American Journal of Plant Sciences
Vol.08 No.10(2017), Article ID:79106,16 pages
10.4236/ajps.2017.810166
Biological Control of Pyricularia oryzae Using Antifungal Compounds Produced by Aspergillus niger
Ali Abdulameer Idan1,2*, Kamaruzaman Sijam1, Jugah Kadir1, Tavga Sulaiman Rashid3, Hayman Kakakhan Awla4, Wael Alsultan1
1Plant Protection Department, Faculty of Agriculture, Universiti Putra Malaysia, Serdang, Malaysia
2Department of Plant Protection, Faculty of Agriculture, University of Kufa, Najaf, Iraq
3Department of Plant Protection, Faculty of Agriculture, University of Salahaddin, Erbil, Iraq
4Department of Plant Protection, Khabat Institute, Erbil Polytechnic University, Erbil, Iraq

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 20, 2017; Accepted: September 12, 2017; Published: September 15, 2017
ABSTRACT
Aspergillus spp. has been widely found as useful microorganism in biotechnology. They have a high ability in the production of secondary metabolites. Therefore, isolates of Aspergillus were isolated from healthy rice field located in Selangor State/Malaysia. The obtained strain (UPMZ01) was conducted against Pyricularia oryzea by applying dual culture and culture filtrate technique. The antagonism of strain UPMZ01 in the dual culture was 81.326% inhibition percentage against P. oryzae given the optimum inhibitory percentage 100% at all concentration of secondary metabolites aged 14 days. The isolate (UPMZ01) was identified as Aspergillus niger with accession number (KY698415). The environment factors such as pH and temperature influencing on production of secondary metabolites. The results were shown that pH at level 5.0 and temperature between 21˚C to 29˚C is the optimum condition for A. niger to produce efficient antifungal metabolites which given 100% PIGR against blast pathogen. The secondary metabolites compounds were identified by Gas Chromatography-Mass Spectrometry (GC-MS). Fifteen compounds were recognized as major compounds which may have the possibility of possessing antifungal characteristics. Most of identified compounds are Oleic Acid, n-Hexadecanoic acid, Hexose, Glycerol, Stearic acid, Tetradecanoic acid, Dodecanoic acid and 5-Hydrxoymethylfurfural.
Keywords:
Blast Disease, Aspergillus sp., Pyricularia oryzae, Antifungal Compounds, GC-MS

1. Introduction
Rice (Oryza sativa L.) is known as the most substantial food in the worldwide. Rice plant exposes to the one of the most devastating disease which has been recognized as a rice blast disease caused by Magnaporthe oryzae (anamorph Pyricularia oryzae) [1] . Thus, blast disease causes serious yield losses in quality and quantity of rice production [2] . Therefore, various synthetic chemicals have been found as the most valuable method in the management of plant diseases. But due to its side effects, chemical pesticides have considered as dangerous substances that can threat the environment and human health. Biological control seems to be an impressive alternative strategy due to environmental safety, low cost commercially and effective against plant diseases [3] . Microbes particularly fungi have commonly been known biologically as a rich source of unique and efficient metabolites. Microorganisms especially fungi have been known as an optimal source of biological and anti-microbial metabolites [4] . The fungus Aspergillus niger has been recognized as the most valuable microorganism in biotechnologies applications [5] . It possesses a high ability in production of secondary metabolites compounds such as organic acids, pectinase, α-amylase, glucose oxidase, glucoamylase and recombinant proteins [6] . These antimicrobial secondary metabolites can be efficient antibiotics against plant pathogens. Hence, the objective of this study is to isolate and identify fungi from rice field and determine their antagonistic activity against P. oryzae. Secondly, to determine the effect of different levels of pH and temperature on the production of antimicrobial compounds by A. niger, and to identify the metabolic compounds using GC-MS chromatogram.
2. Material and Methods
2.1. Isolation of Microorganisms
Rice plant leaves showing symptoms of blast disease were collected from rice plant field located in Tanjung Karang, Selangor state, Malaysia in June 2015 after the observation of pathogen lesions, and then placed into sterilized plastic bags. The samples were transferred to the department of Plant Protection Laboratory in the Faculty of Agriculture, University Putra Malaysia. After collection, the samples were kept at 4˚C after air-drying until needed for the isolation process. The isolation of the pathogens was done using tissue plating technique described by [7] . The selected fungus A. niger was isolated from the collected soil sample by utilizing planting fold dilution method described by [8] . After air-drying, soil sample was sieved and cleaned well. Then, 1 mg of soil transferred to a sterilized tube consists of 10 mL of distilled H2O. Next, Another 1 mL of suspension transferred from the previous tube to another one consists 9 mL of dH2O to reach the final concentration. The procedure continued respectively to reach 10 times of dilution. Finally, 1 mL transferred and distributed on petri dish containing Potatoes Dextrose Agar (OXOID™) medium and grown for 7 - 10 days at 28˚C till use for the further experiments.
