Journal of Biomaterials and Nanobiotechnology
Vol.3 No.1(2012), Article ID:17005,5 pages DOI:10.4236/jbnb.2012.31011
Surface Characterization of Titanium Implants Treated in Hydrofluoric Acid
![]()
1Institute of Metal Physics, Russian Academy of Sciences-Ural Division, Yekaterinburg, Russia; 2Faculty of Physics, University of Osnabrueck, Osnabrueck, Germany; 3Institute of Physics of Advanced Materials, Ufa State Aviation Technical University, Ufa, Russia; 4Ural Federal University, Yekaterinburg, Russia.
Email: danila.korotin@gmail.com, kurmaev@ifmlrs.uran.ru
Received October 4th, 2011; revised November 19th, 2011; accepted December 20th, 2011
Keywords: Commercially Pure Titanium; Surface Composition; Hydrofluoric Acid; Surface Characterization; Biocompatibility
ABSTRACT
The results of XPS measurements of commercially pure titanium (cp-Ti) before and after chemical treatment are presented. We have measured XPS spectra of core levels (Ti 2p, O 1s, C 1s, F 1s) and valence bands of coarse-grained cp-Ti before and after standard acid treatment accepted in dentistry (in 1% HF and 40% HF for 1 min). It is found, that acid treatment of cp-Ti reduces the content of hydrocarbons increasing the surface energy and bio-compatibility of Ti-implants. On the other hand, it is fixed that oxygen concentration on the surface of the acid treated cp-Ti is much higher than for the untreated sample, because the acid treatment removes the contaminated surface layers, increases their reactivity, provides a better passivation and formation of thick protecting TiO2 layer.
1. Introduction
The materials for medical implants are required to be biocompatible, not toxic and also should not cause allergic reactions. They must have high ultimate strength and yield points with desirable low density and low modulus of elasticity. Commercially pure Ti (cp-Ti) (99.85% Ti) meets all these requirements and is considered to be one of the most biocompatible and corrosion resistant metals available for medical applications.
The reaction of biological tissue to biomaterial surfaces and the formation of bonds between implant surfaces and biological tissues are largely dependant on the existence of a high surface energy on the implant surface. Contamination of biomaterial surfaces with hydrocarbons, molecules and elements can reduce the surface energy and, thereby, also the potential bio-to create these components, incorporating the applicable criteria that follow compatibility of an implant. It is known, that commercially pure titanium is highly reactive and its surface composition is substantially different from the bulk [1] and forms spontaneously, within seconds, several oxides when exposed to air, water, or an aqueous biological environment among which TiO2 is the most common one [2].
The interaction of the implant with its biological environment, the formation of material-tissue interface and the long-term success or failure of the integration in the body is now realized to be strongly connected with the surface properties of the implant device. Even within one type of material, such as cp-Ti, the degree of integration depends on the quality of the surface. It is found, that fluoride-modification of titanium medical (dental) implants (performed by chemical treatment in HF-hydrofluoric acid) provides the better implant attachment and improves bone response [3], reduces the healing time needed before loading, as well as significantly improves the bone-to-implant contact [4]. In the present paper the results of surface characterization of cp-Ti after chemical treatment in the hydrofluoric acid (HF) are presented. We have measured XPS core levels (Ti 2p, O 1s, C 1s, F 1s) and valence band spectra before and after standard chemical treatment (in 1% HF, 1 min and in 40% HF, 1 min) which is used in the dentistry before implantation. For each set of measurement the ion etching (with Ar+ ions) was performed in a step by step mode with etching intervals of few minutes (from 1 to 10 min). In this way, the detailed study of composition and chemical state of titanium and oxygen (as well as carbon and fluorine) is performed, what gives us the full information about the surface and the near-surface layers of cp-Ti before and after chemical treatment.
2. Experimental
Commercially pure titanium cp-Ti Grade 4 (Ti: base, C: 0.052%, O: 0.34%, Fe: 0%, N: 0.015% (wt%)) was employed to conduct the research (Figure 1). The coarse grained cp-Ti in the form of discs (10 mm dia and 1 mm thick) was annealed at 950˚C for 1 hr and then etched in hydrofluoric acid (1% HF and 40% HF) during 1 min. The XPS measurements were performed on initial and etched cp-Ti samples using a Perkin Elmer PHI 5600 ci Multitechnique System with monochromatized Al Kα radiation (FWHM = 0.3 eV). The XPS binding energies of untreated cp-Ti, given in Table 1, were determined using the Au 4f7/2 core level (Eb (Au 4f7/2) = 84.0 eV) as a reference. For each set of XPS measurements the ion etching (with Ar+ ions) was performed step by step with intervals of few minutes (from 1 min to 10 min).
