Food and Nutrition Sciences
Vol.4 No.1(2013), Article ID:26766,5 pages DOI:10.4236/fns.2013.41011
Sensory Evaluation of Pigmented Flesh Potatoes (Solanum tuberosum L.)
![]()
1School of Food Science, Washington State University, Pullman, USA; 2United States Department of Agriculture-Agricultural Research Service, Prosser, USA.
Email: boonchew@wsu.edu
Received November 29th, 2012; revised December 19th, 2012; accepted December 26th, 2012
Keywords: Pigmented Potatoes; Antioxidants; Sensory Evaluation; Sensory Preference; Anthocyanins; Carotenoids
ABSTRACT
Pigmented potato cultivars were ranked by a consumer panel for overall acceptance, and acceptance of aroma, appearance, and flavor. Potatoes were analyzed for total phenolics, anthocyanins and carotenoids. Concentrations of total phenolics in yellow and purple potato cultivars were 2-fold greater (P < 0.001) than in the white cultivar. Anthocyanins were low to non-detectable in white and yellow potatoes. Purple potatoes anthocyanin concentration was 20-fold greater (P < 0.001) than in yellow potatoes. Carotenoid concentrations in white and purple potatoes were similar, while yellow potatoes had a 45-fold greater carotenoids concentration compared to white and purple potatoes. Consumers ranked the aroma and appearance of white and yellow potatoes higher than purple (P < 0.05). However, no significant differences were observed in overall acceptance between the potato cultivars. These results suggest that consumers may be willing to consume pigmented potatoes, which are beneficial to health due to their higher antioxidant content.
1. Introduction
High intake of fruits and vegetables rich in antioxidants has been linked to a decreased risk in the development of chronic diseases [1]. Potatoes are the most commonly consumed vegetable in the US Based on consumption patterns and antioxidant capacity, potatoes are an important source of dietary antioxidants [2]. Potatoes have extensive genetic diversity, which has allowed for the cultivation of yellow-, orange-, red-, purple-, and blue-flesh cultivars [3]. These cultivars are rich sources of phenolic acids, anthocyanins and/or carotenoids [4,5].
Phenolic acids, anthocyanins and carotenoids are reported to have multiple beneficial properties, including anti-inflammatory [6-8], anti-carcinogenic [9-11] and cardio-protective [12-14] effects. Consumption of pigmented potatoes rich in these antioxidants may reduce the risk of chronic diseases.
The increased awareness of the health benefits of antioxidants found in fruits and vegetables has promoted the development of breeding programs designed to enhance flavor, diversify color and increase antioxidant concentrations in potatoes [15]. Concentrations of phenolic acids in purple cultivars may be 14 times higher than in yellow cultivars and 33 times higher than in white [5]. Yellow-flesh cultivars are rich in carotenoids, primarily lutein and zeaxanthin [16]. Yellow-flesh potatoes may contain 10 fold higher concentration of total carotenoids compared to white and 3 fold higher than purple-flesh potatoes [3,5]. Sensory studies suggest that color may affect perception of sensory attributes such as aroma, texture and flavor. Consumers often associate color with healthy and appealing food products [17]. β-Carotene has long been added to products such as cheese to enhance color; more recently lutein has been added for its health benefits related to macular degeneration [18]. Polyphenol antioxidants are presently being added to milk products [19] and soups [20] as a functional food ingredient.
We recently reported [21] that consumption of yellowor purple-flesh potatoes decreased inflammation and oxidative damage, and modulated immune response in humans. Diet is linked to the development of chronic diseases, while intake of antioxidants is inversely associated with diseases such as type II diabetes, atherosclerosis and cancer. Fruit and vegetable breeders are continuously attempting to maximize the antioxidant content of produce such as potatoes and carrots. However, consumer acceptance plays a critical role in the overall success of these foods. The purpose of this study was to investigate sensory preferences in potatoes with varying concentrations of phenolic acids, anthocyanins and carotenoids. By ranking the acceptance of the sensory properties of the yellow, purple and white potatoes, the performance of these new pigmented potato cultivars (yellow and purple) could be compared to a traditional cultivar (white). Results from this study will provide potato breeders and consumers invaluable information on consumer acceptability of pigmented potatoes, and possibly make available potatoes with high content of bioactive compounds. Therefore, pigmented potatoes can contribute significantly to total antioxidant intake, and potentially reduce certain chronic diseases. No studies are available on the sensory attributes of different pigmented potatoes. The purpose of this research is to determine the consumer acceptance of aroma, appearance and flavor of white-, yellow-, and purple-flesh potatoes through ranking. Results will provide potato breeders and consumers with important information on the sensory attribute preferences for pigmented potato cultivars.
