American Journal of Plant Sciences
Vol.3 No.6(2012), Article ID:20028,11 pages DOI:10.4236/ajps.2012.36094
Exploring the Insecticidal Potentiality of Amorphophallus paeonifolius Tuber Agglutinin in Hemipteran Pest Management
![]()
Division of Plant Biology, Centenary Campus, Bose Institute, Kolkata, India.
Email: #sampa@bic.boseinst.ernet.in
Received March 7th, 2012; revised March 28th, 2012; accepted April 17th, 2012
Keywords: Amorphophallus paeonifolius Tuber Agglutinin (AMTL); Agglutination; Dissociation Constant (Kd); Insect Bioassay; Thermal Stability Assay; Surface Plasmon Resonance (SPR) Analysis; Brush Boarder Membrane Vesicle (BBMV) Protein
ABSTRACT
Hemipteran group of sap sucking insect pests cause worldwide crop destruction. The role of mannose specific monocot lectins have recently been worked out in hemipteran pest management. The present article demonstrates the insecticidal efficacy of a new mannose specific agglutinin, isolated from tubers of Amorphophallus paeonifolius (AMTL) against a wide range of hemipteran insects. The 25 kDa dimeric protein was found to inhibit the survivability of hemipteran insects namely, Lipaphis erysimi, Aphis gossypii and Dysdercus cingulatus quite efficiently, as analysed by synthetic diet based bioassay experiments. Surface Plasmon Resonance study detected binding of insecticidal AMTL to insect gut brush border membrane vesicle (BBMV) protein, an absolute prerequisite for conferring toxicity against target insects. Further ligand blot analysis spotted a ~74 kDa glycoprotein as putative receptor of AMTL from the total BBMV protein fraction of Lipaphis erysimi. Phylogenetic analysis showed a significant relatedness of AMTL to the previously established monocot lectin Galanthus nivalis agglutinin (GNA) in terms of their conserved mannose binding domains, agglutinating ability of rabbit erythrocytes and insecticidal efficacies. These information project AMTL as a promising candidate in preventing crop loss caused due to hemipteran insect attack.
1. Introduction
Plant phloem sap sucking pests falling under hemipteran class, commonly termed as aphids, are one of the most destructive agricultural pest group known worldwide. Their sap sucking feature not only causes damage of important crop plants but also accounts for additional losses by aiding transmission of disease causing viruses. Chemical control measures adopted for certain aphid species are becoming extremely difficult due to social unacceptability as well as development of insect resistance towards these widely applied insecticides over prolonged period of use. Global policy of Integrated Pest Management (IPM) has been framed taking into account the major concerns against application of huge amount of pesticides which affect both ecology and environment.
The journey from advent to advancement of biotechniques has posed high hopes on the development of novel strategies for Integrated Pest Management (IPM) [1].
Plant lectins are known to be promising components in this regard. The sucking insects feed on phloem sap that contains simple sugars and some free amino acids [2,3]. They are not susceptible to Bt toxins. Alkaline environment within the insect gut and presence of appropriate receptors are the primary requisites for Bt toxin activity but characteristically, the pH of the gut environment of sucking insects is maintained around neutral condition [4].
Lectins are characterized as proteins possessing at least one non-catalytic domain that binds reversibly to specific mono or oligosaccharide [5]. Previous authors have shown that mannose binding lectins bind to glycoproteins present in the peritrophic matrix or other membranous lining of the insect midgut which disrupts digestive processes and nutrient assimilation leading death of the insects [6]. Unfortunately, very little emphasis has been given so far to investigate the exact mechanism of action of these lectins against insects. Hence, knowledge on this aspect is still incomplete. In an attempt to address this problem and explore the potentiality of lectins from Aracean members, protein was purified form Amorphophallus paeeonifolius tubers. The efficacy of this lectin was assayed against Lipaphis erysimi, Aphis gossypii and Dysdercus cingulatus. Interaction study of this protein with the gut membrane of the target insect Lipaphis erysimi was carried out rendering it to be promising as an effective insecticidal agent against sap sucking pests.
2. Materials and Methods
2.1. Plant and Insect Materials
Tubers of local Amorphophallus paeeonifolius plants grown in Institutional experimental fields under natural conditions were used as protein source. Adults and second instar nymphs of Lipaphis erysimi, Aphis gossypii and Dysdercus cingulatus reared on mustard and cotton plants at institutional insectry were used for bioassay experiments.
2.2. Chromatographic Purification, in Gel and Western Analysis of AMTL
Crude protein was isolated from 200 g of fresh tubers following the protocol of Van damme et al. (1995) [5]. This crude protein was subjected to further affinity and ion exchange chromatographic purification as outlined by Majumder et al. (2005) [6]. Molecular mass, oligomeric structure and sample purity was determined by loading the purified protein into gel filtration column of Biosep SEC S2000 (300 × 7.8 mm) phenomenex HPLC column (Phenomenex, Torrance, CA, USA) and monitored (220 nm) using Shimadzu UFLC system (Shimadzu Corporation, Japan). Standard molecular weight markers (Sigma Aldrich, USA) in the range of 14.3 kDa to 66 kDa were used for calibration, prior to sample loading. In all cases the injection volume was 50µl and the flow rate 2 ml∙min–1. The column calibration and elution was done with 10 mM PBS (pH 7.2). All the protein samples crude to purified were analyzed in 15% SDS PAGE [7] and quantified using Bradford method [8]. Western blot analysis was performed using anti AMTL polyclonal antibody raised in rabbit following the protocol of Harlow et al. (1998) [9]. Anti rabbit IgG with HRP conjugate (Sigma Aldrich, USA) was used to probe the primary antibody and the blot developed in Hybond ECL film using Amersham Hybond ECL kit (GE Healthcare, UK).
2.3. Agglutination Assay
100 µl of purified AMTL protein dialyzed against PBS (pH 7.2) was mixed with erythrocyte according to the protocol mentioned by Majumder et al. (2005) [10]. The assay was carried out in a 96 well microtitre plate (Tarsons, India) using two fold dilution series starting from the concentration 200 µg∙ml–1 to 0.78 µg∙ml–1. The agglutination phenomenon was monitored by incubating the reaction sets at 37˚C for 1 hour. The agglutination efficacy was verified by preincubation of AMTL (3.12 ug∙ml–1 and 25 µg∙ml–1) with 20 mM to 500 mM and 50 mM to 2M α-D Mannose respectively.
2.4. Thermal Stability Assay
Thermal stability assay was designed as instructed by Codex Alimentarius Commission, 2003. The stability of AMTL was correlated to its rabbit erythrocyte agglutinating ability. Erythrocytic suspension was prepared according to Majumdar et al. (2005) [6]. AMTL used in this assay was preincubated for 30 minutes under different temperatures (25˚C, 37˚C, 55˚C, 75˚C, 95˚C) and instantly cooled.
