Food and Nutrition Sciences
Vol.4 No.2(2013), Article ID:27732,6 pages DOI:10.4236/fns.2013.42026
Rapid Analysis of Morusin from Ramulus mori by HPLC-DAD, Its Distribution in Vivo and Differentiation in the Cultivated Mulberry Species
![]()
Silk Biotechnology Key Laboratory of Suzhou City, Medical College of Soochow University, Suzhou, China.
Email: mabin047005213@126.com, *sericult@suda.edu.cn
Received December 25th, 2012; revised January 25th, 2013; accepted February 3rd, 2013
Keywords: Morusin; Alcohol Extract; HPLC-DAD; Mulberry; Branch Bark; Root Bark
ABSTRACT
The distribution and content differentiation of morusin in the cultivated species of mulberry by HPLC-DAD are described in this paper. The experimental results showed that morusin is present in all parts of the mulberry bush. The content of morusin was highest in root bark and second highest in branch bark. The difference in morusin content of 20 different species of cultivated mulberry branch bark was significant. The level of morusin was highest in the branch bark of cultivated mulberry No. 404, a tetraploid cultivar, and third in the Husang 32 cultivar of Morus multicaulis. The method used in this study for determining morusin content exhibited good repeatability (RSD 6.02%) and recovery (100.62%). Therefore, the results from this study provide reliable data, for research and development in the future, on the level and distribution of morusin in mulberry in general and the differences found between various cultivated mulberries in particular. Furthermore, the HPLC-DAD method to determine morusin content is fast and reliable and is applicable not only to mulberry bushes but also to other plants.
1. Introduction
Morus (Moraceae) is a small genus found in the temperate and subtropical regions of the northern hemisphere. The silk industry countries, such as Japan, China, North Korea, South Korea, Thailand and Brazil cultivate mulberry bushes on a large scale. In China, the mulberry tree has been widely cultivated and the different components of the bush have been used in traditional Chinese medicine for thousands of years. Recently, the mulberry leaves, which are an important food for silkworm, have also been found to have pharmacological benefits with regards to hyperglycemia and hyperlipidemia [1-3] and have an antioxidant action [4]. The fruit is also an antioxidant and tonic agent [5]. The root bark, called “Sang-Bai-Pi” in Chinese, is considered an important anti-inflammatory [6], antidiabetic [7], antitumor [8] and antinephritis [9] medicine.
Morusin is an isoprenylated flavone, isolated from the root bark of Morus alba L. and the structure of morusin has been identified (Figure 1) [10]. Based on a literature survey, morusin showed strong antimicrobial activity against some species of molds [11] and a weak scavenging activity against superoxide anion radicals [12]. It has been shown to inhibit superoxide anion formation from rat neutrophils stimulated with phorbol myristate acetate (PMA) in a concentration-dependent manner [13]. Morusin has significant inhibitory activity towards the differentiation of 3T3-L1 adipocytes and nitric oxide (NO) production in RAW264.7 cells [6] and shows strong cytotoxicity against HeLa, MCF-7, and Hep3B cell lines [14]. Anti-tumor molecular signaling by morusin in human colorectal cancer cells has also been reported [15]. It has been demonstrated to inhibit arachidonic acid-, PAF-, and collagen-induced platelet aggregation[16] and to have anti-HIV activity [17]. The morusin used in these reports comes from the root or root bark of mulberry.
In China mulberry branches create considerable environmental problems as an agricultural waste. Every year, 10 million tons of mulberry branches form a huge biological resource that has not been fully exploited and utilized. Recently, some reports have clarified that mulberry branch bark mainly contain polyphenolic constituents, including prenylated flavonoids [18], Diels-Alder type adducts [19] and stilbenes [20], with various biological activities such as antimicrobial activity [12] and
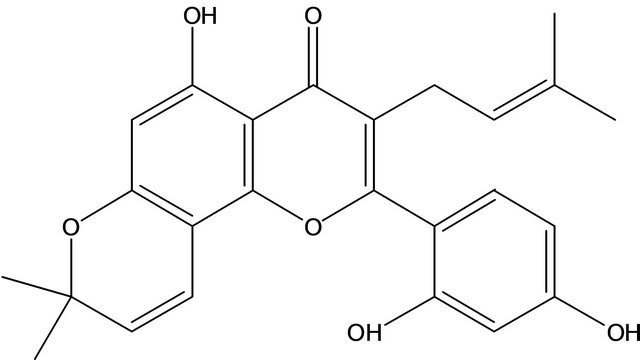
Figure 1. The structure of morusin.
antioxidant activity [21]. So, more rational uses of mulberry branches or its bark as a source of crude drugs to provide a means for recycling this waste have attracted much attention.