2. Materials and Methods
2.1. Potato Cultivars
White- (Russet Burbank), yellow- (PORO3PG6-3), and purple-flesh (PORO4PG82-1) potato (Solanum tuberosum L.) cultivars grown in Toppenish, Washington were used in the study.
2.2. Antioxidant Composition
Whole potatoes (8 kg) were randomly selected, washed, abrasively peeled (1.5 min), cut into 6 mm thick slices, and steam blanched for 8 min at 133˚C. The potatoes were rapidly cooled in an ice-water bath for 8 min and mixed to a uniform consistency in a Hobart mixer (The Hobart Mfg. Co., Troy, OH). The potato slurries were immediately frozen at –35˚C until assay. Potato slurries were analyzed in triplicate for total antioxidant activity, phenolic acid, anthocyanin and carotenoid content.
Total phenolic acids were quantified as previously described [22] with modifications. Briefly, phenolic acids were extracted by homogenizing (OMNI International, Waterbury, CT) 2.45 g potato slurry in 10 mL of a mixture of distilled water and methanol (1:1, v/v) and centrifuging at 16,000 × g for 20 min at 4˚C. The supernatant was collected and 0.5 mL was diluted with 8 mL water. Next, 0.5 mL Folin-Ciocalteu reagent (0.25 N; SigmaAldrich, St. Louis, MO) was added and vortexed. The mixture was allowed to react for 3 min, then 1 mL sodium carbonate (1 N) was added and incubated at RT for 2 h. Absorbance was measured at 725 nm (Beckman DU 640 B, Seattle, WA) and total phenolic concentration expressed as gallic acid equivalent (GAE).
Total anthocyanin content was determined by the pH differential method as previously described, with modifications [23,24]. Briefly, 2.45 g potato slurry was homogenized in 20 mL of a 95% ethanol and 1.5 N HCl mixture (85:15, v/v), incubated for 90 min at 4˚C, and centrifuged at 14,000 × g for 15 min at 4˚C. Five mL of the supernatant was added to 45 mL of 0.025 M potassium chloride buffer (pH 1.0) or 0.4 M sodium acetate buffer (pH 4.5) and equilibrated for 15 min. Absorbance was read at 535 and 700 nm and total anthocyanin concentration expressed as malvidin-3-p-coumarylglycoside equivalents.
Carotenoids were extracted by adding 15 mL acetone to 3 g of potato slurry, vortexing 30 min on a multi-tube vortexer (Scientific Manufacturing Industry model 2601, Emeryville, CA), and centrifuging at 1000 × g for 15 min at 4˚C. This extraction was repeated twice. The acetone was pooled and dried under nitrogen gas at 40˚C. White and purple potato dried residues were dissolved in 1 mL acetone and yellow potatoes in 3 mL acetone. Absorbance was read at 444 nm. Total carotenoids were calculated using the extinction coefficient for lutein, and expressed as µg total carotenoid/g dry matter [25].
2.3. Sensory Evaluation
Freshly harvested potatoes were stored at 4˚C, 95% RH until required for evaluations. On the day of the sensory study, potatoes were allowed to equilibrate to room temperature for 24 h. Potatoes were wrapped in aluminum foil and baked at 204˚C in a conventional oven for 105 min as previously described [26]. After baking, potatoes were divided into 15 - 20 g pieces and were placed in 3-digit random coded glass containers covered with poly wrap and an elastic band in order to retain moisture and aroma. Before sensory evaluation, potato samples were heated for 30 seconds in a microwave oven to serving temperature (50˚C - 55˚C). Potato samples were served in random order.
