Case Reports in Clinical Medicine
Vol.3 No.1(2014), Article ID:42237,5 pages DOI:10.4236/crcm.2014.31011
Melanoma involving the ileum: Report of a case and review of literature
![]()
1Pathology Department, Hospital Universitario Fundación Santa Fe de Bogotá, Bogotá D.C., Colombia; *Corresponding Author: patr.cas@gmail.com
2Clinical Laboratory, Clínica Colsanitas, Bogotá D.C., Colombia
3Faculty of Medicine, Universidad de Los Andes, Bogotá D.C., Colombia
Received 13 November 2013; revised 10 December 2013; accepted 31 December 2013
ABSTRACT
Malignant melanoma accounts for less than 3% of all gastrointestinal tract malignancies, and it is mainly metastatic in origin. In contrast, primary melanoma of the small bowel is an extremely rare entity. We report the case of a 34- year-old male who was diagnosed with melanoma involving the ileum most likely secondary in origin. Histologically, the neoplastic cells involved the entire wall thickness, showed nuclear pleomorphism, nuclear pseudoinclusions, abundant eosinophilic cytoplasm, and were immunoreactive for S100, HMB45, and Melan-A. Electron microscopy showed electron-dense complexes that suggested aggregates of melanin. The final diagnosis was given based on the clinical, radiologic and histopathologic correlation. This case demonstrates the need of clinical and histological immunohistochemistry, electron microscopy and molecular studies together to make a correct diagnosis of spindle cell and epithelioid tumors of the gastrointestinal tract.
Keywords:Melanoma; Ileum; Spindle Cell Tumor; Gastrointestinal Tract
1. INTRODUCTION
Similar morphology can be observed among spindle cell tumors of the gastrointestinal tract; therefore, a definitive diagnosis depends on multiple immunohistochemistry markers [1], molecular biology studies and electron microscopy evaluation. Metastatic malignant melanoma should be ruled out as it is one of the differential diagnoses in spindle cell neoplasms. It represents 1% to 3% of all malignant tumors of the gastrointestinal tract [2].
Herein, we present the case of a patient with a spindle cell tumor of the ileum, in which the histological immunohistochemistry and electron microscopy findings are compatible with melanoma involving the small intestine.
2. CASE REPORT
2.1. Medical History
This was the case of a 34 year-old man complaining of abdominal pain, abdominal discomfort, nausea, and unintentional weight loss. The computed tomography (CT) of the abdomen showed an intestinal mass and two hypodense lesions in the liver. The chest CT showed a hypoechoic lesion localized in the superior lobule of the left lung. An exploratory laparotomy was performed and two masses in the small intestine were found. As a consequence, a partial resection of the ileum was done.
2.2. Macroscopic Findings
Gross examination of the resected small bowel specimen showed two noncontiguous masses, measuring 7.5 cm and 14 cm in their greatest dimension. Both masses derived from the ileum’s wall and protruded into the mesenteric adipose tissue. They had well-defined borders and were ulcerated. Cut section through the greater mass revealed a friable unilocular cystic lesion, filled with hemorrhagic and necrotic material (Figure 1) and a clear

Figure 1. The inset shows the ileum segment with two masses. The main picture depicts the largest mass with a cystic appearance and its relation with the mucosa. The mass protrudes towards the mesenteric fat with a thin, well circumscribed wall.
relationship with the mucosa was not obvious, however the mucosa underlying the larger mass appeared ulcerated. There were no obvious solid components and the wall appeared thin and well circumscribed. Multiple nodules in the adjacent adipose tissue were found and ranged from 0.5 to 1.5 cm in their greatest dimensions.
2.3. Microscopic Findings
Light microscopy showed a neoplastic cell proliferation made of spindle cells that infiltrated the entire wall thickness and was in continuity with the ulcerated mucosa. There was no evidence of an in situ lesion (Figure 2). The cells showed marked nuclear pleomorphism, with pseudoinclusions and abundant eosinophilic cytoplasm without pigment. The mitotic rate was 29 mitosis per 50 high-power fields (40×) with extensive areas of necrosis and some atypical mitosis (Figure 3).
Immunohistochemical studies showed strong positivity of the neoplastic cells for S100 and HMB45 with focal and weak staining for Melan-A (Figure 4). On the other hand, these cells were negative for CD117, desmin, CD34, SMA, and caldesmon. The proliferation index measured with Ki67 was 70% in the most proliferative areas.