2.5. Sugar Specificity Assay of AMTL
AMTL (0.1 mg/ml) was incubated at 25˚C on a Hitachi F-7000 spectrofluorimeter using Sigma cuvette (volume: 1 ml; path length: 1 cm). Titration of solution was performed with 10mM mannose in phosphate buffer (pH 7.2) by adding a small aliquot at a time. After each addition, the solution was stirred for 1 min on a magnetic stirrer, and the fluorescence emission spectrum recorded between 300 and 400 nm using 295 nm as the excitation wavelength. The excitation and emission band passes were 5 nm each. D-glucose was also used for ligand binding experiment in similar manner. The equation for single-site ligand binding measured through changes in the spectroscopic signal [11] is given by ∆F/C = A – Ka∆F where ∆F represents the increase or decrease in fluorescence intensity at a given concentration (C) of the ligand, Ka is the association constant, and A = Ka∆Fmax where ∆Fmax stands for the maximum change in fluorescence intensity. We plotted ∆F/C against ∆F and used the slope (Ka) to calculate the dissociation constant (Kd) for binding of mannose and glucose to AMTL.
2.6. Insect Bioassay of AMTL in Artificial Diet
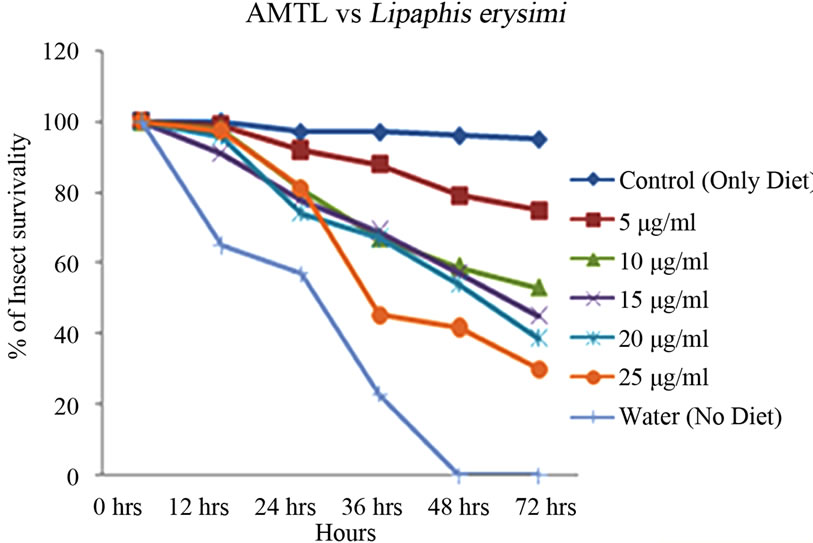
Bioassay was conducted on second instar nymph of Lipaphis erysimi, Aphis gossypii and Dysdercus cingulatus using artificial diet as described by Dadd and Mittler (1976) [10] with some modifications formulated by Bandopadhyay et al. (2001) [12]. The experimental set up was designed according to Majumder et al. (2005) [6]. Average of 30 insects per set were incubated (each in replicates of 5) in liquid artificial diet of 200 µl supplemented with AMTL (5 to 25 µg∙ml–1 with uniform increment of 5 µg∙ml–1), while only diet devoid of AMTL served as the positive control. Percentage of insect survivality was recorded after every 12 hours interval for 72 hours. The LC50 value of AMTL corresponding to each insect was determined by Probit analysis [13].
2.7. Surface Plasmon Resonance Study
Interaction between AMTL and Brush Boarder Membrane Vesicle proteins of target insect Lipaphis erysimi was monitored through Surface Plasmon Resonance (SPR) study. Surface plasmon resonance analysis was executed using a Biacore X100 instrument and CM5 sensor chips (Biacore). The purified AMTL was concentrated through microcon device (Milipore) and subsequently diluted to 2 µg∙µl–1 in 10 mM sodium acetate buffer (pH 4.5). The protein was then immobilized on flow cell 1 on the carboxymethylated dextran surface of CM5 sensor chip by amine coupling using a standard amine-coupling kit (Biacore). The surface of this flow cell was activated for 5 min at a flow rate of 10 µl∙ml–1. Following ligand immobilization on flow cell 1, this flow cell was blocked with a 5 minutes injection of 1 M ethanolamine at a flow rate of 10 µl∙ml–1. The analyte (BBMV proteins) was injected at different concentrations (0 µg—only buffer controls, 7.5, 3.75, 1.875 µg∙60µl–1) at a flow rate of 10 µl∙min–1 for both association and dissociation. The complex was allowed to associate and dissociate for 120 s and 120 s, respectively. HBS-N buffer (10 mM HEPES, pH 7.4, 150 mM NaCl) was used throughout the analysis. The surfaces were regenerated with 1 minute injection of 10 mM glycine-HCl, PH 2.0. The data was analyzed through Biocore X100 Evaluation Software.
2.8. Ligand Blot, Deglycosylation and Mannose Inhibition Assay
Total brush border membrane vesicle (BBMV) protein was isolated according to Bandopadhyay et al. (2001) [12] using the MgCl2 sequential precipitation method suited for luminal membranes. About 20 µg of total BBMV resolved in 12% SDS PAGE was electrophoretically transferred to Hybond C membrane (GE Healthcare, UK) using Hoeffer (Hoefer Inc. Holliston, MA, USA) electroblot apparatus. The membrane was transiently stained with Ponceau S (Sigma Aldrich, USA) to confirm the transfer of proteins onto it. Blocking of membrane was done with 5% non fat milk (Merck, Germany) dissolved in 1X PBST at 37˚C for 1 hour. The membrane was further subjected to incubation with predetermined LC50 of AMTL for 2 hr following extensive washing with 1X PBST. The membrane was then incubated with anti AMTL (1:8000) for 1hour at 37˚C. Further probing of the primary antibody was done with anti rabbit IgG HRP conjugate (1:20,000) (Sigma Aldrich, USA) and developed using Hybond ECL kit on Hybond ECL film (GE Healthcare, Germany).
The effect of α-D Mannose on AMTL and its binding with BBMV receptor was monitored by ligand blot analysis using mannose pre saturated AMTL as probe. The experimental conditions and set up were the same as discussed above. 20 µg of total BBMV of Lipaphis erysimi was subjected to deglycosylation using N Glycosidase F deglycosylation kit (Roche, USA) according to manufacturer’s instructions. Deglycosylated sample was resolved in 12% SDS PAGE and subjected to further ligand blot analysis as mentioned earlier.
2.9. Phylogeny and in Silico Homology Modeling
Phylogram based on relationship between AMTL (EU08 3312) and other GNA related monocot mannose binding lectins was obtained using BLAST and multiple alignment programs. In silico model of AMTL was generated using 3D JIGSAW considering GNA lectin as template. Structure and orientation of mannose binding pockets were predicted and the models viewed using Pymol 3D Graphics viewer.