Despite a large number of studies on the structure and pharmacological activity of morusin, the morusin content distribution and varietal differences between mulberry stem/branch (Ramulus mori) and bark have rarely been examined. The aim of this study was to evaluate the morusin content of mulberry branch bark and establish a method for determining the morusin content of mulberry branch bark. The contents of morusin in the samples were assayed by reversed-phase high performance liquid chromatography (RP-HPLC) with DAD detection.
2. Materials and Methods
2.1. Chemicals and Materials
The solvents used for the extraction of morusin samples were analytical grade anhydrous alcohol and Milli-Q (Millipore Australia Pty. Ltd., North Ryde, New South Wales, Australia) distilled water, while those used for the HPLC analysis were HPLC grade acetonitrile and MilliQ distilled water. The morusin standard was purchased from VWR (Shanghai standardization for the traditional research center. Product Number 30-2010).
2.2. Equipment
The Shimadzu LC-20A HPLC system consists of a computer-controlled system with upgraded LC-20A software and an SCL-10A VP System controller. Other accessories were two LC-20AT Shimadzu Liquid Chromatography Pumps, a CTO-20A Column Oven, a RF-20A high sensitivity fluorescence detector and an SPD M20A Diode Array Detector.
2.3. HPLC Chromatographic Conditions
A Varian C18 column (Pursuit XRs 5m, C-18, 150 ´ 4.6 mm, 5 μm) fitted with diode array detection from 200 to 400 nm for morusin with mobile phase consisting of Milli-Q distilled water and 70% acetonitrile [22] was used.
2.4. Preparation of the Standard Sample Solution
0.20 mg of a standard morusin sample was dissolved in 8 ml of anhydrous alcohol of which 25 μg·ml−1 of standard sample solution was used as the standard solution in HPLC analysis. The same standard solution was diluted 5-fold with ethanol to create the 5.0 μg·ml−1 standard solution used for the UV spectroscopy analysis. The reference solution was ethanol. The morusin was measured in the 200 - 400 nm UV absorption spectra range. 25 μg·ml−1 of standard sample solution was diluted 50-fold by ethanol in order to prepare 0.5 μg·ml−1 solution for fluorescent spectroscopy.
2.5. Sample Preparation
Twenty varieties of mulberry were sampled on August 15, 2010 from Soochow University. The 1 year old leaves, branches and roots of Husang 32 were washed with water, their bark and xylem placed in a vacuum freeze-drying machine for 4d, following which they were ground into a fine powder. The samples were then stored in polyethylene bags in the freezer at −20˚C.
2.6. Preparation of the Sample Solution
Each sample (14.7549 g) was extracted with anhydrous alcohol for 15 min, filtered through nylon mesh, and the residue ground with anhydrous alcohol twice. This process was repeated 3 times. The filtrate was then centrifuged at 3000 rpm for 20 min and evaporated to dryness under vacuum. The sample was made up to a volume of 30 ml with anhydrous alcohol. Finally, the samples were stored in sealed glass vials in a refrigerator at 4˚C prior to analysis.
2.7. HPLC Analysis
The sample solution was analyzed by HPLC, using a Shimadzu LC-20A HPLC-DAD with a C18 reversed phase column and a Metaguard column (4.6 mm Metasil AQ 5U C18 120A). The sample solution was filtered through a 0.45 μm nylon membrane filter (Millipore) and 20 μl was injected into the liquid chromatograph. The flow rate was set at 1.0 ml·min−1. The temperature of the column oven was set at room temperature. The mobile phase consisted of solvent B (acetonitrile) and solvent A (Milli-Q distilled water) at 70/30 (v/v). The spectra were recorded in the 200 to 400 nm range and the absorbance of the effluent was monitored at 269 nm.
2.8. Identification of Morusin
Twenty microlitres of the sample extract solution or the standard sample solution were injected into the chromatograph using a water: acetonitrile mix (30:70) as the mobile phase, at room temperature. The chromatographic variables (flow rate, quantification parameter) and the analytical parameters (sensitivity, reproducibility, recovery, detection limit) were studied. Finally, 20 different species of cultivated mulberry branch bark and five different components of the mulberry were analyzed for their morusin content.