Electron microscopy showed large tumor cells with pleomorphic nuclei, some with prominent nucleolus, presence of numerous mitochondria with abundant reticuli, and some electron-dense complexes that suggested aggregates of melanin (Figure 5).
Histological findings and the electron microscopy suggested a final differential diagnosis between a mela-

Figure 2. H & E Stain (4×). The neoplastic cells infiltrate the entire wall thickness and were in continuity with ulcerated mucosa.

Figure 3. H & E Stain (40×). Neoplastic cells were spindle cell shaped with marked nuclear pleomorphism, nuclear pseudo inclusions and abundant eosinophilic cytoplasm without pigment and a high mitotic rate.
noma involving the ileum and intestinal clear cell sarcoma. An attempt to look for translocation t(2; 22) (q32.3; q12) EWS-CREB1 fusion gene through real-time PCR from paraffin embedded tissue was done but DNA quality sample was poor. However, given the clinical presentation of abdominal, hepatic and lung masses along with the reactivity of the melanocyte specific markers (S100, HMB-45 and Melan-A) and the presence of electrondense granules, we favored the diagnosis of melanoma most likely secondary.
We were not able to get additional history or follow up; hence, we could not know if the patient had a primary cutaneous melanoma or a regressed one. A disclaimer

Figure 4. Immunohistochemical studies showed strong positivity of the neoplastic cells for S100 and HMB45 (40×).
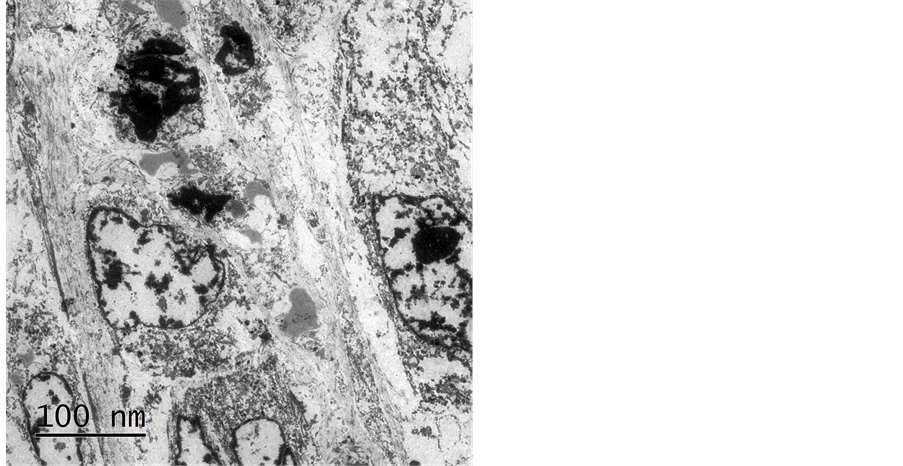
Figure 5. Electron microscopy showed some electron-dense complexes that suggested aggregates of melanin.
was done that in the absence of a known primary melanoma, this tumor could represent a primary intestinal melanoma.
3. DISCUSSION
Malignant melanoma accounts for 1% to 3% of all gastrointestinal malignancies [3]. Most gastrointestinal tract melanomas are metastatic, being their most common primary site the cutaneous melanoma [4]. A great discussion regarding the origin of these tumors has developed, since several cases of primary melanomas of the gastrointestinal system have been described, particularly in the ileum and anus [2,4]. Some suggest that these neoplasms arise from the Schwann cells of the gastrointestinal tract or from the melanoblastic cells of the neural crest, which migrate to the distal ileum through the omphalomesenteric canal [2,5].
Up to 60% of patients that die from melanoma are found to have metastases at autopsy [3]; however, the diagnosis of the metastases is made in less than 4% of living patients, since there are no specific symptoms early in the disease or they are asymptomatic [6,7]. Although it is important to distinguish primary from metastatic melanoma, establishing the exact origin of these tumors may be difficult, with a reported incidence of gastrointestinal metastases from unknown primary to be less that 9% [7]. Some researchers consider all gastrointestinal tract melanomas to be metastatic in origin, based on the fact that some cutaneous melanomas suffer spontaneous regression [2,8]; nevertheless, the presence of a precursor lesion or melanosis further suggest the possibility that there might be primary gastrointestinal tract melanomas [9].