2.2. Pathogenicity Test of the Pathogen
The pathogenicity test was done according to separated leaf assay technique [9] . Leaf fragments of 2 mm in size were cut from two isolated 10 days old colonies and placed onto the center of two-month-old rice plant leaf, and then incubated at room temperature (27˚C ± 2˚C). After 7 days of incubation, lesion development was observed and the length was measured.
2.3. DNA Identification of the Microorganisms
Fungi were grown on PDA at 28˚C ± 2˚C for 3 - 4 days in order to obtain a pure fungal mycelia mats before the producing of spores that may cause contamination during DNA extraction. Fungi mycelia were cut from the colony and separated from the PDA medium using laboratory forceps in order to obtain clean fungal mycelia, then placed onto a sterilized filter paper for 30 min to be dried. Fungi mycelia transferred to a collection tube 1.5 ml and grinded using tissue grinder. DNA was extracted by the modified CTAB method by [10] . The obtained genomic DNA was incubated in TE buffer and stored at −20˚C for further use. The region (ITS 5.8S rDNA) was used to amplify the genomic DNA [11] . The universal primers (ITS1 and ITS4, synthesized by 1st base company SdnBhd, Malaysia) were assayed to amplify the DNA [12] . PCR amplification was applied in PCR tube containing 25 µl (taq. PCR) Master Mix, 15 µl free nuclease water, 3 µl of each Forward primer ITS1 and Reverse ITS4 then 4 µl of template DNA. The PCR reaction was done due to the following cycle condition: Initial denaturation at 94˚C for 3 minutes; 30 cycles of 94˚C for 30 sec denaturation, 45 sec for annealing at 55˚C and 72˚C for 1 minute elongation 35 cycle; and a final extension step of 72˚C for 5 minutes. Electrophoresis gel technique was conducted to screening PCR products, volume of 5 µl of PCR amplified product was investigated on (1.5% Agarose gel TopVision™ eluted with 100 ml of 1X TBE buffer) using 4 µl of DNA safe dye for visualization. Sequencing technology reaction was performed at 1st Base Company SdnBhd, Malaysia. The sequencing results were clarified by utilizing BioEdit sequence alignment editor [13] . The acquired sequence from reverse primer was transformed to reverse complement and then aligned with acquired sequence from the forward primer to obtain the consensus sequence. GenBankin formations were used to analyze and identify the species of fungal isolate sequence by BLASTn alignment. The software Mega 7 was utilized to analyze the phylogenetic tree of identified fungi.
2.4. Antagonistic Activity
The dual culture technique on PDA medium (Gupta et al., 2001) was used in this experiment to select the most potent fungal isolates from the soil that show better activity against P. oryzae. Disks of about 5 mm were cut from A. niger isolate 4 days-old and inoculated at 1 cm distance from the edge of the Petri dish containing PDA medium. On the other side of the plates, disks of 5 mm size cut from the pathogen isolate of 7 days old were placed at 1 cm from the other edge of the Petri dish. The disk containing the pathogen mycelium was used to prepare the controls on separate PDA plates. All the experiments were conducted in triplicates and incubated at room temperature for 7 days. After the incubation period, the radial growth of each fungus was measured and calculated using the formula below.
2.5. Culture Filtrate Method
The culture filtrate method was used to determine the secondary metabolites efficiency of the selected isolates against blast pathogen. The experiment was conducted according to the method described by [14] with some modification. Firstly, disks of about 5 mm containing the A. niger isolate was inoculated into 250 mL of PDB (OXOID™) prepared in sterilized 500 mL conical flasks and incubated at 26˚C ± 2˚C on an orbital rotary incubator shaker at 120 rpm for 14 days. After every 7 days of incubation, three volumes (50, 25 and 12.5 mL) of fungal supernatant were taken from A. niger culture. The collected supernatants were transferred to 50 mL test tubes and centrifuged at 5000 rpm for 5 min to remove remaining mycelial fragments that might be present in the supernatant. After centrifugation, the supernatants were transferred to a new set of tubes and then filtered twice using sterilized Whatman No. 1 filter paper; followed by filtering through 0.45 and 0.22 µm pore biological membrane filters. Molten PDA medium was used to make up the final concentration of the filtrates to 100% (v/v). The filtrates were poured into Petri plates and inoculated with 5 mm disks of P. oryzae placed at the center of the plates. Finally, for the positive control, sterilized dH2O with the same concentration and volume was mixed with molten PDA to obtain a final concentration of 100% (v/v). All the treatments were performed in triplicates and incubated at 26˚C ± 2˚C for 10 days. The growth inhibition percentage was measured at the end of the incubation period.