3. Results and Discussion
XPS wide scans of coarse-grained cp-Ti before and after chemical treatment in hydrofluoric acid (1% and 40% HF) are presented in Figure 2. The XPS analysis shows, that dental implant surfaces consist of titanium, oxygen and carbon. As can be seen (Figure 2 and Table 2), C/Ti intensity ratio estimated from XPS survey spectra decreased from 6.3 to 5.3 after treatment in 1% HF (for 1 min) and from 6.3 to 2.9 after treatment in 40% HF (for 1 min). Therefore, the acid treatment reduces the contamination of cp-Ti surface with hydrocarbons, and increases the surface energy and potential bio-compatibility of the Tiimplant.
The concentration profiles of carbon, oxygen and titanium on the surface of untreated and chemically treated cp-Ti (Figure 3) show, that the acid etched surface is much cleaner than the untreated surface. The carbon contamination for the acid etched surface is much lower and the oxygen concentration is much higher than for the untreated sample. The curves of the acid etched surface are much flatter than those of the untreated surface, what means that the oxide in the near-surface region is much more homogeneous than in the untreated sample. The surface contamination of the acid treated sample can practically be removed within very few sputter cycles, whereas for the untreated sample the contamination reaches deeper into the bulk.
According to XPS measurements of high-energy resolved Ti 2p3/2, 1/2 spectra (Figure 4), the Ti 2p3/2 and Ti 2p1/2 peaks are located at 459.2 eV and 464.9 eV both before and after acid treatment and can be attributed to Ti4+ [5]. No Ti3+ shoulder at lower binding energy on the Ti 2p3/2 peak is detected, suggesting that all samples have a stoichiometric TiO2 surface. As can be seen, XPS Ti 2p spectra consist of high-energy shake up satellites at 477.8 eV and 472.4 eV which are typical for TiO2 [6]. It is in

Figure 1. Microstructure of the initial coarse grained cp-Ti.
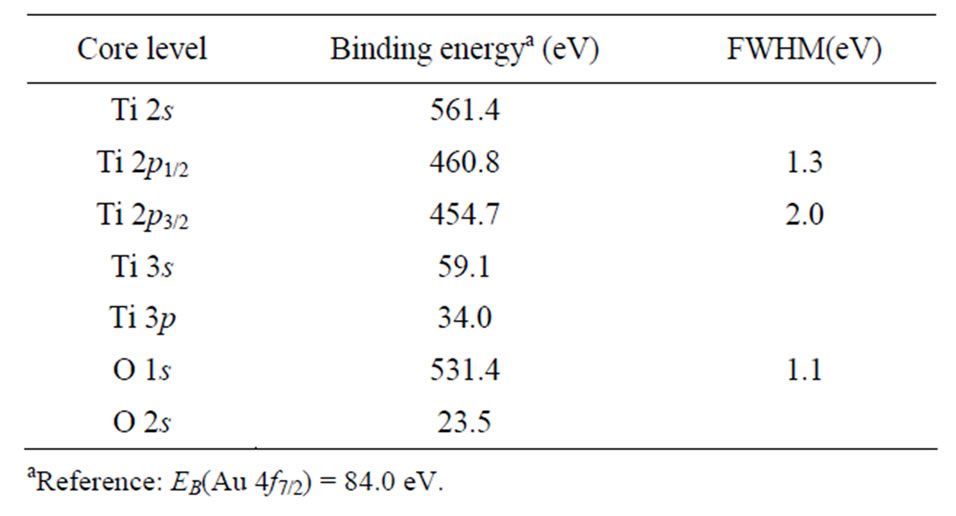
Table 1. XPS binding energies of untreated cp-Ti.