Any subtype of cutaneous melanoma can metastasize to the gastrointestinal system, even the superficial spreading melanoma. As a matter of fact, this subtype is the one most likely to disseminate to the small intestine [2] and it represents 60% to 80% of all the reported cases [2,4]. Regardless that there are no clear pathogenic mechanisms that can explain metastases to the gastrointestinal tract, it is believed that its rich blood supply [3], plus the abnormal overexpression of the chemokine receptor CCR9 in the metastases of the small intestine may be fundamental in this process [10].
Clinical presentation of metastatic lesions is similar to that of any other gastrointestinal tumor. Symptoms are nonspecific and they include weight loss, fatigue, abdominal pain, gastrointestinal bleeding, nausea, vomiting, mass, intestinal obstruction, and intussuception [6].
Macroscopically, metastatic lesions may be pigmented or amelanotic and they present four different morphologic patterns: polypoid, cystic, infiltrating and exoenteric [2]. The polypoid pattern is the one most commonly found in small bowel metastasis [11], in contrast to our case where the tumor was cystic.
Histologically, the tumors may adopt a variety of patterns: spindle cell, epithelioid, and pleomorphic. Among the differential diagnosis (Table 1) one must consider the
Table 1 . Differential diagnosis of spindle cell and epithelioid tumors of the gastrointestinal tract.
*PDGFR-α: Platelet Derived Growth Factor Receptor-α.
gastrointestinal stromal tumors (GIST) [12], schwannomas, smooth muscle tumors, fibrous solitary tumor, and the soft tissue clear cell sarcoma [13].
Atypia and pleomorphism are unusual morphologic findings of GIST, and immunohistochemical studies are required to distinguish it from melanoma. It should be kept in mind that some melanomas may express CD117, in fact there are reports with up to 63% of cases showing CD117 positivity [14]. Something similar is seen with the epithelioid GIST, which may be positive for Melan-A in up to 33% of cases [14]. S100 is positive in up to 5.3% of spindle cell pattern GISTs; conversely, epithelioid pattern GISTs does not show reactivity for this marker. The only melanocyte marker that does not show immunoreactivity in any GIST subtype is HMB45. Melanomas are always negative for smooth muscle cell markers which can differentiate easily from the smooth muscle neoplasms [15] (Table 1).
Inflammatory polyps are among the benign spindle cell lesions that one could find in the ileum. These lesions usually have admixed inflammatory cells, mainly eosinophils, they are submucosal and have no atypia or mitosis, immunohistochemistry shows diffuse positivity for CD34 and variable staining for muscle markers. Schwannomas are usually found in the stomach and rarely in the ileum. Areas of palisading nuclei, lymphoid aggregates at the periphery and some degree of atypia can be seen. Typically no mitoses are noted and these lesions are usually S100 positive, but negative for other melanoma markers.
The most difficult differential diagnosis of melanoma of the ileum is with clear cell sarcoma (CCS). Clear cell sarcoma was first described as a soft tissue tumor by Enzinger in 1965, and is also known as malignant melanoma of soft tissue [16]. It is commonly found in the fascia, aponeurosis, and tendons of lower limbs of adolescents and young adults [16,17]. Morphologically and immunohistochemical studies are similar to melanoma; however they do not show the BRAF mutation and are known to present a recurrent translocation t(12; 22) (q13; q12), which fuses the EWS gene with the ATF1 gene. Recently a gastrointestinal CSS counterpart has been described. This entity has epithelioid cells; however some spindle cells and giant cells can be noted. The immunohistochemistry findings show a strong positivity for S100 and negativity for other melanoma markers (Melan-A and HMB45) differing from their soft tissue counterpart. These tumors can show a variant chromosomal translocation between EWS (22q12) and CREB1 (2q32.3), presumably a t(2; 22) (q32.3; q12) that can be find by real-time PCR characteristic to the gastrointestinal location and different from the soft tissue counterpart.
4. CONCLUSION
We report the case of a cystic and mostly necrotic mass with histological findings and immunohistochemical studies compatible with the melanoma involving the small bowel. Due to the lack of clinical history and an in situ component, it was not possible to determine if this was a primary or a metastatic lesion. Electron microscopy studies support the diagnosis of a melanoma. However, no molecular confirmation to rule out CSS of gastrointestinal origin was possible.
REFERENCES
- Turner, M.S. and Goldsmith, J.D. (2009) Best practices in diagnostic immunohistochemistry: Spindle cell neoplasms of the gastrointestinal tract. Archives of Pathology & Laboratory Medicine, 133, 1370-1374.