2.6. Minimum Inhibition Concentration (MIC)
Minimum inhibitory concentrations (MICs) were conducted to determine the lowest concentration of secondary metabolites of A. niger in which no growth of P. oryzae can be detected in the experiment. Concentrations less than 12.5% (v/v) were performed with following of fold serial dilutions. A. niger was incubated for 14 days in incubator shaker at 26˚C ± 2˚C. After incubation, volumes (6.25, 3.125 and 1.562 ml) were collected and filtered using (Watman No.1) filter paper then 0.45 µm followed by 0.22 µm millix syringe filter. Agar diffusion method was assayed to all filtered volumes and adjusted to the final concentration 100% (v/v) with molted PDA (OXIOD™). Each plate received a 5 mm disk of P. oryzae, and then incubated at 26˚C ± 2˚C for 10 days. The inhibition percentage was measured.
2.7. The Effect of pH and Temperature on A. niger Secondary Metabolites
The production of soil fungal secondary metabolites is affected by different factors such as the temperature and pH [15] . This experiment was conducted to determine the optimum circumstances in producing of secondary metabolites of A. niger. Therefore, A. niger was grown on PDB distributed in 4 conical flasks and pH medium adjusted to (3, 5, 7 and 9) respectively by adding HCL and NaOH [4] . The measurement of pH was by pH meter. Flasks were kept at 26˚C ± 2˚C for 14 days to let A. niger grows in different level of pH. Besides, PDB was added to other 4 conical flasks inoculated with A. niger then incubated under various temperature (21˚C, 25˚C, 29˚C and 34˚C) for 14 days to find the optimum temperature for producing of metabolites. Volume 12.5 ml of each treatment (pH and temperature) was filtered then added to 85.5 ml of molten PDA to provide the final concentration 100% (v/v). Lastly, volumes poured in petri dishes then inoculated by 5 mm mycelial disks of 5 days old colony of P. oryzae. Treatments incubated for 10 days then percentage of radial growth was measured.
2.8. Identification of Volatile Compounds by GC-MS
About 5 mL of fungal secondary metabolites of A. niger aged 14 days on PDB was filtered and sent to the Department of Chemistry, Faculty of Sciences, University Putra Malaysia for GC-MS dissection analysis. The GC-MS analysis of the sample was done using SHIMADZU GC-MS QP 2010 plus with EI electron impact ion source of 70 eV employing a BPX5 Fused Silica Capillary Column (30 m × 0.25 m ID × 0.25 FILM THICKNESS). The temperature program was at the range of 100˚C for 5 minutes to 250˚C for 10 minutes. The purge flow program was 3.0 mL/min. The retention time and peak of areas were recorded by integrating electronically. The obtained peaks were compared and matched with the institute of National Standards and Technology (NIST 08 and NIST 08s) library and correlated with the distributed data that have been published.
3. Results
3.1. Isolation of Microorganisms
The two obtained isolates of P. oryzae were named as UPMZPO and UPMZPO1. These strains identified morphologically as a simple whitish mycelium toward the colony center, moderately and become greyish brown to dark looking for the rest of the colony, and single (or fasciculate) conidiophores [16] . These two isolates were revealed typical characteristics of blast pathogen. Besides, pure isolates of A. niger colony aged 7 days was observed as grayish black to black colony with white mycelia at the edge of the fungus colony [17] .
3.2. Pathogenicity Test
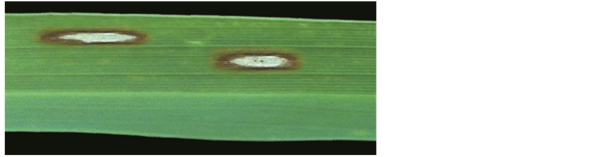
The two obtained isolates were shown virulence pathogenicity on rice plant leaf (Figure 1). The isolate UPMZPO was selected for the further testes according to lesion length on the surface of rice leaf. The disease symptoms appear as brown lesions while on the sensitive leaves, diamond-form or spindle-form spots.