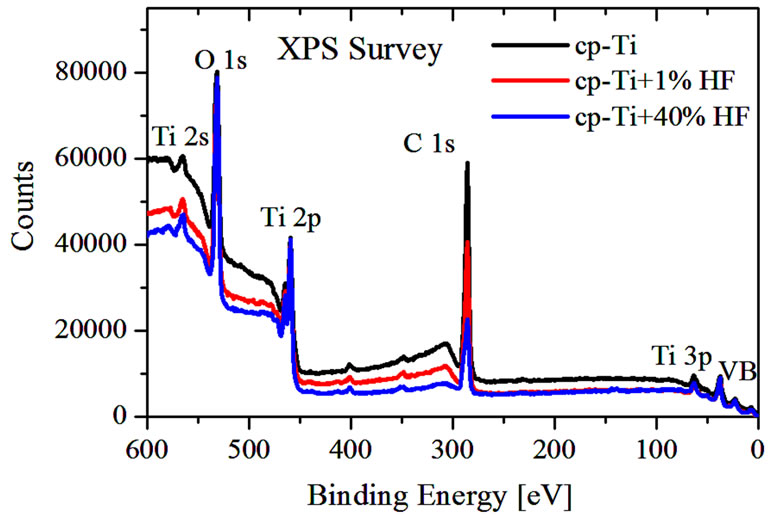
Figure 2. XPS wide scans of coarse-grained cp-Ti before and after chemical treatment in hydrofluoric acid (1% and 40% HF).
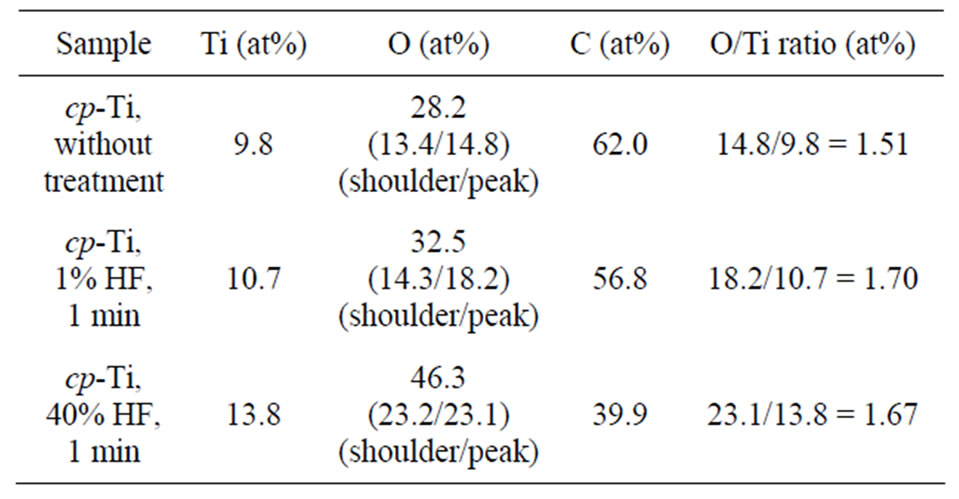
Table 2. Concentration of Ti, C and O estimated from highenergy resolved XPS spectra.