- Lens, M., Bataille, V. and Krivokapic, Z. (2009) Melanoma of the small intestine. The Lancet Oncology, 10, 516-521. http://dx.doi.org/10.1016/S1470-2045(09)70036-1
- Blecker, D., Abraham, S., Furth, E.E. and Kochman, M.L. (1999) Melanoma in the gastrointestinal tract. The American Journal of Gastroenterology, 94, 3427-3433. http://dx.doi.org/10.1111/j.1572-0241.1999.01604.x
- Schuchter, L.M., Green, R. and Fraker, D. (2000) Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Current Opinion in Oncology, 12, 181-185. http://dx.doi.org/10.1097/00001622-200003000-00014
- Panagiotou, I., Brountzos, E.N., Bafaloukos, D., Stoupis, C., Brestas, P. and Kelekis, D.A. (2002) Malignant melanoma metastatic to the gastrointestinal tract. Melanoma Research, 2, 169-173. http://dx.doi.org/10.1097/00008390-200204000-00010
- Korkolis, D.P., Apostolaki, K., Gontikakis, E., Plataniotis, G.D., Siskos, D., Xinopoulos, D., et al. (2008) Primary malignant melanoma of the duodenum: Aggressive management and long-term survival of an unusual oncologic entity. Southern Medical Journal, 101, 836-839. http://dx.doi.org/10.1097/SMJ.0b013e31817dfd75
- Manouras, A., Genetzakis, M., Lagoudianakis, E., Markogiannakis, H., Papadima, A., Kafiri, G., et al. (2007) Malignant gastrointestinal melanomas of unknown origin: Should it be considered primary? World Journal of Gastroenterology, 13, 4027-4029.
- McGovern, V.J. (1975) Spontaneous regression of melanoma. Pathology, 7, 91-99. http://dx.doi.org/10.3109/00313027509092702
- Mishima, Y. (1967) Melanocytic and nevocytic malignant melanomas. Cellular and subcellular differentiation. Cancer, 20, 632-649. http://dx.doi.org/10.1002/1097-0142(1967)20:5<632::AID-CNCR2820200510>3.0.CO;2-7
- Letsch, A., Keilholz, U., Schadendorf, D., Assfalg, G., Asemissen, A.M., Thiel, E., et al. (2004) Functional CCR9 expression is associated with small intestinal metastasis. Journal of Investigative Dermatology, 122, 685- 690. http://dx.doi.org/10.1111/j.0022-202X.2004.22315.x
- Bender, G.N., Maglinte, D.D., McLarney, J.H., Rex, D. and Kelvin, F.M. (2001) Malignant melanoma: Patterns of metastasis to the small bowel, reliability of imaging studies, and clinical relevance. The American Journal of Gastroenterology, 96, 2392-2400. http://dx.doi.org/10.1111/j.1572-0241.2001.04041.x http://dx.doi.org/10.1016/S0002-9270(01)02604-1
- Gabali, A.M., Priebe, P. and Ganesan, S. (2008) Primary melanoma of small intestine masquerading as gastrointestinal stromal tumor: A case report and literature review. The American Journal of Surgery, 74, 318-321.
- Taminelli, L., Zaman, K., Gengler, C., Peloponissios, N., Bouzourene, H., Coindre, J.M., et al. (2005) Primary clear cell sarcoma of the ileum: An uncommon and misleading site. Virchows Arch 447, 772-777. http://dx.doi.org/10.1007/s00428-005-0019-y
- Venkataraman, G., Quinn, A.M., Williams, J. and Hammadeh, R. (2005) Clear cell sarcoma of the small bowel: A potential pitfall. Case report. APMIS 113, 716-719. http://dx.doi.org/10.1111/j.1600-0463.2005.apm_243.x
- Garcia, J.J., Kramer, M.J., Mackey, Z.B., O’Donnell, R.J. and Horvai, A.E. (2006) Utility of CD117 immunoreactivity in differentiating metastatic melanoma from clear cell sarcoma. Archives of Pathology & Laboratory Medicine, 130, 343-348.
- Segal, N.H., Pavlidis, P., Noble, W.S., Antonescu, C.R., Viale, A., Wesley, U.V., et al. (2003) Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. Journal of Clinical Oncology, 21, 1775-1781. http://dx.doi.org/10.1200/JCO.2003.10.108
- Comin, C.E., Novelli, L., Tornaboni, D. and Messerini, L. (2007) Clear cell sarcoma of the ileum: Report of a case and review of literature. Virchows Arch, 451, 839-845. http://dx.doi.org/10.1007/s00428-007-0454-z