3. Results
3.1. Purification and Western Blot of AMTL
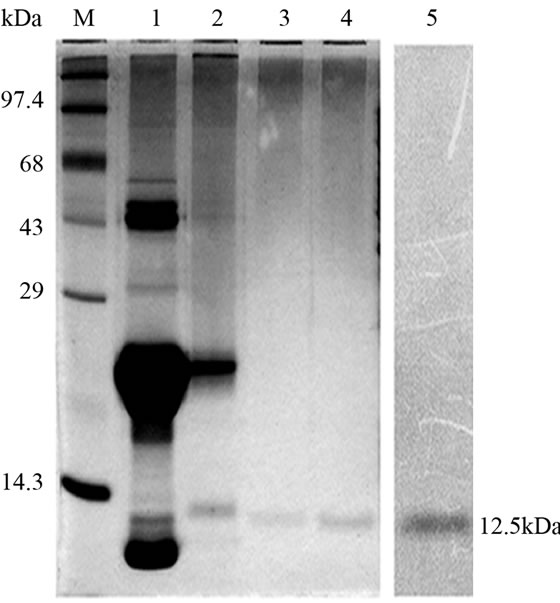
Lectin isolated from Amorphophallus paeonifolius tubers were initially purified through affinity chromatography (Figure 1(a)). The fractions (no: 2 to 7) showing higher densities were pooled, concentrated and subjected to further ion exchange chromatographic purification. Purified samples showed elution of major protein peak of 25kDa at 4.45 mins (Figure 1(b), inset) along with minor fraction of other contaminated proteins when subjected to pass through gel filtration column. The major peak was collected, concentrated and passed through the same gel filtration column giving a single peak of purified stable dimeric AMTL at 4.47 mins (Figure 1(b)). All the crude and purified lectin fractions were subjected to 15% SDS PAGE analysis showing ~12.5 kDa protein band which was further verified by western blot analysis (Figure 1(c)).
3.2. Hemagglutination and Mannose Inhibition Assay
Hemagglutination assay was performed with purified AMTL on rabbit erythrocytes. The erythrocytes started to agglutinate at a concentration of as low as 3.12 µg∙ml–1 of AMTL (Figure 1(d), lane 8), although complete agglutination was found at 25 ug∙ml–1 protein. The agglutinating property was however found to be completely reverted by pre incubation of AMTL with α-D mannose (Figure 1(e), lane 6 and Supplementary Figure 2).
 (a)
(a)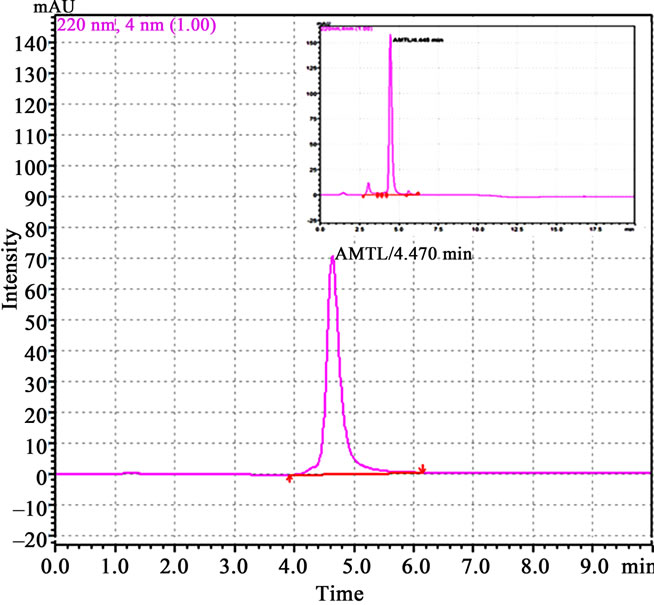 (b)
(b)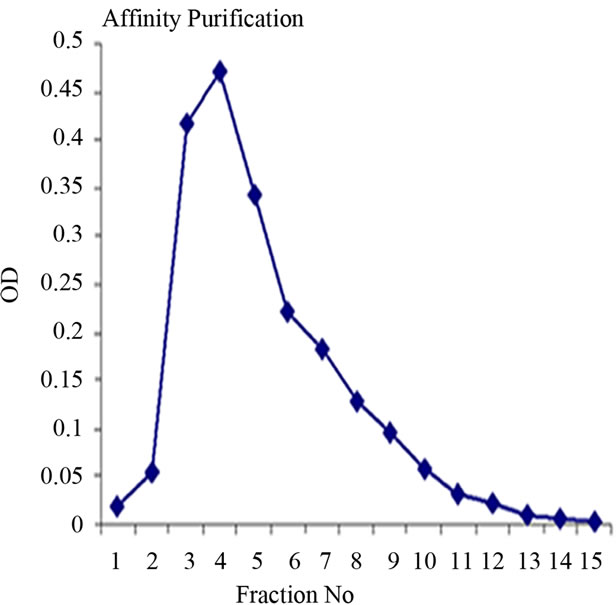 (c)
(c) (d)
(d)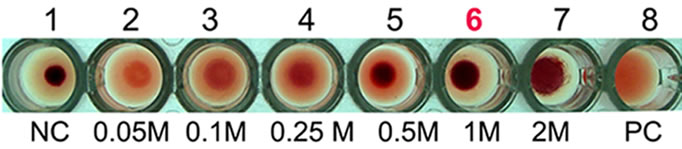 (e)
(e) (f)
(f)
Figure 1. Purification, haemagglutination, mannose inhibition and thermal stability assay of native AMTL. (a) Spectrophotometric representation (graphical) of affinity purified AMTL showing differential protein density (O.D) in different eluent fractions; (b) Graphical view of HPLC purification of 25 kDa pure native protein showing elution at 4.47 mins. (Inset) HPLC purification graph of ion exchange purified AMTL with major peak fraction of 25 kDa eluted at 4.45 mins; (c) SDS PAGE (15%) profile of crude and purified fractions of AMTL. Lane M: Standard protein molecular weight marker; Lane 1: Crude AMTL fraction; Lane 2: Affinity purified AMTL fraction; Lane 3: Ion exchange purified AMTL fraction; Lane 4: HPLC purified AMTL fraction; Lane 5: Western Blot of AMTL Showing ~12.5 kDa band; (d) Haemagglutination assay of native AMTL using rabbit erythrocytes. Well 1: No protein control; Well 2 to 10: Represent rabbit erythrocyte incubated with different AMTL concentrations (200 microgram∙ml–1 to 0.78 microgram∙ml–1 in serial 2 fold dilutions); Well 8: showing the onset (minimal dose for) of agglutination (3.12 microgram∙ml–1) AMTL concentration; (e) Inhibition studies of agglutination by mannose sugar. Well 1: No protein control; Well 2 to 7: Rabbit erythrocyte incubated with 25 microgram∙ml–1 AMTL presaturated with different concentrations of α-D mannose (50 mM to 2 M); Well 8: Positive control with rabbit erythrocyte & 25 microgram∙ml–1 AMTL but no mannose; Well 6: Showing complete inhibition of agglutination at 1M mannose concentration; (f) Thermal stability assay of AMTL. Well 1: Normal temperature control; Well 2-6: Rabbit erythrocyte incubated with different temperature treated AMTL (25 microgram∙ml–1 concentration); Well 4: Showing the loss of protein function at approximately >55˚C.
3.3. Thermal Stability Assay
Thermal stability assay was carried out with AMTL (25 µg∙ml–1 on rabbit erythrocytes. Complete loss of agglutination of AMTL was observed at approximately 55˚C (Figure 1(f), lane 4) that determined the characteristic thermolabile nature of AMTL.