3.3. Molecular Identification
The DNA purity and quantity were identified by NanoDrop 2000 c spectrophotometer (Thermo Fisher Scientific, USA). Based on screening of gel electrophoresis, DNA fragments for the isolate UPMZ01 and UPMZPO were amplified at range (582 and 527 bp) respectively. Measurement was according to molecular weight marker (100 bp ladder) as in (Figure 3). Investigations of DNA sequencing were obtained by NCBI Blast. The first isolates identified as Aspergillus niger named as UPMZ01. The partial sequence of 18s rDNA of UPMZ01 isolate was shown up to 100% similarity to A. niger with an accession (KU729033). While the pathogen isolate identified as Magnaportha oryzea (Pyriculariaoryzae) were shown 100% similarity to the accession (AB274418) P. oryzae. Lastly, phylogenetic tree for the identified fungi was made by using Mega 7 software (Figure 2). The identified fungi were recorded in GenBank with accessions (KY698415 and KY698417).
3.4. Antagonistic Activity
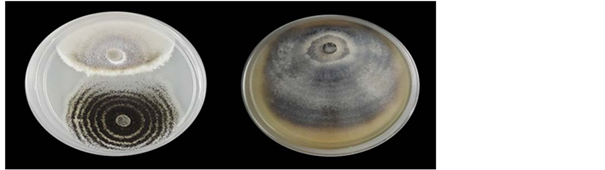
The dual culture result was shown a clear inhibition zone of the pathogen P. oryzae. The percentage inhibition of redial growth is up to 81.326% compared with the control plate (Chart 1 & Figure 3).
3.5. Culture Filtrate
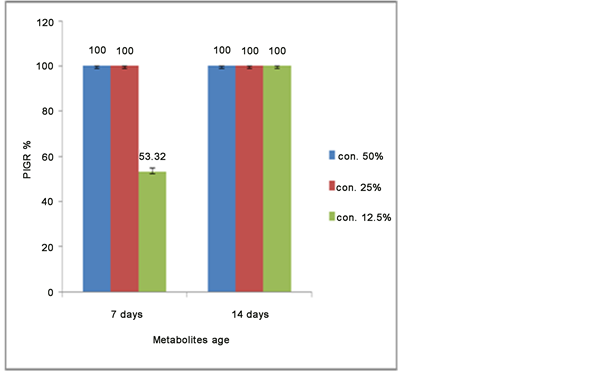
The results were observed from the agar diffusion method of filtered metabolites of UPMZ01 strain at the concentrations (12.5, 25 and 50 mL) shown (100%, 100% and 53.32%) percent inhibition growth respectively. These results were at 7 days of metabolites age dissolved in broth medium. While, the observations was done at 14 days of metabolites age (100%, 100% and 100%) of inhibition growth to all treatments. All were obtained results, compared with negative control plates (0% inhibition) which were had the similar concentration volume of sterilized distilled water as showing in Figure 4 and Figure 5.
Figure 1. Blast disease lesions caused by P. oryzae on rice plant leave.


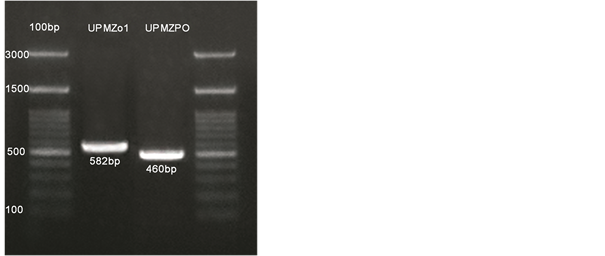
Figure 2. (a) and (b) phylogenetic tree for the identified fungi using maximum likelihood method. And (c) Gel electrophoresis profiles of ITS1-ITS4 5.8S rRNA locale amplified from genomic DNA. The strains UPMZ01 and UPMZPO fragmented at 582 and 460 base pairs respectevly. Ladder = Molecular weight marker (100 PB ladder). Agarose gel loaded (1.5%).
Figure 3. Inhibition of P. oryzae in dual culture method. Left plate = A. niger; right plate = control.
Figure 4. The effect of A. niger secondary metabolites on mycelial growth of P. oryzae using agar diffusion method. The plates from (a1) to (a3) treated with metabolites at conc. (12.5%, 25% and 50%) 7 days old respectively. Plates from (b1) to (b3) treated with metabolites 14 days old. The plates (c1) to (c3) as control conducted with dH2O at the similar concentration of treated fungus.
Figure 5. Mean value of percentage inhibition growth of P. oryzae mycelium in different concentration of A. niger antifungal metabolites. Vertical bars represent the standard error.