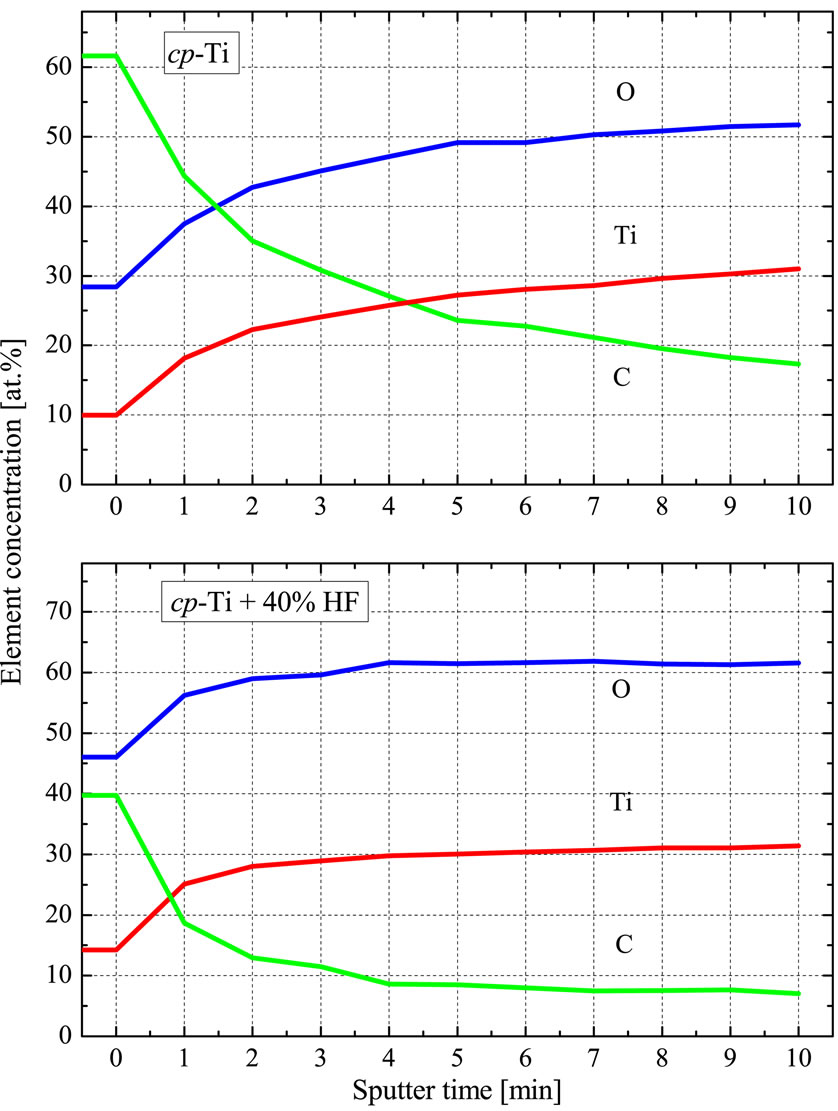
Figure 3. The concentration profiles of carbon, oxygen and titanium on the surface of untreated and chemically treated cp-Ti.
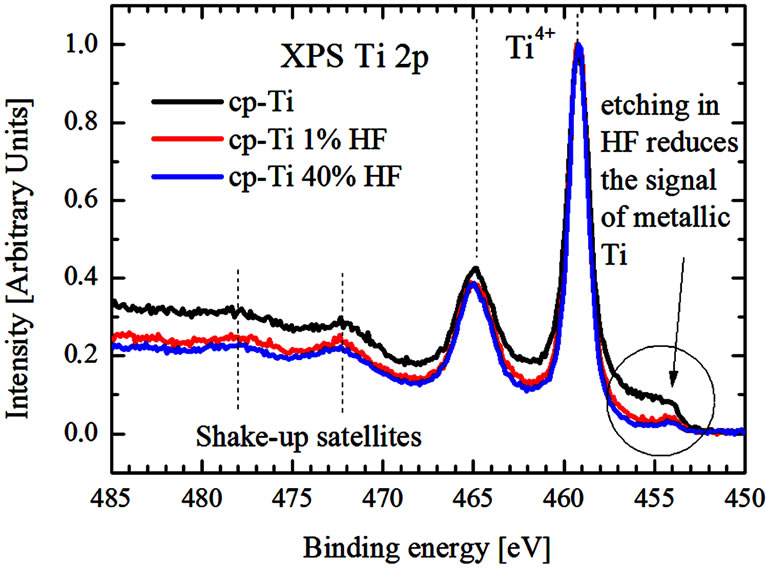
Figure 4. XPS Ti 2p-spectra of initial and acid treated cp-Ti.
agreement with the theoretical thermodynamics, which specify, that the free energy of formation of TiO2 is favored over other titanium oxides [7]. XPS Ti 2p spectra of untreated and chemically treated cp-Ti measured depending on time of Ar+-ion etching (1 min - 10 min) are found to be quite different (Figure 5). One can see, that the nearsurface region of untreated cp-Ti is composed by superposition of TiO2, Ti2O3, TiO and Ti-metal, whereas the contributions of Ti2O3, TiO and Ti-metal are strongly reduced for chemically treated sample. It means that the TiO2 concentration in the surface region of the acid treated sample is significantly higher than in the untreated sample.