For each attribute, potato cultivars were ranked by 60 untrained panelists (29 males and 31 females, ages 18 - 62). The Institutional Review Board of Washington State University approved all study procedures. Panelists were asked to rank cultivars in order of acceptance with 1 = most accepted and 3 = least accepted for aroma, appearance, flavor, and overall quality. Unsalted crackers and reagent grade water were provided for cleansing the palate between samples.
2.4. Statistical Analysis
Panelist ranking of potato cultivar aroma, appearance, flavor and overall quality were reported as rank sum using Compusense sensory software (version 4.6; Compusense Inc., Ontario, Canada). The most preferred samples were represented by the lowest value (sum of panelist responses). Statistical significance was analyzed by Friedman’s analysis of ranked sums and means separation was determined by Tukey’s HSD. Total phenolics, anthocyanins and carotenoids among cultivars were compared by ANOVA using the General Linear Models procedure of SAS (version 8; SAS Institute, Cary, NC). Differences in treatment means were compared using protected LSD. Statistical significance was established at P < 0.05.
3. Results and Discussion
The purpose of this study was to determine the acceptability of white-, yellowand purple-flesh potatoes by consumers, compare the sensory evaluations of the pigmented potatoes to the commonly consumed white potato, and relate antioxidant concentrations to consumer acceptability. To our knowledge, this is the first study on sensory preferences for different pigmented potato cultivars and relating the antioxidant profiles to the sensory preferences. The antioxidant concentrations of the potato cultivars are shown in Table 1.
Yellow and purple cultivars had the highest concentration of total phenolics (3.2 and 3.1 mg/g, respectively), and concentrations were 2-fold greater (P < 0.001) than in the white cultivar (1.5 mg/g). Anthocyanins were not detectable in the white cultivar, and low in the yellow cultivar. The purple potatoes had a 20-fold greater (P < 0.001) concentration of anthocyanins than the yellow. White and purple cultivars had the same concentration of carotenoids (1.3 μg/g), while yellow potatoes had a 45-fold greater (58.1 μg/g; P < 0.001) concentration of carotenoids compared to both white and purple potatoes.
Sensory rank sum values for sensory attributes of the different potato cultivars are shown in Table 2. Based on aroma and appearance, white and yellow potatoes were ranked as significantly more acceptable (P < 0.001) compared to purple potatoes. No significant differences were observed between the potatoes based on flavor acceptance or overall acceptance.
Based on appearance and aroma, the purple potato was ranked as the least accepted (P < 0.001) potato compared
Table 1. Antioxidant concentrations of white-, yellow-, and purple-flesh potatoes.
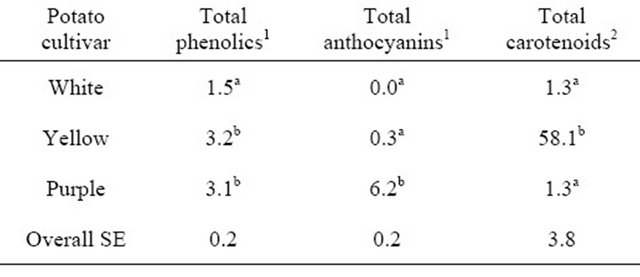
1g/kg; 2mg/kg; a,bDifferent letters denote significant difference (P < 0.001).
Table 2. Rank sum values for acceptance of aroma, appearance and flavor of baked white-, yellow-, and purple-flesh potatoes as evaluated by the consumer panel (n = 60)1.
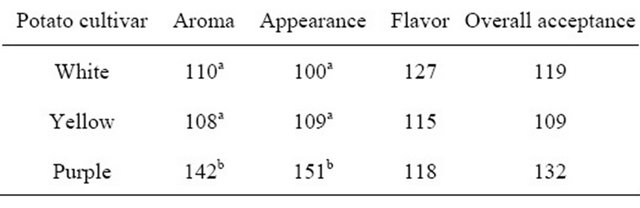
1The more accepted cultivar is represented by the lower value. a,bDifferent letters denote significant difference (P < 0.05).
to other potatoes. The purple-flesh potatoes had high (P < 0.001) concentrations of anthocyanins compared to white and yellow cultivars, which are responsible for purple skin and flesh color. The color of food plays a major role in perception of aroma, flavor and texture: color tends to give the perception of stronger odor intensity in foods when compared to non-colored counterparts [27]. The lower ranking of aroma acceptance in the purple potatoes may be due to color perception or to a high concentration of volatile compounds compared to white or yellow potatoes. Volatile concentrations and corresponding aroma values have been shown to significantly differ among white potato cultivars [28], and it is also possible that volatile concentrations differ among pigmented cultivars. However, the concentration of volatiles in the potatoes was not measured in this study. Although purple potatoes had the lowest ranking for appearance and aroma, panelists did not rank the purples potatoes as significantly lower in flavor compared to the white and yellow potatoes.