3.6. Minimum Inhibition Concentration (MIC)
The observed results were shown mycelium growth of P. oryzae in the plate with concentration 6.25% (v/v) of A. niger metabolites with inhibition percentage 42.85%. The inhibition percentage reduced respectively based on the decreasing of aqueous metabolites solution in Table 1 and Figure 6.
3.7. The Effect of pH and Temperature on A. niger Secondary Metabolites
The treatments were shown significantly different results. Treatment with pH 5.0 was observed with 100% PIGR to the mycelia of tested pathogen while the treatment with PH 3.0 was given 83.803% PIGR. The pH 7.0 treatment was given 42.33% PIGR and pH 9.0 was shown inhibition percentage 13.26%. The Figure 7 and Figure 8 showed the PIGR mean of treatments.
The temperature test showed that the metabolites antimicrobial product of A. niger resulted under temperature range between 21˚C to 29˚C have been revealed inhibitory percentage up to 100% against P. oryzae (Figure 9 and Figure 10), while the treated metabolites under temperature 34˚C has been given inhibition 55.66˚C. As a result were observed, the range between 21˚C and 29˚C is the most efficient temperature for the production of A. niger antimicrobial metabolites.
3.8. Identification of Volatile Compounds by GC-MS
The analysis of aqueous secondary metabolites was performed at the Department of Chemistry, Faculty of Science at University Putra Malaysia. The
Table 1. The minimum inhibition concentration of UPMZ01 metabolites on the growth inhibition of P. oryzae by serial dilutions method.
Means of PIGR with the same letter are not significantly different.




Figure 6. Plates (a), (b) and (c) show the MIC as following concentrations (6.25, 3.125, 1562) respectively, and (d) is the control.
Figure 7. The effect of different levels of pH on A. niger antimicrobial metabolites in the inhibition of targeted pathogenic fungus P. oryzae.





Figure 8. The effect of different levels of pH 3, 5, 7 and pH 9 as in (a), (b), (c) and (d) respectively on secondary metabolites of the isolate UPMZ01 and the inhibition effect on the pathogen P. oryzae. (n) is the control.





Figure 9. The effectsin different temperatures ((a) = 21˚C, (b) = 25˚C, (c) = 29˚C and (d) = 34˚C) on antimicrobial metabolites of the isolate UPMASN01 on mycelium growth of the pathogen P. oryzae, N is the control.
obtained results indicated of 15 compounds were detected and identified by using mass spectrometry linked with gas chromatography. Most of them are fatty acids. The area and retention time of each presented compound was showed in Table 2.
4. Discussion
Two isolates named as UPMZPO and UPMZPO01 were shown typical characteristic morphological growth of blast disease. The fungus Pyricularia oryzae has been recognized as a simple whitish mycelium color toward the colony center, moderately become greyish brown to dark looking for the rest of the colony, and
Figure 10. The effect of various temperature on the A. niger antimicrobial metabolites against the pathogen P. oryzae.
Table 2. The presence of non-volatile compound analyzed by GC-MS chromatogram according retention time.
single (or fasciculate) conidiophores. The revealed characteristics have been agreed with [16] . In the conducted dual culture test, A. niger was revealed a clear inhibition against P. oryzae. Dual culture test can be performed as a typical test for the election of a biological control agent. Investigations have observed a cumulative impact due to producing of antimicrobial, lytic enzymes and metabolites [18] . [19] reported that A. niger was revealed inhibition growth 66.29% and 59.26% of Trigonospila cingulate and Stereumhirsutum respectively. Culture filtrate method was performed to evaluate the efficiency of secondary metabolites in controlling of P. oryzae growth in vitro. According to obtained results, the concentration of A. niger secondary metabolites 12.5% (v/v) at 14 days old was strongly revealed the optimum inhibition percentage 100% against P. oryzae mycelium growth. Meanwhile, (a1) was significantly different from (a2) and (a3) due to the difference between concentrations (Figure 4). And 7 days of incubation period may not enough for the UPMZ01 isolate to produce concentrated metabolites at 12.5%. Whereas, there was no significant variance between the concentrations 25% and 50%(v/v) along 14 days of metabolites age, which means the 25% (v/v) concentration and above were enough concentration of bioactive secondary metabolites to be effective (100%) against mycelium growth of the P. oryzae. The difference between the percentages of inhibition at the similar concentration of secondary metabolites was due to the period of incubation time. Therefore, it may influence on antimicrobial substance and fungal exudates during the incubation period. A recent study was done by [20] investigated that A. niger metabolites