Figures 6 and 7 display the high-resolution XPS C 1s
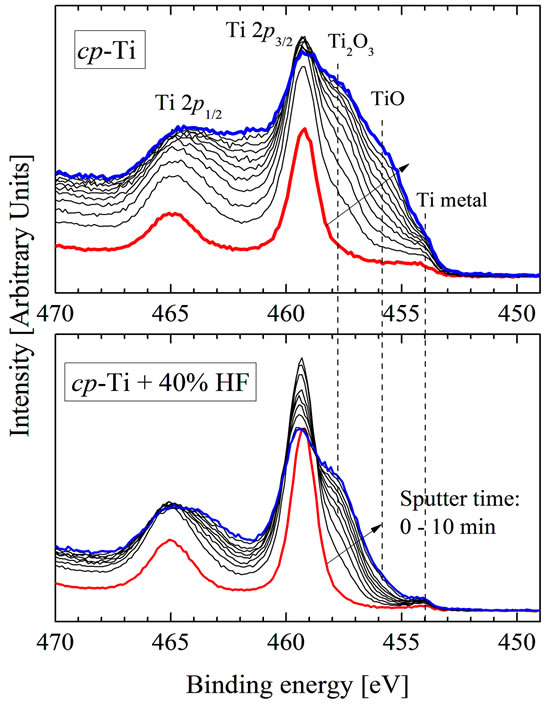
Figure 5. XPS Ti 2p-spectra of untreated (upper panel) and chemically treated (lower panel) cp-Ti in dependence of etching time.
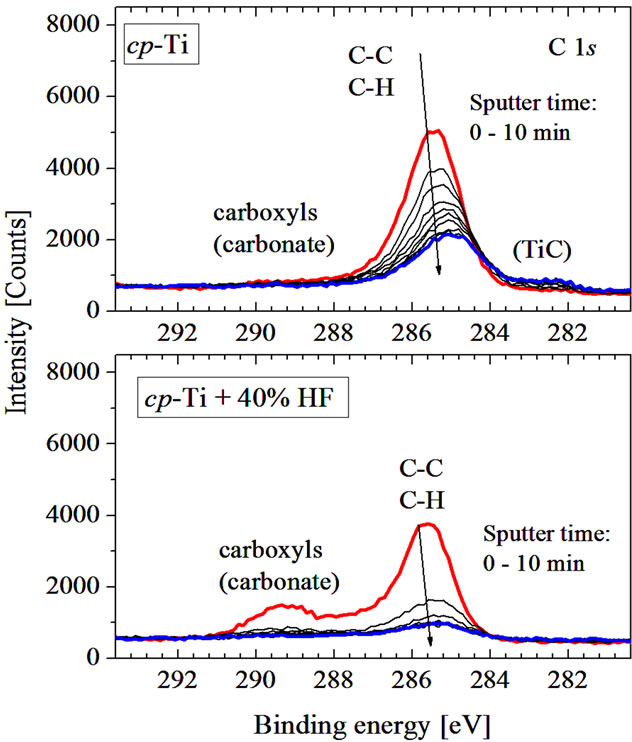
Figure 6. XPS C 1s spectra of initial (upper panel) and acid treated (lower panel) cp-Ti in dependence of time etching.
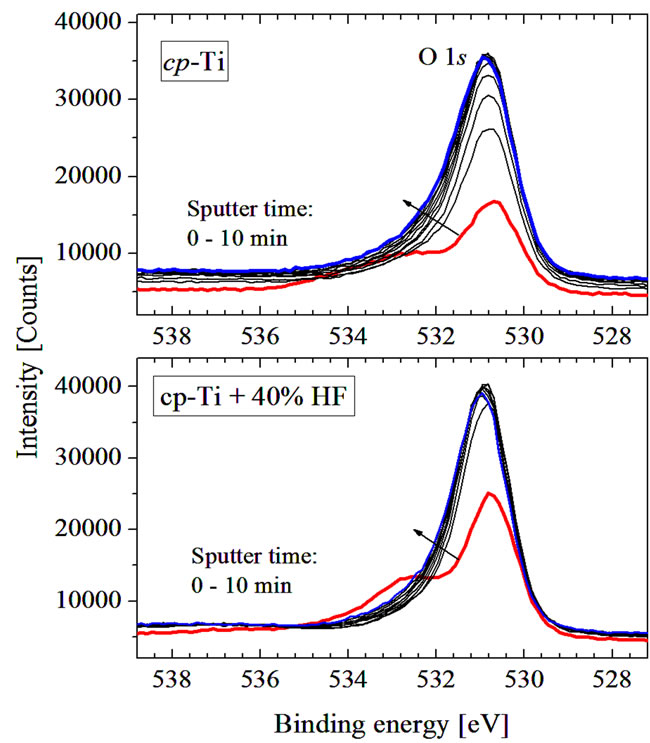
Figure 7. XPS O 1s spectra of untreated (upper panel) and chemically treated (lower panel) cp-Ti in dependence of time etching.