White potatoes were ranked as the most favorable in appearance. These results may be explained by the familiarity of consumers with white potatoes. Consumers would likely be more familiar with white potatoes compared to pigmented potatoes, thus the rankings of the white potato for aroma and appearance acceptance were more favorable. The pigmented potatoes (yellow and purple) were higher (P < 0.001) in total phenolics compared to the yellow potatoes. Yellow potatoes were higher (P < 0.001) in total carotenoids and purple potatoes were higher (P < 0.001) in total anthocyanins, compared to white potatoes. White Russet-like potatoes are the most commonly consumed potatoes in the US [2]. These data suggest that the aroma and the appearance of the pigmented potatoes may be a deterrent to their acceptability but if consumers actually consume the potatoes, flavor and overall acceptance are not significantly different from the white potato. This result was supported by panelist comments regarding purple potatoes that indicated that the dark purple coloring of potatoes was not considered desirable. This trend may be explained by the relatively recent introduction of colored potatoes into the US market [29].
Panelist ranked the appearance of yellow potatoes between white and purple potatoes, with white and yellow potatoes not being significantly different. Comments from panelists suggested that the yellow color potato flesh was positively associated with sweet potatoes, and therefore many panelists found yellow potatoes more acceptable. Sensory research involving carrots has demonstrated that consumers consistently preferred orange and white carrots over purple carrots, when evaluated for sensory attributes similar to those used in this study [30].
Compared to purple-flesh potatoes, yellow-flesh was ranked as more acceptable for aroma and appearance but not significantly different from white potatoes. Yellow potatoes have the highest amount of carotenoids (P < 0.001) compared to the other cultivars, and this may have influenced aroma and flavor. Carotenoids are known to produce aromas via enzymatic and non-enzymatic pathways; volatile compounds have been found in carotenoid-containing fruits, vegetables, and white wines [31]. Panelists may have perceived carotenoid-derived aromas from yellow potatoes as more acceptable, thereby ranking them higher than purple potatoes.
This study showed that in terms of antioxidant concentration, including total phenolics, anthocyanins and carotenoids, large differences were observed between white, yellow and purple potatoes. When these potatoes were ranked for the acceptance of sensory attributes by a consumer panel, results showed that purple potatoes were ranked as least accepted for appearance and aroma compared to yellow and white potatoes. Since color is known to have an effect on sensory perception of different foods, this may have played a role in the favorable flavor perception of yellow and purple potatoes. However, no significant differences were observed between the three potatoes for flavor and overall acceptance. These results indicated that pigmented potatoes are acceptable to consumers. Therefore, consumption of pigmented potatoes can potentially provide additional health benefits due to the higher content of phenolic acids, anthocyanins and carotenoids.
4. Acknowledgements
Funding for this project was received from the Washington State Potato Commission and the US Potato Board.
REFERENCES
- J. K. Willcox, J. K. Ash and G. L. Catignanani, “Antioxidants and the Prevention of Chronic Disease,” Critical Reviews in Food Science and Nutrition, Vol. 44, No. 4, 2004, pp. 275-295. doi:10.1080/10408690490468489
- O. K. Chun, D.-O. Kim, N. Smith, D. Schroeder, J. T. Han and C. Y. Lee, “Daily Consumption of Phenolics and Total Antioxidant Capacity from Fruit and Vegetables in the American Diet,” Journal of the Science of Food and Agriculture, Vol. 85, No. 10, 2005, pp. 1715-1724. doi:10.1002/jsfa.2176
- C. R. Brown, D. Culley, C.-P. Yang, R. Durst and R. Wrolstad, “Variation of Anthocyanin and Carotenoid Contents and Associated Antioxidant Values in Potato Breeding Lines,” Journal of the American Society for Horticultural Science, Vol. 130, No. 2, 2005, pp. 174- 180.