were conducted as a biocontrol agent against Fusariumoxysporumf sp. lycopersici and showed a good inhibition percentage in different concentrations. In addition, some of Aspergillus spp. was used as a biocontrol agent in controlling of several diseases. [3] demonstrated that (glucose oxidase) extracted from A. tubingensis was successfully inhibited the growth of Fusariumsolani. The fungal growth characterization is essential to recognize the optimum conditions for fungus behaviors. The fungus A. niger strain UPMZ01 was experimented under various levels of pH and temperature. Based on obtained results, the pH 5 for fungal biomass growth yield was efficient to reach the optimum antimicrobial production. The fungal activity also exhibited as pH-dependent. The various levels of pH medium have an obvious effect on the antimicrobial and secondary metabolites production. The concentration of hydrogen ion may have an explicit effect on cell behavior, or it can act indirectly due to the variance of dissociation degree of substances in the growth medium. Hence, the alteration of pH level is also substantial for the enzyme activities of microbes, production of substances intermediate, their solubility and dissociation [15] . Therefore, pH 5.0 is the optimum level for the production of antimicrobial by A. niger which that given a 100% inhibition against blast pathogen mycelial growth at 12.5% (v/v) aged 14 days of aqueous secondary metabolites. The incubation temperature is considered as a physical factor that can play different role of impact on microorganisms growth and its production of secondary metabolites [15] . Besides, the present study is to determine the effect of temperature on the production of fungal secondary metabolites. The results were observed in Chart 2 and Figure 2 revealed no significant between the treated metabolism under from 21˚C to 29˚C which were given 100% inhibitory percentage against mycelium growth of P. oryzae, while the effect of the temperature on fungal metabolism under 35˚C was given 55.66% inhibitory percentage. The findings of the current study are consistent with those of [21] , who found the insecticidal compounds that produced by Asspergillus sp. at 25˚C. Moreover, the incubation temperature under 27˚C was conducted for the production of antimicrobial antibiotic from Aspergillus sp. [22] . According to those obtained results, the most interesting finding was that the optimum incubation temperature in the production of antimicrobial at the range in between 21˚C to 29˚C for the fungus A. niger to produce efficient secondary metabolites against blast pathogen. The aqueous extract of A. niger secondary metabolites were analyzed using GC-MS. Some of obtained compounds were identified as fatty acids. Theses fatty acids have been utilized as antimicrobial agents since a long term previously. The properties that possessed by fatty acids are well recognized to be antibacterial and antifungal agents [23] . [24] stated that oleic acid has been shown antimicrobial activity in a reduction from 84% to 14% of living bacteria Staphylococcus aureus treated with 0.1% of oleic acid. Moreover, n-hexadecanoic acid known also (palmitic acid) extracted from Canthiumparviflorum leaves were investigated to show antimicrobial activity against fungi and gram positive and negative bacteria significantly reported by [25] . Fatty acid methyl esters extract includes (oleic acid, n-hexadecanoic acid and stearic acid) was assayed and shown successfully inhibition activities against (Candida albicans, Candida tropicalis, Candida krusei, Candida parapsilosis, Micrococcus luteus, Pseudomonas aeruginosa, Escherichia coli, Bacillus subtilis, Bacillus pumilus and Bacillus subtilis) investigated by [26] . Hence, the presence of fatty acids in our obtained results has a potentiality in the reduction of mycelium growth of P. oryzae. Hence, the presence of fatty acids in our obtained results has a high potentiality in the reduction of mycelium growth of P. oryzae. Glycerol compound was presented in our GC-MS analysis. Glycerol is a common component known as a natural antimicrobial utilized in food and cosmetics technologies. [27] found that a low concentration of glycerol can inhibit different staphylococci. Besides, HMF (5-Hydrxoymethylfurfural) and Hexose also existed is our GC-MS results (Table 2). [28] reported that no growth of fungus Pichia stipites when exposed to 60 mM of HMF. The (5-Hydrxoymethy lfurfural) is created by dehydration of hexoses [28] . These two compounds are damaging microorganisms by decreasing biological and enzymes activities, protein inhibitors and DNA breakdown and are recognized as the most powerful inhibitors [28] . However, in this study, the investigations were proven the effect of the different antimicrobial compounds included fatty acids in the reduction of mycelium growth of the P. oryzae.