3.4. Sugar Specificity of AMTL
Dissociation constant (Kd) of AMTL was calculated to be 0.13 μM for mannose and 0.40 μM for glucose which indicated high affinity of AMTL towards mannose rather than glucose (Figures 2(a) and (b)).
3.5. Artificial Diet Based Insect Bioassay
Artificial diet based toxicity assay conducted on insects like Lipaphis erysimi, Aphis gossypii and Dysdercus cingulatus showed increase in mortality with increasing concentrations of AMTL over a period of 72 hours (Figures 3(a)-(c)). The lethal concentration (LC50) determined by Probit analysis were 13.47 ± 0.230 µg∙ml–1, 7.78 ± 0.202 µg∙ml–1 and 14.05 ± 0.270 µg∙ml–1 for L. erysimi, A. gossypii and D. cingulatus respectively (Table 1).
3.6. Surface Plasmon Resonance Study
The adjusted sensorgram (Figure 4(a)) recorded in SPR study showed stable interaction between Liphaphis erysimi BBMV receptor proteins and AMTL. The stable binding feature was detected after evaluation of sensorgram using Biocore X100 Evaluation Software. BBMV proteins was shown to interact stably (represented by 105 RU) at a concentration of 7.5 ug per 60 ul Liphaphis erysimi BBMV
 (a)
(a)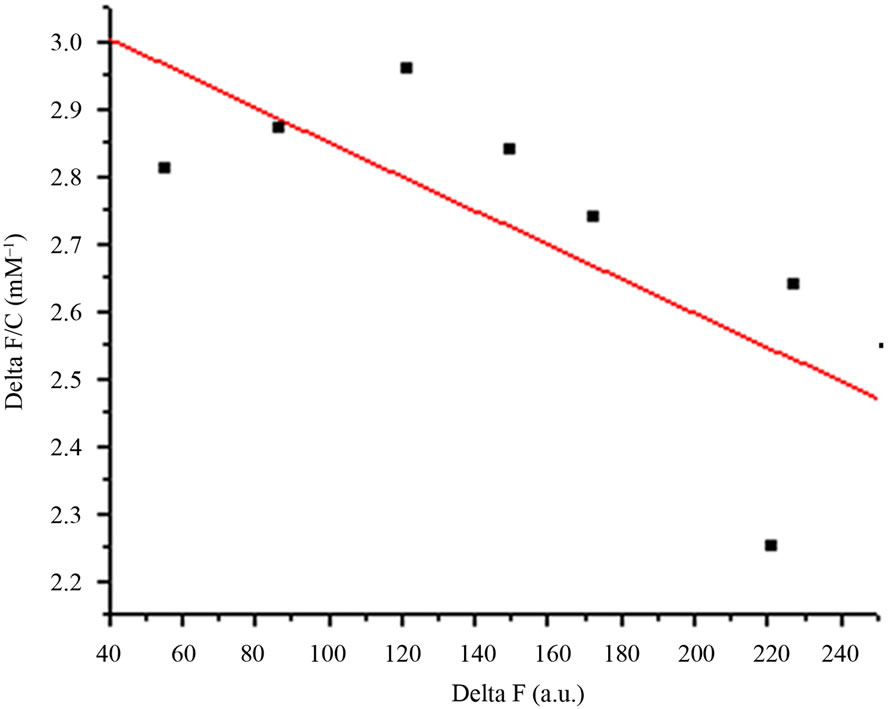 (b)
(b)
Figure 2. Comparative analysis (determination of kd) showing affinity of AMTL for mannose and glucose. (a) Binding of mannose with AMTL with dissociation constant 0.13 µM; (b) Binding of glucose with AMTL with dissociation constant 0.4 µM.
 (a)
(a)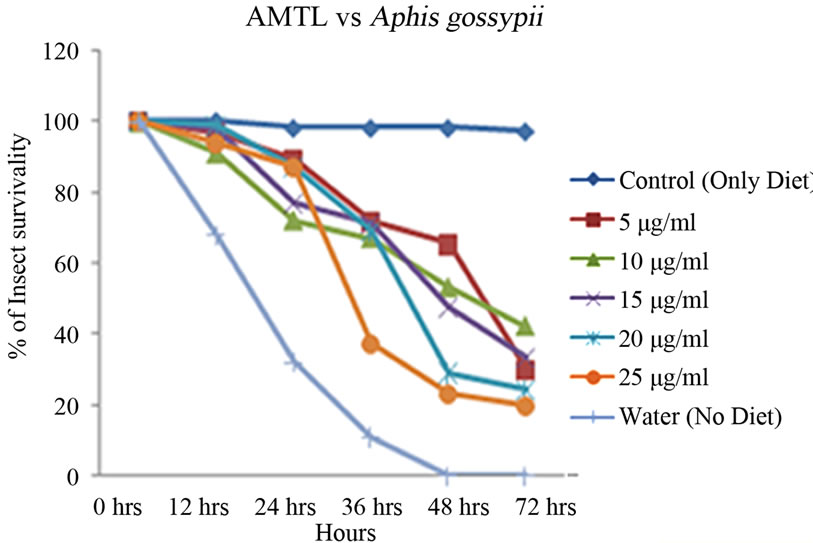 (b)
(b)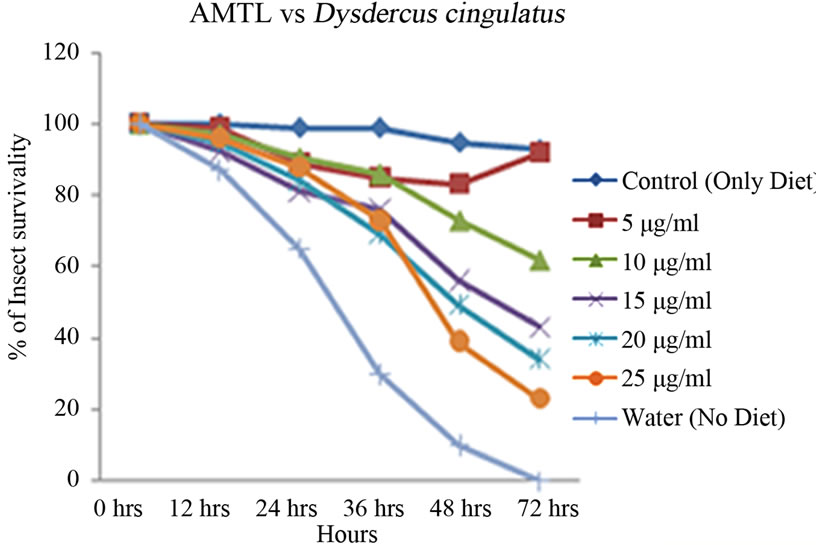 (c)
(c)
Figure 3. Insect bioassay in artificial diet. Insect mortality graph at different concentrations of AMTL in synthetic diet recorded over 72 hours in (a) Lipaphis erysimi; (b) Aphis gossypii; and (c) Dysdercus cingulatus.

Table 1. Toxicity assay of native AMTL determined against Lipaphis erysimi, Dysdercus cingulatus and Aphis gossypii.