The identification of morusin in the branch bark of cultivated mulberry was carried out by comparing the HPLC analysis of the UV spectra and retention times of the biosamples with the morusin standard.
2.9. Quantification of the Morusin
Quantification of morusin was carried out using the external standard method. Standard morusin solutions, at various concentration levels, were injected into the HPLC system and the peak areas, and thus the calibration curves and response factors, were recorded under the same conditions as the samples. The concentration of a compound was calculated as the peak area of the compound × response factor.
3. Results and Analysis
3.1. The Morusin Standard Curve
Morusin was identified by its retention time and the UV absorbance of purified standards. Morusin standard solutions with concentrations of 2, 5, 10, 15, 20 and 25 μg·ml−1 were injected into the HPLC column (Figure 2), By plotting the peak-area (y) vs. concentration (x, μg·ml−1), the regression equations produced for morusin and its correlation coefficients (r) were as follows: y = 5477910 + 36086.7079x, R = 0.99538. Morusin recorded in that range and peak area showed a high correlation (x: morusin micrograms; y: peak area). Linearity ranged between 0.3 and 30 μg·ml−1.
3.2. Chromatographic and Spectral Characteristics of Morusin
As shown in Figure 3, the morusin ethanol standard sample was determined by HPLC analysis and diode array detection. Morusin on a C18 reversed-phase column showed good retention behavior and a peak at the retention time of 13.96 min (Figure 3). The HPLC chromatographic behavior of the alcohol extract of the branch bark of the cultivated mulberry Husang 32 is shown in Figure 3. The peak time at 13.78 min was very close to the retention time of the morusin standard sample.
The results also show that the peak UV absorption spectrum of the branch bark ethanol extract of Husang 32 was almost the same as the standard morusin sample with

Figure 2. Standard curve of morusin concentration and its absorption at 269 nm.
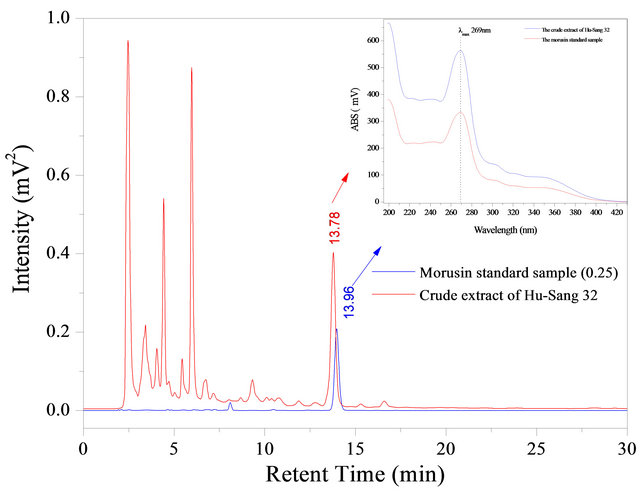
Figure 3. HPLC chromatogram of the standard sample morusin and alcohol extract of mulberry branch bark (Hu-Sang 32). Upper, the UV-Vis absorption spectra corresponding to the two peaks (morusin standard sample and the ethanol extract of the bark).
a maximum absorption peak at 269 nm. This demonstrates that the peak of the Husang 32 mulberry ethanol extract was morusin.
3.3. The Distribution of Morusin in the Mulberry
The bark and xylem of mulberry were extracted using anhydrous alcohol in order to analyze the distribution of morusin in the mulberry, leaves, roots and branches. The extracts were analyzed using HPLC and DAD and the results are shown in Figure 4. There was a significant difference in morusin content between the different components of the mulberry bush.
The morusin level in the root bark was up to 867.04 mg·g−1 whereas mulberry branch bark, mulberry branch xylem, and mulberry root xylem had a much lower content with the content in mulberry leaves being less than one-twentieth of the content in the root bark and content of morusin in mulberry branch bark being just one of third of the morusin content found in the root bark. In general, one hectare of mulberry bushes could produce more than 15 tonnes of dry mulberry branches per year in China. The dry weight of branches accounts for 30% of the total weight of a mulberry bush. The morusin content in the branch bark is 0.027% on a dry weight basis. Therefore, 4.05 kg of morusin could be obtained from one hectare of mulberry bushes.