5. Conclusion
The investigations which were obtained in vitro revealed that the A. niger strain (UPMZ01) from rice rhizosphere soil possesses the potentiality in growth inhibition of rice blast pathogen P. oryzae in dual culture assay and culture filtrate with various concentrations. The aqueous extract of A. niger secondary metabolites were found to be a successful unique agent against P. oryzae growth. The MIC was observed to find the minimum concentration of aqueous metabolism against blast pathogen growth. The bioactive fungus A. niger was characterized in different levels of pH and temperature. The proper temperature and pH were recorded to be the optimum circumstances for the production of antimicrobial compounds. The compounds were analyzed using GC-MS technique. The volatile compounds were obtained from GC-MS analytical recorded the presence of natural fatty acids. Fatty acids are widely used in biosciences and industrial synthesis. These compounds strongly have a potential to be utilized as biocontrol agents instead of chemical pesticide. However, the fungus A. niger strain UPMZ01 was greatly found to control rice blast pathogen P. oryzae and it can be an alternative natural product instead of chemicals and synthetic antibiotics in controlling of fungi diseases.
Cite this paper
Idan, A.A., Sijam, K., Kadir, J., Rashid, T.S., Awla, H.K. and Alsultan, W. (2017) Biological Control of Pyricularia oryzae Using Antifungal Compounds Produced by Aspergillus niger. American Journal of Plant Sciences, 8, 2445-2460. https://doi.org/10.4236/ajps.2017.810166
References
- 1. Li, Q., et al. (2011) Suppression of Magnaporthe Oryzae by Culture Filtrates of Streptomyces Globisporus JK-1. Biological Control, 58, 139-148.https://doi.org/10.1016/j.biocontrol.2011.04.013
- 2. Hayasaka, T., Fujii, H. and Ishiguro, K. (2008) The Role of Silicon in Preventing Appressorial Penetration by the Rice Blast Fungus. Phytopathology, 98, 1038-1044.https://doi.org/10.1094/PHYTO-98-9-1038
- 3. Kriaa, M., Hammami, I., Sahnoun, M., Azebou, M.C., Triki, M.A. and Kammoun, R. (2015) Biocontrol of Tomato Plant Diseases Caused by Fusarium Solani Using a New Isolated Aspergillus Tubingensis CTM 507 Glucose Oxidase. Comptes Rendus Biologies, 338, 666-677. https://doi.org/10.1016/j.crvi.2015.05.007
- 4. Mathan, S., Subramanian, V. and Nagamony, S. (2013) Optimization and Antimicrobial Metabolite Production from Endophytic Fungi Aspergillus Terreus KC 582297. European Journal of Experimental Biology, 3, 138-144.
- 5. Schuster, E., Dunn-Coleman, N., Frisvad, J. and Van Dijck, P. (2002) On the Safety of Aspergillus niger—A Review. Applied Microbiology and Biotechnology, 59, 426-435.https://doi.org/10.1007/s00253-002-1032-6
- 6. Lopes, F.C., et al., (2011) Production of Proteolytic Enzymes by a Keratin-Degrading Aspergillus niger. Enzyme Research, 2011, 1-9. https://doi.org/10.4061/2011/487093
- 7. Sesma, A. and Osbourn, A.E. (2004) The Rice Leaf Blast Pathogen Undergoes Developmental Processes Typical of Root-Infecting Fungi. Nature, 431, 582-586.https://doi.org/10.1038/nature02880
- 8. Kumar, P.K.R., Hemanth, G., Niharika P.S. and Kolli, S.K. (2015) Isolation and Identification of Soil Mycoflora in Agricultural Fields at Tekkali Mandal in Srikakulam District. International Journal of Advances in Pharmacy, Biology and Chemistry, 4, 484-490.
- 9. Guleria, S., Aggarwal, R., Thind, T.S. and Sharma, T.R. (2007) Morphological and Pathological Variability in Rice Isolates of Rhizoctonia solani and Molecular Analysis of their Genetic Variability. Journal of Phytopathology, 155, 654-661.https://doi.org/10.1111/j.1439-0434.2007.01291.x
- 10. Allen, G.C., Flores-Vergara, M.A., Krasnyanski, S., Kumar, S. and Thompson, W.F. (2007) A Modified Protocol for Rapid DNA Isolation from Plant Tissues USING cetyltrimethylammonium Bromide. Nature Protocols, 1, 2320-2325.https://doi.org/10.1038/nprot.2006.384
- 11. Sahnoun, M., Bejar, S., Sayari, A., Ali, M., Kriaa, M. and Kammoun, R. (2012) Production, Purification and Characterization of Two-Amylase Isoforms from a Newly Isolated Aspergillus Oryzae Strain S2. Process Biochemistry, 47, 18-25.
- 12. White, T.J., Bruns, S., Lee, S. and Taylor, J. (1990) Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: PCR Protocols: A Guide to Methods and Applications, Academic Press, 315-322.
- 13. Hall, T. (1999) BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95-98.