 (a)
(a) (b)
(b)
Figure 4. Surface plasmon resonance sensorgram. Sensorgram of binding of AMTL with the Liphaphis erysimi BBMV proteins. Panel (a): Adjusted sensorgram of real time binding of Liphaphis erysimi BBMV proteins with the immobilized AMTL; Panel (b): Binding analysis of BBMV proteins with the immobilized AMTL.
proteins used in the experiment (Figure 4(b)).
3.7. Ligand Blot and Sugar Specificity Assay
BBMV preparations of Lipaphis erysimi separated in SDS PAGE (12%) (Figure 5, lane 1) was subsequently transferred to Hybond–C membrane .Then the membrane was incubated with purified AMTL and subsequently probed with anti AMTL antisera which detected the presence of ~74 kDa major putative receptor protein of AMTL (Figure 5, lane 2). Similar ligand assay performed independently with 1M α-D mannose saturated AMTL highlighted the absence of binding signal suggesting N linked mannosylated oligosaccharide mediated interaction between AMTL and the putative receptor protein of L. erysimi (Figure 5, lane 3).
3.8. Deglycosylation and Subsequent Ligand Blot Assay
Total BBMV fraction was subjected to further ligand blot assay after deglycosylation of the proteins. The ligand blot assay using anti AMTL antisera demonstrated complete loss of binding affinity to the deglycosylated receptor protein (~74 kDa) to AMTL (Figure 5, lane 5).
3.9. Phylogenetic Analyses and in Silico Homology Modeling of AMTL With GNA Related Lectins
Phylogenetic relatedness of AMTL (EU083312) with GNA related lectins was monitored using multialign program which showed that lectins belonging to GNA superfamily may be clustered in 2 major groups (Supplementary Figure 1). Each cluster was subdivided in two sub clusters (Supplementary Figure1). AMTL (highlighted) and GNA (highlighted) though grouped together in a large sub cluster but were found to be somewhat dis-
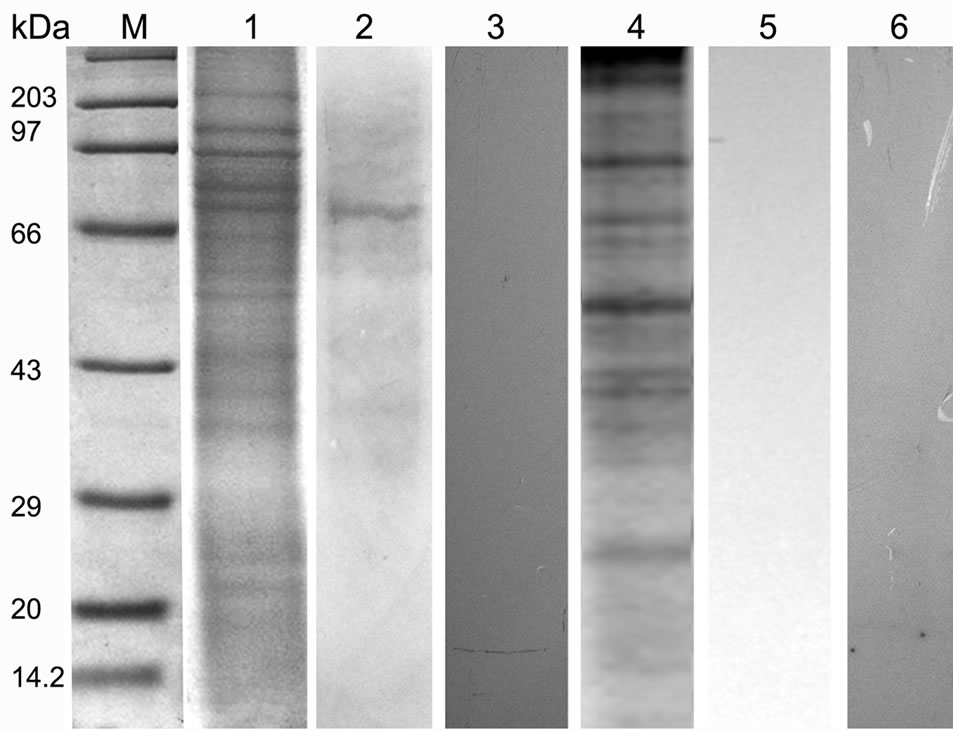
Figure 5. SDS PAGE (12%) profile showing ligand blot, mannose inhibition, deglycosylation assay of total BBMV protein of Lipaphis erysimi. Lane M: Standard protein molecular weight marker; Lane 1: Coomassie stained total BBMV fraction of Lipaphis erysimi; Lane 2: Ligand blot analysis of total BBMV of Lipaphis erysimi showing putative ~74 kDa receptor of AMTL; Lane 3: Ligand blot assay of the same AMTL used was presaturated with α-D mannose; Lane 4: Total protein profile of the BBMV (Coomassie stained) after deglycosylation experiment; Lane 5: Ligand blot assay with deglycosylated BBMV protein; lane 6: Negative control: Ligand blot of total BBMV without AMTL probe.
tant members when divided into further separate groups Supplementary Figure 1). Homology modeling of AMTL (Figure 6(a)) using GNA as template (Figure 6(b)) showed the three mannose substrate binding pockets in each monomer of AMTL to be similar to GNA (Figures 6(c)-(e)). The mannose or substrate binding pocket comprising of QXDXNXVXY residues were found to be conserved in both AMTL & GNA. The mannose subdomain 1 spanning from 89th to 97th amino acid residue in GNA while 92nd to 100th amino acid resuidue in AMTL.
