetes. Journal of Clinical Endocrinology and Metabolism, 89, 2078-2084. doi:10.1210/jc.2003-031907
- Seino, Y., Fukushima, M. and Yabe, D. (2010) GIP and GLP-1, the two incretin hormines: Similarities and differences. Journal of Diabetes Investigation, 1, 8-23. doi:10.1111/j.2040-1124.2010.00022.x
- Nyström, T., Gutniak, M.K., Zhang, Q., Zhang, F., Holst, J.J., Ahrén, B. and Sjöholm, A. (2004) Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. American Journal of Physiology—Endocrinology and Metabolism, 287, E1209-E1215. doi:10.1152/ajpendo.00237.2004
- The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus (2010) Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetology International, 1, 2-20. doi:10.1007/s13340-010- 0006-7
- Nishimura, A. and Sawai, A. (2006) Determination of adiponectin in selum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clinica Chimica Acta, 371, 163-168. doi:10.1016/j.cca.2006.03.008
- Watanabe, K., Oba, K., Suzuki, T., Ouchi, M., Suzuki, K., Futami-Suda, S., Sekimizu, K., Yamamoto, N. and Nakano, H. (2011) Oral glucose loading attenuates endothelial function in normal individual. European Journal of Clinical Investigation, 41, 465-473. doi:10.1111/j.1365-2362.2010.02424.x
- Corretti, M.C., Anderson, T.J., Benjamin, E.J., Celermajer, D., Charbonneau, F., Creager, M.A., Deanfield, J., Drexler, H., Gerhard-Herman, M., Herrington, D., Vallance, P., Vita, J., Vogel, R., International Brachial Artery Reactivity Task Force (2005) Guideline for the Ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology, 39, 257-265. doi:10.1016/S0735-1097(01)01746-6
- Potenza, M.A., Gagliardi, S., Nacci, C., Carratu’, M.R. and Montagnani, M. (2009) Endothelial dysfunction in diabetes: From mechanism to therapeutic targets. Current Medicinal Chemistry, 16, 94-112. doi:10.2174/092986709787002853
- Balas, B., Baig, M.R., Watson, C., Dunning, B.E., Ligueros-Saylan, M., Wang, Y., He, Y.L., Darland, C., Holst, J.J., Deacon, C.F., Cusi, K., Mari, A., Foley, J.E. and DeFronzo, R.A. (2007) The dipeptidyl peptidase 4 inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. Journal of Endocrinology and Metabolism, 92, 1249-1255. doi:10.1210/jc.2006-1882
- Nonaka, K., Kakikawa, T., Sato, A., Okuyama, K., Fujimoto, G., Kato, N., Suzuki, H., Hirayama, Y., Ahmed, T., Davies, M.J. and Stein, P.P. (2008) Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Research and Clinical Practice, 79, 291-298. doi:10.1016/j.diabres.2007.08.021
- Scott, R., Loeys, T., Davies, M.J. and Engel, S.S. (2008) Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes, Obesity and Metabolism, 10, 959-969. doi:10.1111/j.1463-1326.2007.00839.x
- Hermansen, K., Kipnes, M., Luo, E., Fanurik, D., Khatami, H., Stein, P., Sitagliptin Study 035 Group (2007) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes, Obesity and Metabolism, 9, 733-745. doi:10.1111/j.1463-1326.2007.00744.x
- Di Flaviani, A., Picconi, F., Di Stefano, P., Giordani, I., Malandrucco, I., Maggio, P., Palazzo P., Sgreccia, F., Peraldo, C., Farina, F., Frajese, G. and Frontoni, S. (2011) Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care, 34, 1605-1609. doi:10.2337/dc11-0034
- Kato, T., Inoue, T. and Node, K. (2010) Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: An acarbose and nateglinide comparative study. Cardiovascular Diabetology, 9, 12. doi:10.1186/1475-2840-9-12
- Lim, S., Choi, S.H., Shin, H., Cho, B.J., Park, H.S., Ahn, B.Y., Kang, S.M., Yoon, J.W., Jang, H.C., Kim, Y.B. and Park, K.S. (2012) Effect of a Dipeptidyl Peptidase-IV Inhibitor, des-fluoro-sitagliptin, on neointimal formation after balloon injury in rats. PLoS One, 7, e35007. doi:10.1371/journal.pone.0035007
- Schulze, M.B., Shai, I., Rimm, E.B., Li, T., Rifai, N. and Hu, F.B. (2005) Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes, 54, 534-539. doi:10.2337/diabetes.54.2.534
- Pischon, T., Girman, C.J., Hotamisligil, G.S., Rifai, N., Hu, F.B. and Rimm, E.B., (2004) Plasma adiponectin levels and risk of myocardial infarction in men. Journal of the American Medical Association, 291, 1730-1737. doi:10.1001/jama.291.14.1730
- Ouchi, N. and Walsh, K. (2007) Adiponectin as an antiinflammatory factor. Clinica Chimica Acta, 380, 24-30. doi:10.1016/j.cca.2007.01.026
- Azuma, K., Kawamori, R., Toyofuku, Y., Kitahara, Y., Sato, F., Shimizu, T., Miura, K., Mine, T., Tanaka, Y., Mitsumata, M. and Watada, H. (2006) Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 2275-2280. doi:10.1161/01.ATV.0000239488.05069.03
- Oeseburg, H., de Boer, R.A., Buikema, H., van der Harst, P., van Gilst, W.H. and Silljé, H.H. (2010) Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 1407-1414. doi:10.1161/ATVBAHA.110.206425
- Liu, H., Dear, A.E., Knudsen, L.B. and Simpson, R.W. (2009) A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular ahhesion molecules. Journal of Endocrinology, 20, 59-66. doi:10.1677/JOE-08-0468
- Arakawa, M., Mita, T., Azuma, K., Ebato, C., Goto, H., Nomiyama, T., Fujitani, Y., Hirose, T., Kawamori, R. and Watada, H. (2010) Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes, 59, 1030-1037. doi:10.2337/db09-1694
NOTES
*Competing Interests: the authors declare that they have no conflict of interest.