- C. R. Brown, “Antioxidants in Potato,” American Journal of Potato Research, Vol. 82, No. 2, 2005, pp. 163-172. doi:10.1007/BF02853654
- C. M. Andre, M. Oufir, C. Guignard, L. Hoffman, J. Hausman, D. Evers and Y. Larondelle, “Antioxidant Profiling of Native Andean Potato Tubers (Solanum tubersosum L.) Reveals Cultivars with High Levels of β-Carotene, α-Tocopherol, Chlorogenic Acid, and Petanin,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 26, 2007, pp. 10839-10849. doi:10.1021/jf0726583
- M. D. dos Santos, M. C. Almeida, N. P. Lopes and G. E. de Souza. “Evaluation of Anti-Inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid,” Biological & Pharmaceutical Bulletin, Vol. 29, 2006, No. 11, pp. 2236-2240.
- A. Rossi, I. Serraino, P. Dugo, R. Di Paola, L. Mondello, T. Genovese, D. Morabito, G. Dugo, L. Sautebin, A. P. Caputi and S. Cuzzocrea, “Protective Effects of Anthocyanins from Blackberry in a Rat Model of Acute Lung Inflammation,” Free Radical Research, Vol. 37, No. 8, 2003, pp. 891-900. doi:10.1080/1071576031000112690
- E. H. Lee, D. Faulhaber, K. M. Hanson, W. Ding, S. Peters, S. Kodali and R. D. Granstein, “Dietary Lutein Reduces Ultraviolet Radiation-Induced Inflammation and Immunosupression,” Journal of Investigative Dermatology, Vol. 122, No. 2, 2004, pp. 510-517. doi:10.1046/j.0022-202X.2004.22227.x
- W. Yi, J. Fischer, G. Krewer and C. C. Akoh, “Phenolic Compounds from Blueberries Can Inhibit Colon Cancer Cell Proliferation and Induce Apoptosis,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 18, 2005, pp. 7320-7329. doi:10.1021/jf051333o
- M. C. Lazze, M. Savio, R. Pizzala, O. Cazzalini, P. Perucca, A. I. Scovassi, L. A. Stivala and L. Bianchi, “Anthocyanins Induce Cell Cycle Perturbations and Apoptosis in Different Human Cell Lines,” Carcinogenesis, Vol. 25, No. 8, 2004, pp. 1427-1433. doi:10.1093/carcin/bgh138
- J. S. Park, B. P. Chew and T. S. Wong, “Dietary Lutein from Marigold Extract Inhibits Mammary Tumor Development in BALB/c Mice,” Journal of Nutrition, Vol. 128, No. 10, 1998, pp. 1650-1656.
- F. Natella, M. Nardini, F. Belelli and C. Scaccini, “Coffee Drinking Induces Incorporation of Phenolic Acids into LDL and Increases the Resistance of LDL to ex Vivo Oxidation in Humans,” American Journal of Clinical Nutrition, Vol. 86, No. 3, 2007, pp. 604-609.
- Y. Chang, K. Huang, A. Huang, Y. Ho and C. Wang, “Hibiscus Anthocyanin-Rich Extract Inhibited LDL Oxidation and oxLDL-Mediated Macrophage Apoptosis,” Food and Chemical Toxicology, Vol. 44, No. 7, 2006, pp. 1015-1023. doi:10.1016/j.fct.2005.12.006
- J. Milde, E. F. Elstner and J. Grassmann, “Synergistic Effect of Phenolics and Carotenoids on Human LowDensity Lipoprotein Oxidation,” Molecular Nutrition & Food Research, Vol. 51, No. 8, 2007, pp. 956-961. doi:10.1002/mnfr.200600271
- E. Peabody, “Veggies Reinvented: Breeding Vegetables with More Flavor and Nutrients,” Agricultural Research, Vol. 55, 2007, pp. 4-7.