 (a)
(a)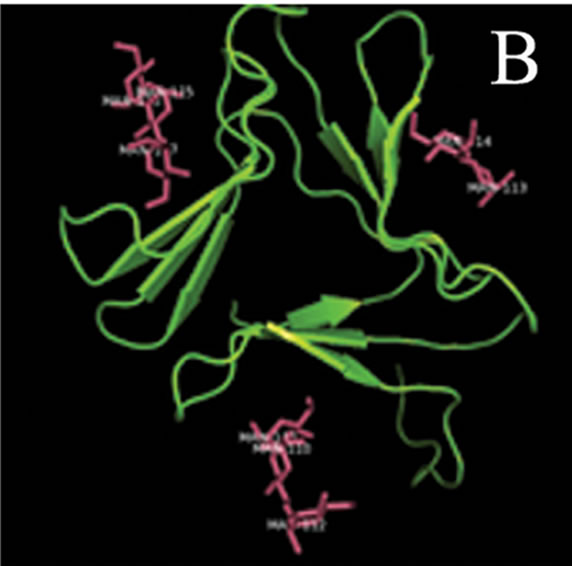 (b)
(b)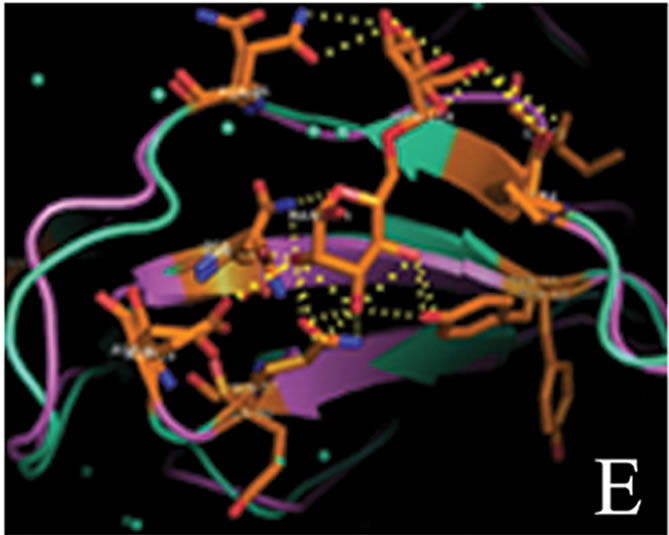 (c)
(c)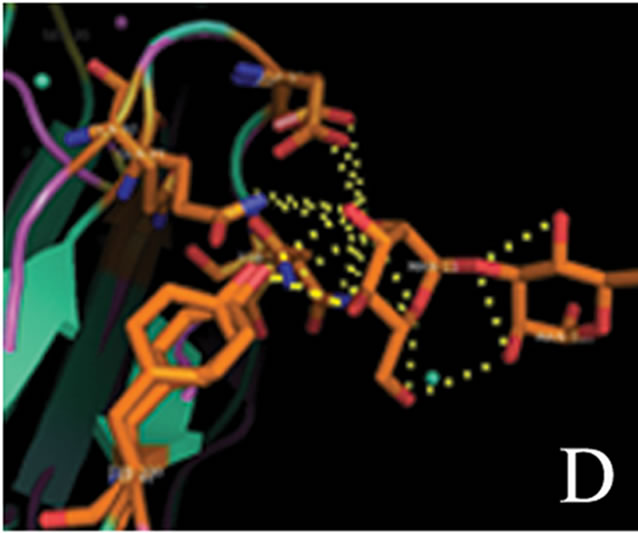 (d)
(d)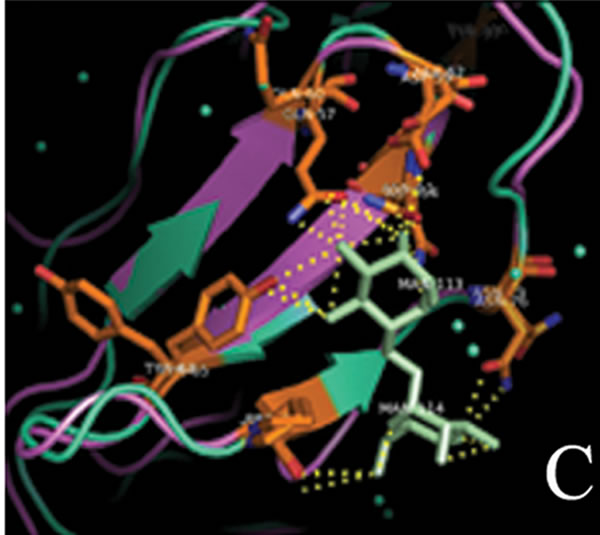 (e)
(e)
Figure 6. Models showing structural resemblance of AMTL with GNA lectin. Aligned AMTL and GNA (a); Crystal structure of GNA with three substrate mannose residues (b); Structural alignment of AMTL and GNA showing aligned mannose binding subdomain 3(c), subdomain 2(d) and subdomain 1(e).
The second subdomain traversed 57th to 65th amino acid position in GNA and 60th to 68th amino acid position in AMTL. The third subdomain occupied 26th to 34th amino acid in GNA, whereas it spanned 28th to 36th amino acid in AMTL.
4. Discussion
Plant lectins are reported to be effective components in hemipteran pest management. Snowdrop mannose specific lectin (GNA) isolated from Galanthus nivalis is renowned as one of the well worked out member having adverse effects on the development, fecundity as well as survivality of hemipteran insects [14-17]. However, keeping the public debate and social concern in mind, GNA a product of non food plant invited further look out for optional edible sources of lectins as insecticidal [3]. In the following years the efficacy of other monocot mannose specific lectins isolated from different sources such as Allium sativum (ASAL), Diffenbechia sequina (DEA), Colocasia esculentum (CEA), Arum maculatum (ATL) against hemipteran pests have been documented [12, 18-22]. But the mode of action of these lectins still remains elusive. At the same time the effectivity of a single insecticidal protein is thought to decrease over time and generation as a result of insect behavioural reorientation leading to host manipulation; a defensive strategy implemented by the insects in due course of evolution [23]. Focussing on this issue in the present report, the potentiality of an edible lectin isolated from Amorphophallus paeonifolius tuber (AMTL) conferring toxicity to different members of hemipteran class of insects has being illustrated.
4.1. The Native Protein Characterization
Similar to different monocot mannose binding GNA related lectins identified earlier by the present investigating group [6,12,18,19], AMTL a newly identified lectin was also found to be of stable dimeric nature with 25 kDa MW which resolved as approximately 12.5 kDa monomeric band in 15% SDS PAGE and was further validated in western blot analysis. The lectin like nature of AMTL was confirmed by haemagglutination assay. The agglutination property was however reverted by preincubating 3.12 ug/ml (minimal concentration for agglutination) of AMTL with 150 mM of α-D mannose and 25 ug/ml (concentration causing complete agglutination) of AMTL with 1 M of α-D mannose suggesting AMTL to have similar kind of specificity towards mannose sugars comparable with other previously reported lectins from our group [6, 12] . The complete agglutinating concentration of AMTL was further used to monitor the thermal stability of the protein which was found to be stable up to 55˚C.
Previous studies have established the fact that monocot mannose binding lectin ASAL binds to alpha D-mannose with calculated Kd 0.06 mM for single mannose moiety [24]. Since native AMTL belongs to monocot mannose binding lectin superfamily, binding of AMTL towards mannose was assumed. However whether AMTL had similar affinity towards other sugars, apart from mannose, was analysed using Kd calculation which showed that undoubtedly AMTL showed much higher affinity towards mannose than glucose. This data further revalidated the mannose mediated inhibition of haemagglutination.
The potentiality of AMTL as an effective insecticidal protein was analysed further by bioassays conducted on L. erysimi (mustard aphid), A. gossypii (cotton aphid) and D. cingulatus (red cotton bug). The LC50 value of AMTL against mustard aphid was reported to be lower compared to ATL and ASAL [6,12] whereas the LC50 value against Red Cotton Bug was calculated to be similar to ASAL but lower than DEA and CEA [18] indicating that the degree of effectivity of a particular lectin on a wide range of insect pest is variable. However, the LC50 value of AMTL against cotton aphid was 7.78 µg∙ml–1 which to the best of our knowledge is the first significant report of documenting the efficacy of a mannose specific monocot lectin against cotton aphid.
4.2. Interaction of AMTL with Insect Gut Membrane Receptor
The insecticidal efficacy of AMTL triggered a further inquest for looking in depth into the mode of action of the protein. Lectins, well established as potential insecticidal toxins are assumed to cause detrimental effects when bound to insect midgut epithelial cell [12]. Hence, whatever be the mechanism of action of lectins, binding of the protein to insect plays pivotal role in conferring resistance against herbivory. In the present study SPR sensogram revealed a stable interaction between AMTL and L. erysimi membrane receptor proteins thus paving the path towards identification of its cognate insect receptor of AMTL.