3.4. The Difference in Morusin Content between Mulberry Varieties
This experiment tested the branch bark of 20 varieties of cultivated mulberry and found that morusin levels were highest in the root bark. The morusin content in different varieties of cultivated mulberries was investigated by longitudinal comparison. The results are shown in Table 1 and Figure 5.

Figure 4. The distribution of morusin in various parts of mulberry.
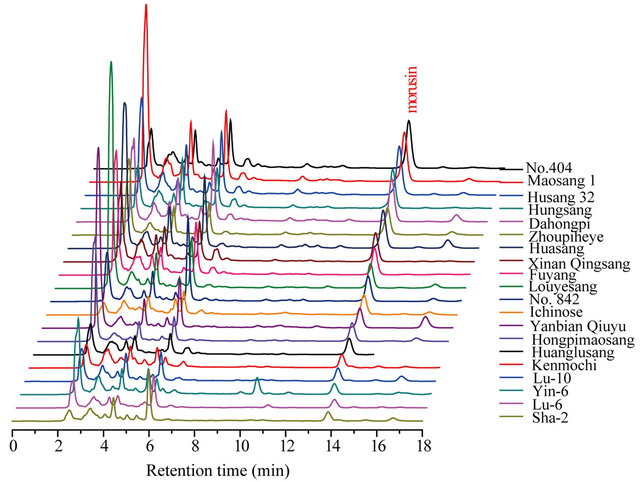
Figure 5. The content difference and HPLC pattern of morusin in 20 kinds of cultivated mulberry branch bark.
Table 1. The content of morusin in 20 kinds of different species of mulberry branch bark.

Table 1 and Figure 5 show that, there is a significant difference in the branch bark morusin content between the 20 varieties. On a dry weight basis, the branch bark of No. 404 (tetraploid cultivated mulberry) contained the highest concentration of morusin at up to 314.83 μg·g−1, with Husang 32 ranking third. The lowest morusin content was found in the Guangdong cultivar Sha-2 from M. atropurpurea Roxb at about 37.24 mg·g−1. The results therefore show that No. 404 and Husang 32 branch barks have the greatest potential for commercial morusin extraction.
3.5. Extraction Recovery of Morusin in Mulberry Branch Bark
In order to determine the accuracy of the results, an ethanol extraction of Ichinose, a cultivar from M. alba Linn, was used as a test sample solution. The sample solution divided into three parts and the morusin content in these samples was determined. The content of morusin was calculated by the linear equation produced from the standard samples. 250 μg of the morusin standard sample was added to the test sample solution before blending as part of a recovery study. The experimental results showed that the extraction recovery rate for morusin in mulberry branch bark was 100.62%, with a relative standard deviation of ±4.96% (Table 2). This is a high recovery bearing in mind the complexity of the analyses. The recovery test of morusin in mulberry branch bark exhibits very good precision.
3.6. The Reproducibility of the HPLC for the Determination of Morusin
The sample extraction solution of the branch bark of Xinan Qingsang, a cultivar from M. multicaulis Perr, was measured by HPLC-DAD five times and the content of morusin was found to be 73.13, 70.15, 66.41, 64.76, 63.19 μg·g−1, respectively, with an average of 67.53 μg·g−1, (±4.06 μg·g−1) and a RSD = 6.02%. This indicates that the HPLC determination of the morusin in mulberry branch bark shows good repeatability (Table 3).
4. Conclusion
This experiment compared the branch bark, branch xylem, leaf, root bark and root xylem of cultivated species of mulberry. Husang 32 and the branch bark from 20 other cultivated species of mulberry were used as experimental material. First, anhydrous alcohol was used to obtain an extract and then HPLC was used to determine the content of morusin from the alcohol extraction. The experiment uses a morusin standard sample to draw a standard linear equation in order to determine the content of morusin in the unknown samples. The experiment showed that the content of morusin in different plant components differs by up to 20 times. The content was lowest in the leaves whereas it was highest in the root bark at about 867.04 mg·g−1 (dry weight). The branch bark content was the second highest at around 270.08 mg·g−1, The experiment also shows that the morusin levels found in the branch bark differed between species. The content in the branch bark of cultivar No.404 was the highest (314.83 mg·g−1), the content of Husang 32 (270.08 mg·g−1,) was third while the Guangdong mul-
Table 2. The recovery trial of morusin in mulberry bark.