and O 1s core levels spectra of initial and chemically treated cp-Ti in dependence of Ar+-ion etching. One can see that the hydrocarbons (C-C, C-H) give the main contribution to the C 1s peak, while the less intensive high energy shoulder is formed by carboxyl/carbonate groups [8] (Figure 6). Chemical treatment strongly reduces carbon contamination especially in the near-surface region.
Both initial and treated cp-Ti samples show an intensive O 1s peak from Ti-O bonds at 530.6 eV and a shoulder at higher energy which can be attributed to the contributions of OH and H2O species [9], the content of which is not strongly changed after chemical treatment (Figure 7). One can see, that O 1s signal is not practically reduced with the ion etching giving additional evidence of formation of thick TiO2 layer on the surface of chemically treated cp-Ti. It has been suggested, that the hydroxyl groups contribute to bone growth because calcium ions bind with the acidic groups and phosphate molecules bind with the basic groups of hydroxyapatite [8].
XPS F 1s spectra of cp-Ti after chemical treatment in HF (not shown) are found to be very similar to those of Ti immersed in solution containing fluoride [10]. The formation of titanium fluoride (TiF4) on the surface of cpTi after acid treatment is not clearly detected.
In Figure 8 the XPS valence bands of initial and chemically treated cp-Ti are presented. They consist of four peaks located at 14.5 eV, 7.4 eV, 5.5 eV and 0.9 eV. In agreement with previous studies we assign the feature located at 7.4 eV to O 2p-Ti 3d hybridized states, while the 5.5 eV feature has mainly O 2p character [11]. One can see, that the ratio of the two peaks located at 7.4 and 5.5 eV is found to be very similar to that of TiO2 [5]. C 2s-states are fixed at E = 14.5 eV and are seen mainly in the spectrum of untreated cp-Ti where the contribution of carbon contamination is the highest (see Figures 1 and 5). The peak at 0.9 eV can be attributed to a contribution of 3d-states of metallic Ti [12]; the relative intensity of this peak is not changed after etching which is consistent with XPS Ti 2p-measurements (see Figure 3).
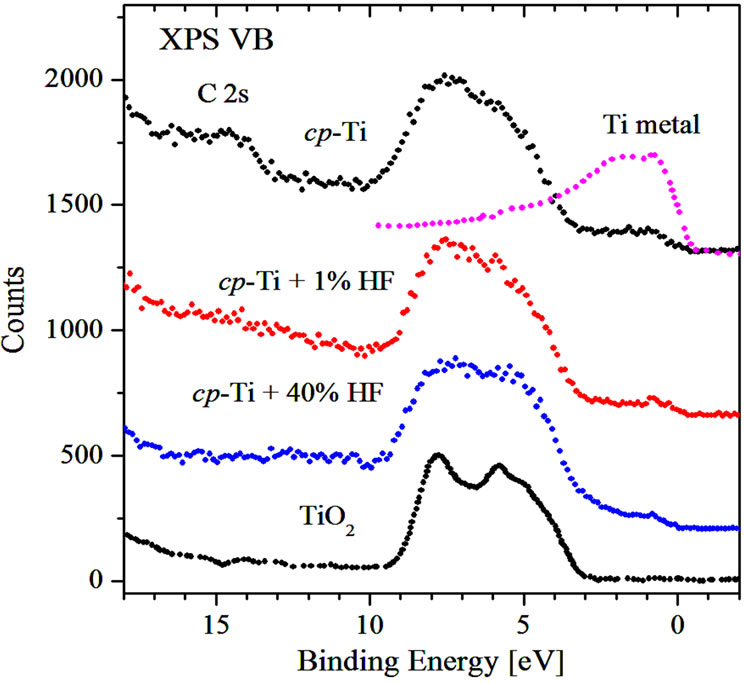
Figure 8. XPS valence bands of initial and acid treated cp-Ti.