4.3. Identification, Nature and Specificity of the Insect Receptor
Ligand blot analysis performed with BBMV of L. erysimi highlighted a ~74 kDa putative receptor of AMTL. Previous receptors identified from L. erysimi using anti ATL and anti ASAL as probe were found to be ~40 kDa and ~56 kDa proteins respectively [6,12]. Further experiments conducted with AMTL presaturated with α-D mannose showed loss of binding to the ~74 kDa putative receptor of L. erysimi revalidating the specificity of the protein towards mannose residues. Inability of detecting any such receptor protein in ligand blot after deglycosylating the receptor protein was indicative of glycospecific nature of the receptor. Since the hemipteran insects rely mostly on free amino acids for their nutrition rather than on digestive enzymes like other lepidopteran, dipteran or coleopteran class of insects, the possibility of AMTL binding preferentially to such sparingly available digestive enzymes can be statistically ruled out [12]. Previous reports suggested that ASAL interacts with a chaperonin like protein symbionin (SymL) a GroEL homologue, encoded by mutuallistic Buchnera aphidicola that are known to reside in the gut of L. erysimi for supplying them free essential amino acids. SymL is also known to interact with the Read through domain (RTD) of pathogenic viruses. Earlier reports have shown the efficiency of ASAL in gaining over the competition by binding with SymL, thus indirectly obstructing viral transmission [25]. However similar role of AMTL demands further experimental authentication.
4.4. Relative Position of AMTL in the Phylogenetic Tree of GNA Related Plant Lectins
The phylogeny of AMTL (EU083312) and its relatedness with GNA like mannose binding lectins were studied which showed both GNA and AMTL though grouped together under large major cluster, fell apart when further subdivided into minor sub clusters. This distance could probably be attributed to their oligomerization status (AMTL-dimeric, GNA-tetrameric) and sequence dissimilarity (Suppl Figure 1). However when subjected to in silico structural alignment both AMTL and structurally solved GNA were found to be almost aligned in respect to their substrate binding sites known to be encoded by the conserved QDNVY motif [26].
5. Conclusion
The above results potentiate the incorporation of AMTL as a promising insecticidal protein effective against sap sucking hemipteran pests. Interaction of insecticidal components to the insect midgut receptor remains the primary criterion for conferring toxicity [27] which was revalidated by the binding experiment using SPR. The cognate insect receptor of AMTL was identified to be a ~74 kDa, glycoprotein. AMTL could probably have anti metabolic effects on the insect mid gut causing either nutrient leaching [28] or anti absorption detrimental effects as shown in case of Giardia cyst wall protein 1 resembling the characteristic features of lectin [29]. Such ambiguity regarding the exact mode of action of this lectin can however be removed by detailed structural and functional characterization of the insect receptor which needs to be addressed at length. Nonetheless, the structural as well as functional resemblance between AMTL and insecticidal GNA provides a strong platform for AMTL to be enlisted as a promising insecticidal candidate protein effective against sap sucking insects.
6. Acknowledgements
We would like to thank Dr. K. R. Kranthi, Central Institute of Cotton Research Nagpur, for his critical suggestions during the insect bioassay experiments. We would like to extend our special thanks to Prof. Kalipada Das, Dept of Chemistry, Bose Institute for his suggestions during data interpretation of sugar specificity assay. Authors are thankful to Bose Institute for infrastructural facility and to Department of Biotechnology, Government of India for financial support (Sanction no—BT/PR9593/ AGR/02/444/07 dt. 17.09.2007 and BT/01/CoE/06/03 dt. 04.12.2006). Technical support of Mr. Arup Kumar Dey, Mr. Swarnava Das and Mr. Sudipta Basu are duly acknowledged.
REFERENCES
- J. Waage, “What Does Biotechnology Bring to Integrated Pest Management?” Biotech Develop Monitor, No. 32, 1997.
- T. Watanabe and H. Kitagawa, “Photosynthesis and Translocation of Assimilates in Rice Plants Following Phloem Feeding by the Planthopper Nilaparvata lugens (Homoptera: Delphacidae),” Journal of Economic Entomology, Vol. 93, No. 4, 2000, pp. 1192-1198. doi:10.1603/0022-0493-93.4.1192
- E. Fitches, D. Wiles, A. E. Douglas, G. Hinchliffe, N, Audsley and J. A. Gatehouse, “The Insecticidal Activity of Recombinant Garlic Lectins Towards Aphids,” Insect Biochemistry and Molecular Biology, Vol. 38, No. 10, 2008, pp. 905-915. doi:10.1016/j.ibmb.2008.07.002
- C. Deraison, I. Darboux, L. Duportets, T. Gorojankina, Y. Rahbe and L. Jouanin, “Cloning and Characterization of a Gut-Specific Cathepsin L from the Aphid Aphis gossypii,” Insect Molecular Biology, Vol. 13, No. 2, 2004, pp. 165- 177. doi:10.1111/j.0962-1075.2004.00474.x
- W. J. Peumans and E. J. M. Vandamme, “Lectins as Plant Defense Proteins,” Plant Physiology, Vol. 109, No. 2, 1995, pp. 347-352. doi:10.1104/pp.109.2.347
- P. Majumder, H. A. Mondal and S. Das, “Insecticidal Activity of Arum Maculatum Tuber Lectin and Its Binding to the Glycosylated Insect Gut Receptors,” Journal of Agriculture and Food Chemistry, Vol. 53, No. 17, 2005, pp. 6725-6729.
- U. K. Laemmli, “Cleavage of Structural Proteins during Assembly of Head of Bacteriophage-T4,” Nature, Vol. 227, No. 5259, 1970, pp. 680-688. doi:10.1038/227680a0
- M. M. Bradford, “Rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing Principle of Protein-Dye Binding,” Anual Review of Biochemistry, Vol. 72, No. 1-2, 1976, pp. 248-254.
- E. Harlow, “Antibodies: A Laboratory Manual,” Cold Spring Harbour Laboratory, New York, 1988, pp. 60-67.
- R. Dadd and T. Mittler, “Permanent Culture of an Aphid on a Totally Synthetic Diet,” Cellular and Molecular Life Sciences, Vol. 22, No. 12, 1976, pp. 832-833.
- A. Fersht, “Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding,” Cambridge University Press, New York, 1999, p. 208.
- S. Bandyopadhyay, A. Roy and S. Das, “Binding of Garlic (Allium sativum) Leaf Lectin to the Gut Receptors of Hemipteran Pests Is Correlated to Its Insecticidal Activity,” Plant Science, Vol. 161, No. 5, 2001, pp. 1025-1033. doi:10.1016/S0168-9452(01)00507-6
- H. Chi, “Computer Program for the Probit Analysis,” National Chung Hsing University, Taichung, 1997.
- A. Gatehouse, R. Down, K. Powell, N. Sauvion, Y. Bahbe, C. A. Newell, A. Merryweather, W. D. O. Hamilton and J. A. Gatehouse, “Transgenic Potato Plants with Enhanced Resistance to Peach-Potato Aphid Myzus persicae,” Entomologia Experimentalis et Applicata, Vol. 79, No. 3, 1996, pp. 295-307.