- D. E. Breithaupt and A. Bamedi, “Carotenoids and Carotenoid Esters in Potatoes (Solanum tubersosum L.): New Insights into an Ancient Vegetable,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 24, 2002, pp. 7175-7181. doi:10.1021/jf0257953
- R. Baker and C. Gunther, “The Role of Carotenoids in Consumer Choice and the Likely Benefits from Their Inclusion into Products for Human Consumption,” Trends in Food Science and Technology, Vol. 15, 2004, pp. 484-488. doi:10.1016/j.tifs.2004.04.0094
- S. T. Jones, K. J. Aryana and J. N. Losso, “Storage Stability of Lutein During Ripening of Cheddar Cheese,” Journal of Dairy Science, Vol. 88, No. 5, 2005, pp. 1661-1670. doi:10.3168/jds.S0022-0302(05)72838-1
- L. G. Axten, M. W. Wohlers and T. Wegrzyn, “Using Phytochemicals to Enhance Health Benefits of Milk: Impact of Polyphenols on Flavor Profile,” Journal of Food Science, Vol. 73, No. 6, 2008, pp. H122-126. doi:10.1111/j.1750-3841.2008.00808.x
- R. Llorach, F. A. Tomas-Barberan and F. Ferreres, “Functionalisation of Commercial Chicken Soup with Enriched Polyphenol Extract from Vegetable By-Products,” European Food Research and Technology, Vol. 220, No. 1, 2005, pp. 31-36. doi:10.1007/s00217-004-1054-7
- K. L. Kaspar, J. S. Park, C. R. Brown, B. D. Mathison, R. Navarre and B. P. Chew, “Pigmented Potato Consumption Alters Oxidative Stress and Inflammatory Damage in Men,” Journal of Nutrition, Vol. 141, No. 1, 2011, pp. 108-111. doi:10.3945/jn.110.128074
- T. Swain and W. E. Hillis, “The Phenolic Constituents of Prunus domestica I.—The Quantitative Analysis of Phenolic Constituents,” Journal of the Science of Food and Agriculture, Vol. 10, No. 2, 1959, pp. 63-68. doi:10.1002/jsfa.2740100110
- T. Fuleki and F. J. Francis, “Quantitative Methods for Anthocyanins. 1. Extraction and Determination of Total Anthocyanin in Cranberries,” Journal of Food Science, Vol. 33, No. 1, 1968, pp. 72-77. doi:10.1111/j.1365-2621.1968.tb00887.x
- J. Lee, R. W. Durst and R. E. Wrolstad, “Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study,” Journal of AOAC International, Vol. 88, No. 5, 2005, pp. 1269-1278.
- G. Britton, “General Carotenoid Methods,” Methods in Enzymology, Vol. 111, 1985, pp. 113-149. doi:10.1016/S0076-6879(85)11007-4
- T. D. Boylston, J. R. Powers, K. M. Weller and J. Yang, “Comparison of Sensory Differences of Stored Russet Burbank Potatoes Treated with CIPC and Alternative Sprout Inhibitors,” American Journal of Potato Research, Vol. 78, No. 2, 2001, pp. 99-107. doi:10.1007/BF02874765
- C. M. Christensen, “Effects of Color on Aroma, Flavor and Texture Judgments of Foods,” Journal of Food Science, Vol. 48, No. 3, 1983, pp. 787-790. doi:10.1111/j.1365-2621.1983.tb14899.x
- M. J. Oruna-Concha, S. C. Duckham and J. M. Ames, “Comparison of Volatile Compounds Isolated from the Skin and Flesh of Four Potato Cultivars after Baking,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 5, 2001, pp. 2414-2421. doi:10.1021/jf0012345
- K. B. Stelljes, “Colorful Potatoes Offer Nutrition, Variety,” Agricultural Research, Vol. 49, 2001, p. 6.
- R. L. Surles, N. Weng, P. W. Simon and S. A. Tanumihardjo, “Carotenoid Profiles and Consumer Sensory Evaluation of Specialty Carrots (Daucus carota L.) of Various Colors,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 11, 2004, pp. 3417-3421. doi:10.1021/jf035472m
- P. Winterhaulter and R. L. Rouseff, “Carotenoid-Derived Aromatic Compounds: An Introduction,” In: P. Winterhalter and R. L. Rouseff, Eds., Carotenoid-Derived Aromatic Compounds, American Chemical Society, Washington DC, 2002, pp. 1-13.