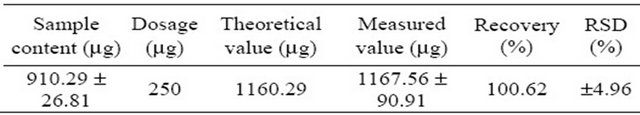
Table 3. Morusin reproducibility of the HPLC analysis.

berry Sha-2 content was the lowest (37.24 mg·g−1). The method described here to determine the content of morusin exhibited excellent repeatability (67.53 mg·g−1, ±4.06) and recovery rates (100.62%, ±4.96). So the morusin from the branch bark of No. 404 and Husang 32 can be commercially extracted and use made of its antitumor, antibacterial and anti-HIV properties which will further utilize and improve the efficient development of Mulberry bark. The present results not only provide reliable data about morusin in mulberry branch bark for research and development in the future, but also demonstrate a good method for the quality control of morusin production by HPLC-DAD.
5. Acknowledgements
The authors gratefully acknowledge the earmarked fund for China Agriculture Research System (CARS) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
REFERENCES
- B. Andallu and N. Ch. Varadacharyulu, “Antioxidant Role of Mulberry (Morus indica L. cv. Anantha) Leaves in Streptozotocin-Diabetic Rats,” Clinica Chimica Acta Vol. 338, No. 1-2, 2003, pp. 3-10. doi:10.1016/S0009-8981(03)00322-X
- B. Andallu, B. Radhika and V. Suryakantham, “Effect of Aswagandha, Ginger and Mulberry on Hyperglycemia and Hyperlipidemia,” Plant Foods for Human Nutrition, Vol. 58, No. 3, 2003, pp. 1-7. doi:10.1023/B:QUAL.0000040352.23559.04
- K. Murata, K. Yatsunami, E. Fukuda, et al., “Antihyperglycemic Effects of Propolis Mixed with Mulberry Leaf Extract on Patients with Type 2 Diabetes,” Alternative Therapies in Health and Medicine, Vol. 10, No. 3, 2004, pp. 78-79.
- C. Y. Lee, S. M. Sim and H. M. Cheng, “Systemic Absorption of Antioxidants from Mulberry (Morus alba L.) Leaf Extracts Using an in Situ Rat Intestinal Preparation,” Nutrition Research, Vol. 27, No. 8, 2007, pp. 492-497. doi:10.1016/j.nutres.2007.06.004
- Q. Du, J. Zheng and Y. Xu, “Composition of Anthocyanins in Mulberry and Their Antioxidant Activity,” Journal of Food Composition and Analysis, Vol. 21, No. 5, 2008, pp. 390-395. doi:10.1016/j.jfca.2008.02.007
- Z. G. Yang, K. Matsuzaki, S. Takamatsu and S. Kitanaka, “Inhibitory Effects of Constituents from Morus alba var. Multicaulis on Differentiation of 3T3-L1 Cells and Nitric Oxide Production in RAW264.7 Cells,” Molecules, Vol. 16, No. 7, 2011, pp. 6010-6022. doi:10.3390/molecules16076010
- A. N. Singab, H. A. El-Beshbishy, M. Yonekawa, T. Nomura and T. Fukai, “Hypoglycemic Effect of Egyptian Morus alba Root Bark Extract: Effect on Diabetes and Lipid Peroxidation of Streptozotocin-Induced Diabetic Rats,” Journal of Ethnopharmacology, Vol. 100, No. 3, 2005, pp. 333-338. doi:10.1016/j.jep.2005.03.013
- S. Yoshizawa, M. Suganuma, H. Fujiki, T. Fukai, T. Nomura and T. Sugimura, “Morusin, Isolated from Root Bark of Moms alba L., Inhibits Tumour Promotion of Teleocidin,” Phytotherapy Research, Vol. 3, No. 5, 1989, pp. 193-195. doi:10.1002/ptr.2650030508
- T. Fukai, K. Satoh, T. Nomura and H. Sakagami, “Antinephritis and Radical Scavenging Activity of Prenylflavonoids,” Fitoterapia, Vol. 74, No. 7-8, 2003, pp. 720- 724. doi:10.1016/j.fitote.2003.07.004