- K. S. Powell, J. Spence, M. Bharathi, J. A. Gatehouse and A. M. R. Gatehouse, “Immunohistochemical and Developmental Studies to Elucidate the Mechanism of Action of the Snowdrop Lectin on the Rice Brown Planthopper, Nilaparvata lugens (Stal),” Journal of Insect Physiology, Vol. 44, No. 7-8, 1998, pp. 529-539. doi:10.1016/S0022-1910(98)00054-7
- N. T. Loc, P. Tinjuangjun, A. M. R. Gatehouse, P. Christou and J. A. Gatehouse, “Linear Transgene Constructs Lacking Vector Backbone Sequences Generate Transgenic Rice Plants Which Accumulate Higher Levels of Proteins Conferring Insect Resistance,” Molecular Breeding, Vol. 9, No. 4, 2002, pp. 231-244. doi:10.1023/A:1020333210563
- S. Ramesh, D. Nagadhara, V. D. Reddy and K. V. Rao, “Production of Transgenic Indica Rice Resistant to Yellow Stem Borer and Sap Sucking Insects, Using SuperBinary Vectors of Agrobacterium tumafaciens,” Plant Science, Vol. 166, No. 4, 2004, pp. 1077-1085. doi:10.1016/j.plantsci.2003.12.028
- A. Roy, S. Banerjee, P. Majumder and S. Das, “Efficiency of Mannose-Binding Plant Lectins in Controlling a Hemipteran Insect, the Red Cotton Bug,” Journal of Agricultural Food Chemistry, Vol. 50, No. 23, 2002, pp. 6775-6779. doi:10.1021/jf025660x
- P. Majumder S. Banerjee and S. Das, “Identification of Receptors Responsible for Binding of the Mannose Specific Lectin to the Gut Epithelial Membrane of the Target Insects,” Glycoconjugate Journal, Vol. 20, No. 9, 2004, pp. 525-530. doi:10.1023/B:GLYC.0000043288.72051.7c
- P. Saha, P. Majumder, I. Dutta, T. Ray, S. C. Roy and S. Das, “Transgenic Rice Expressing Allium sativum Leaf Lectin with Enhanced Resistance against Sap-Sucking Insect Pests,” Planta, Vol. 223, No. 6, 2006, pp. 1329-1343. doi:10.1007/s00425-005-0182-z
- D. Chakraborti, A. Sarkar, H. A. Mondal and S. Das, “Tissue Specific Expression of Potent Insecticiadal, Allium sativum Leaf Agglutinin (ASAL) in Important Pulse Crop, Chickpea (Cicer arietinum L.) to Resist the Phloem Feeding Aphis craccivora,” Transgenic Research, Vol. 18, No. 4, 2009, pp. 529-544. doi:10.1007/s11248-009-9242-7
- S. Sengupta, D. Chakraborti, H. A. Mondal and S. Das, “Selectable Antibiotic Resistance Marker Gene Free Transgenic Rice Harbouring the Garlic Leaf Lectin Gene Exhibits Resistance to Sap Sucking Planthoppers,” Plant Cell Report, Vol. 29, No. 3, 2010, pp. 261-271. doi:10.1007/s00299-010-0819-7
- M. S. Chen, “Inducible Direct Plant Defense against Insect Herbivores: A Review,” Insect Science, Vol. 15, No. 2, 2008, pp. 101-114. doi:10.1111/j.1744-7917.2008.00190.x
- N. Banerjee, S. Sengupta, A. Roy, P. Ghosh, K. Das and S. Das, “Functional Alteration of a Dimeric Insecticidal Lectin to a Monomeric Antifungal Protein Correlated to Its Oligomeric Status,” PLoS ONE, Vol. 6, No. 4, 2011. doi:10.1371/journal.pone.0018593
- S. Banerjee, D. Hess, P. Majumder, D. Roy and S. Das, “The Interactions of Allium sativum Leaf Agglutinin with a Chaperonin Group of Unique Receptor Protein Isolated from a Bacterial Endosymbiont of the Mustard Aphid,” Journal of Biological Chemistry, Vol. 279, No. 22, 2004, pp. 23782-23789. doi:10.1074/jbc.M401405200
- N. R. Chandra, G. Ramachandraiah, K. Bachhawat, T. K. Dam, A. Surolia and M. Vijayan, “Crystal Structure of a Dimeric Mannose-Specific Agglutinin from Garlic: Quaternary Association and Carbohydrate Specificity,” Journal of Molecular Biology, Vol. 285, No. 3, 1999, pp. 1157-1168. doi:10.1006/jmbi.1998.2353
- A. E. Douglas, D. R. Price, L. B. Minto, E. Jones, K. V. Pescod, C. L. Francois, J. Pritchard and N. Boonham, “Sweet Problems: Insect Traits Defining the Limits to Dietary Sugar Utilisation by the Pea Aphid, Acyrthosiphon pisum,” Journal of Experimental Biology, Vol. 209, No. 8, 2006, pp. 1395-1403. doi:10.1006/jmbi.1998.2353
- A. Koornneef and C. M. Pieterse, “Cross Talk in Defense Signaling,” Plant Physiology, Vol. 146, No. 3, 2008, pp. 839-844. doi:10.1104/pp.107.112029
- A. Chatterjee, A. Carpentieri, D. M. Ratner, E. Bullitt, C. E. Costello, P. W. Robbins and J. Samuelson, “Giardia Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer,” PLoS Pathogogy, Vol. 6, No. 8, 2010.

Supplementary Figure 1. Dendogram showing relatedness of AMTL with GNA.
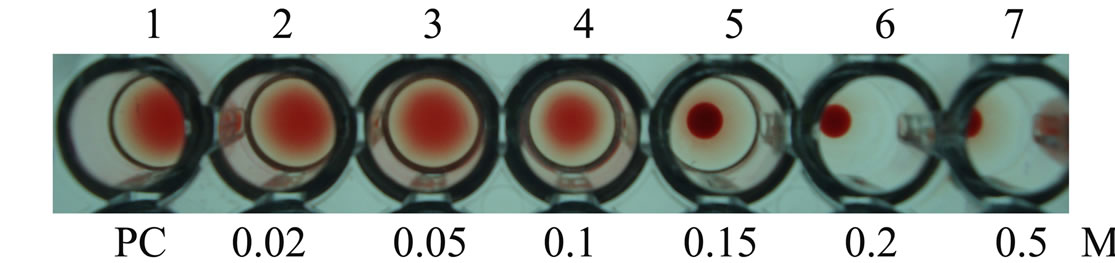
Supplementary Figure 2. Inhibition studies of agglutination by mannose sugar. Well 1: AMTL protein control; Well 2 to 7: Rabbit erythrocyte incubated with 3.12 microgram∙ml–1 AMTL presaturated with different concentrations of α-D mannose (20 mM to 0.5 M); Well 5: Showing complete inhibition of agglutination at 150 mM mannose concentration.
NOTES
*Equal contribution.
#Corresponding author.