4. Conclusion
To conclude, we have performed a full surface characterization of commercially pure Ti (cp-Ti) before and after chemical treatment in hydrofluoric acid with help of the XPS measurements of core levels and valence bands. It is found, that after acid treatment the content of hydrocarbons is reduced, what increases the surface energy and potential of bio-acceptability of a Ti-implant. According to our measurements, the acid treatment reduces the level of surface contaminations and induces the formation of thick TiO2 oxide layer.
5. Acknowledgements
We acknowledge the support of the Russian Science Foundation for Basic Research (Project 11-02-00022).
REFERENCES
- D. C. Smith, “Surface characterization of implant materials: biological implications,” In: J. E. Davies, Ed., The Bone-Biomaterial Interface, University of Toronto Press, Toronto, 1991, pp. 3-18.
- B. Kasemo and J. Lausmaa, “Metal selection and surface characteristics,” In: P.-I. Branemark, G. A. Zarb and T. Alberktsson, Eds., Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry, Quintessence, Chicago, 1985, pp. 99-116.
- J. E. Ellingsen, C. B. Johansson, A. Wennerberg and A. Holmen, “Improved retention and bone-to-implant contact with fluoride-modified titanium implants,” The International Journal of Oral & Maxillofacial Implants, Vol. 19, No. 5, 2004, pp. 659-666.
- J. E. Ellingsen, “Pre-treatment of titanium implants with fluoride improves their retention in bone,” Journal of Materials Science: Materials in Medicine, Vol. 6, No. 12, 1995, pp. 749-753. doi:10.1007/BF00134312
- S. Bartkowski, M. Neumann, E. Z. Kurmaev, V. V. Fedorenko, S. N. Shamin, V. M. Cherkashenko and S. N. Nemnonov, A. Winiarski and D. C. Rubie, “Electronic structure of titanium monoxide,” Physical Review B, Vol. 56, No. 16, 1997, pp. 10656-10667. doi:10.1103/PhysRevB.56.10656
- H. B. Jones, “Teeth and bones: application of surface science to dental materials and related biomaterials,” Surface Science Report, Vol. 42, No. 3-5, 2001, pp. 75- 205. doi:10.1016/S0167-5729(00)00011-X
- C. M. Chan, S. Trigwell and T. Ouerig, “Oxidalion of a NiTi Alloy,” Surface and Interface Analysis, Vol. 15, No. 6, 1990, pp. 349-354. doi:10.1002/sia.740150602
- Y.-J. Park, H.-J. Song, I. Kim and H.-S. Yang, “Surface characteristics and bioactivity of oxide film on titanium metal formed by thermal oxidation,” Journal of Materials Science: Materials in Medicine, Vol. 18, No. 4, 2007, pp. 565-575. doi:10.1007/s10856-007-2303-7
- J.-H. Yi, C. Bernard, F. Variola, S. F. Zalzal, J. D. Wuest, F. Rosei and A. Nanci, “Characterization of a Bioactive Nanotextured Surface Created by Controlled Chemical Oxidation of Titanium,” Surface Science, Vol. 600, No. 19, 2006, pp. 4613-4621. doi:10.1016/j.susc.2006.07.053
- S. Takemoto, M. Hattori, M. Yoshinari, E. Kawada and Y. Oda, “Suppression of fluoride-induced corrosion of titanium by albumin in oral modified environment,” Journal of Biomedical Materials Research Part B: Applied Biomaterials, Vol. 87, No. 2, 2008, pp. 475-481. doi:10.1002/jbm.b.31129
- U. Diebold, “The surface science of titanium dioxide,” Surface Science Reports, Vol. 48, No. 5-8, 2003, pp. 53-229. doi:10.1016/S0167-5729(02)00100-0
- H. Höchst, P. Steiner, G. Reiter and S. Hüfner, “XPS Valence Bands of Ti, Zr, Nb, Mo and Hf,” Zeitschrift für Physik B Condensed Matter, Vol. 42, No. 3, 1981, pp. 199-204. doi:10.1007/BF01422023