- Y. S. Chi, H. G. Jong, K. H. Son, H. W. Chang, S. S. Kang and H. P. Kim, “Effects of Naturally Occurring Prenylated Flavonoids on Enzymes Metabolizing Arachidonic Acid: Cyclooxygenases and Lipoxygenases,” Biochemical Pharmacology, Vol. 62, No. 9, 2001, pp. 1185-1191. doi:10.1016/S0006-2952(01)00773-0
- H. Y. Sohn, K. H. Son, C. S. Kwon, G. S. Kwon and S. S. Kang, “Antimicrobial and Cytotoxic Activity of 18 Prenylated flavonoids Isolated from Medicinal Plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai,” Phytomedicine, Vol. 11, No. 7-8, 2004, pp. 666-672. doi:10.1016/j.phymed.2003.09.005
- V. Kuete, D. C. Fozing, W. F. G. D. Kapche, et al., “Antimicrobial Activity of the Methanolic Extract and Compounds from Morus mesozygia Stem Bark,” Journal of Ethnopharmacology, Vol. 124, No. 3, 2009, pp. 551-555. doi:10.1016/j.jep.2009.05.004
- T. Fukai, K. Satoh, T. Nomura and H. Sakagami, “Antinephritis and Radical Scavenging Activity of Prenylflavonoids,” Fitoterapia, Vol. 74, No. 7-8, 2003, pp. 720- 724. doi:10.1016/j.fitote.2003.07.004
- N. T. Dat, P. T. X. Binh, L. T. P. Quynh, C. V. Minh, H. T. Huong and J. J. Lee, “Cytotoxic Prenylated Flavonoids from Morus alba,” Fitoterapia, Vol. 81, No. 8, 2010, pp. 1224-1227. doi:10.1016/j.fitote.2010.08.006
- J. C. Lee, S. J. Won, C. L. Chao, et al., “Morusin Induces Apoptosis and Suppresses NF-κB Activity in Human Colorectal Cancer HT-29 Cells,” Biochemical and Biophysical Research Communications, Vol. 372, No. 1, 2008, pp. 236-242. doi:10.1016/j.bbrc.2008.05.023
- H. H. Ko, S. M. Yu, F. N. Ko, C. M. Teng and C. N. Lin, “Bioactive Constituents of Morus australis and Broussonetia papyrifera,” Journal of Natural Products, Vol. 60, No. 10, 1997, pp. 1008-1011. doi:10.1021/np970186o
- S. D. Luo, J. Nemec and B. M. Ning, “Anti-HIV Flavonoids from Morus alba,” Acta Botanica Yunnanica, Vol. 17, No. 1, 1995, pp. 89-95.
- J. K. Cho, Y. B. Ryu, M. J. Curtis-Long, et al., “Inhibition and Structural Reliability of Prenylated Fiavones from the Stem Bark of Morus Lhou on β-Secretase (BACE-1),” Bioorganic & Medicinal Chemistry Letters, Vol. 21, No. 10, 2011, pp. 2945-2948. doi:10.1016/j.bmcl.2011.03.060
- G. Ni, Q. J. Zhang, Y. H. Wang, R. Y. Chen, Z. F. Zheng and D. Q. Yu, “Chemical Constituents of the Stem Bark of Morus cathayana,” Journal of Asian Natural Products Research, Vol. 12, No. 6, 2010, pp. 505-515. doi:10.1080/10286020.2010.489817
- S. C. Sun, R. Y. Chen and D. Q. Yu, “Structures of Two New Benzofuran Derivatives from the Bark of Mulberry Tree (Morus macroura Miq.),” Journal of Asian Natural Products Research, Vol. 3, No. 4, 2001, pp. 253-259. doi:10.1080/10286020108040364
- L. W. Chang, L. J. Juang, B. S. Wang, et al., “Antioxidant and Antityrosinase Activity of Mulberry (Morus alba L.) Twigs and Root Bark,” Food and Chemical Toxicology, Vol. 49, No. 4, 2011, pp. 785-790. doi:10.1016/j.fct.2010.11.045
- M. C. Pascual-Marti, A. Salvador, A. Chafer and A. Berna, “Supercritical Fluid Extraction of Resveratrol from Grape Skin of Vitis inifera and Determination by HPLC,” Talanta, Vol. 54, No. 4, 2001, pp. 735-740. doi:10.1016/S0039-9140(01)00319-8
NOTES
*Corresponding author.