- 14. Dennis, C. and Webster, J. (1971) Antagonistic Properties of Species-Groups of Trichoderma: II. Production of Volatile Antibiotics. Transactions of the British Mycological Society, 57, 363-369.
- 15. Jain, P. and Pundir, R.K. (2011) Effect of Fermentation Medium, pH and Temperature Variations on Antibacterial Soil Fungal Metabolite Production. Journal of Agricultural Technology, 7, 247-269.
- 16. Srivastava, D., Kumar, D., Pandey, P., Khan, N.A. and Singh, K.N. (2014) Morphological and Molecular Characterization of Pyricularia oryzae Causing Blast Disease in Rice (Oryza sativa) from North India. International Journal of Scientific and Research Publications, 4, 1-9.
- 17. Nyongesa, B.W., Okoth, S. and Ayugi, V. (2015) Identification Key for Aspergillus Species Isolated from Maize and Soil of Nandi. Advances in Microbiology, 5, 205-229. https://doi.org/10.4236/aim.2015.54020
- 18. Compant, S., Duffy, B., Nowak, J., Cle, C. and Barka, E.A. (2005) Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects Minireview Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and. Applied and Environmental Microbiology, 71, 4951-4959. https://doi.org/10.1128/AEM.71.9.4951-4959.2005
- 19. Tiwari, C.K., Parihar, J. and Verma, R.K. (2011) Potential of Aspergillus niger and Trichoderma viride as Biocontrol Agents of Wood Decay Fungi. Journal of the Indian Academy of Wood Science, 8, 169-172. https://doi.org/10.1007/s13196-012-0027-x
- 20. Dewan, M.M. and al deen Dewan, Z.N.K. (2009) The Effect of Aspergillus niger, Trichoderma harzianum and Their Mixture Exudates on the Viability of Fusarium oxysporum f. sp. lycopersici and the Growth of Tomato Seedlin. Kufa Journal for Agricultural Science, 12, 1-12.
- 21. Suzuki, K., Kuwahara, A., Yoshida, H., Fujita, S., Nishikiori, T. and Nakagawa, T. (1997) NF00659A1, A2, A3, B1 and B2, Novel Antitumor Antibiotics Produced by Aspergillus sp. NF 00659. I. Taxonomy, Fermentation, Isolation and Biological Activities. The Journal of Antibiotics, 50, 314-317. https://doi.org/10.7164/antibiotics.50.314
- 22. Fang, F., Ui, H., Shiomi, K., Masuma, R. and Yamaguchi, Y. (1997) Two New Components of the Aspochalasins Produced by Aspergillus sp. The Journal of Antibiotics, 50, 919-925. https://doi.org/10.7164/antibiotics.50.919
- 23. Bush, K. (1997) Antimicrobial Agents. Current Opinion in Chemical Biology, 1, 169-175.
- 24. Stenz, L., et al. (2008) Impact of Oleic Acid (Cis-9-Octadecenoic Acid) on Bacterial Viability and Biofilm Production in Staphylococcus aureus. FEMS Microbiology Letters, 287, 149-155. https://doi.org/10.1111/j.1574-6968.2008.01316.x
- 25. Krishnan, K.R., James, F. and Mohan, A. (2016) Isolation and Characterization of n-Hexadecanoic Acid from Canthium parviflorum Leaves. Journal of Chemical and Pharmaceutical Research, 8, 614-617.
- 26. Agoramoorthy, G., Chandrasekaran, M., Venkatesalu, V. and Hsu, M.J. (2007) Blind-Your-Eye Mangrove from India. Brazilian Journal of Microbiology, 38, 739-742. https://doi.org/10.1590/S1517-83822007000400028
- 27. Projan, S.J., Brown-skrobot, S. and Schlievert, P.M. (1994) Glycerol Monolaurate Inhibits the Production of 1-Lactamase, Toxic Shock Syndrome Toxin-i, and Other Staphylococcal Exoproteins by Interfering with Signal Transduction. Journal of Bacteriology, 176, 4204-4209. https://doi.org/10.1128/jb.176.14.4204-4209.1994
- 28. Liu, Z.L., Slininger, P.J., Dien, B.S., Berhow, M.A., Kurtzman, C.P. and Gorsich, S.W. (2004) Adaptive Response of Yeasts to Furfural and 5-Hydroxymethylfurfural and New Chemical Evidence for HMF Conversion to 2,5-Bis-Hydroxymethylfuran. Journal of Industrial Microbiology & Biotechnology, 31, 345-352. https://doi.org/10.1007/s10295-004-0148-3