Pharmacology & Pharmacy
Vol.4 No.2(2013), Article ID:29754,9 pages DOI:10.4236/pp.2013.42029
Microdoses Levels of Butadiene Diepoxide (BDO2) Induced Toxicity in Prostate Cancer Cells
![]()
1Department of Environmental Toxicology, Southern University, Baton Rouge, USA; 2Department of Biological Sciences, Baton Rouge, USA; 3Department of Chemistry, Southern University, Baton Rouge, USA.
Email: wesley_gray@subr.edu
Copyright © 2013 Sowmya Koppula et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 16th, 2013; revised February 20th, 2013; accepted April 3rd, 2013
Keywords: BDO2; Cytotoxicity; Gene Expression; Prostate; Micro-Dose
ABSTRACT
Low-level exposure to environmental pollutants such as BDO2 contributes directly and indirectly to an increase in PCa. The aim of this study was to define the cellular changes associated with micro-doses of Butadiene Diepoxide (BDO2) in prostate cancer cells. We observed that micro-doses of BDO2 resulted in doseand time-dependent increases in cytotoxicity and increased expression of prostate tumor markers in LNCaP(AR+) and DU145(AR−) cells. There was an increased sensitivity of DU145(AR−) cells to BDO2 toxicity which was reversed by transient transfection of AR into theses cell. Exposure of prostate cells to BDO2 increases cytotoxicity, and apoptosis, which correlates with increases in caspases and Bcl2 protein and mRNA levels. In cell DU145(AR−) cell transient transfected with a functional AR, the levels of cytotoxicity and caspase activity were decreased in the presence of BDO2, but BDO2-induced apoptotic protein expression was unaltered. This study provides evidence that micro-doses of BDO2 modulate prostate cell toxicity by promoting apoptosis and tumor gene expression.
1. Introduction
1,3-Butadiene is a gas used commercially in the production of styrene-butadiene rubber, plastics, and thermoplastic resins with the major environmental source been incomplete combustion of fuels from mobile sources (e.g., automobile exhaust). Tobacco smoke can be a significant source of 1,3-butadiene in indoor air. The reactive intermediates 1,2-epoxy-3-butene, 1,3,4-diepoxybutane, and 3,4-epoxy-1, 2-butanediol all play significant roles in the toxicity of 1,3-butadiene. These metabolites are capable of reacting with macromolecules such as DNA to induce a variety of genotoxic effects in mice and rats as well as in human cells in vitro [1-6]. The metabolism and genetic toxicity of 1,3-butadiene and its oxidative metabolites in humans and rodents is well established. Experimental animal studies support the theory that butadiene and its metabolites are human carcinogenic agents [3, 7-10]. Theses animal studies have suggest a specie difference in the carcinogenicity of 1,3-butadiene in mice and rats. Tumor induced by 1,3-butadiene occurs in the hematopoietic system—heart (hemangiosarcomas), lung, preputial gland, liver, mammary gland, ovary, and kidney and prostate [9-13]. Although the tumors induced by 1,3-butadiene in these tissues are thought to be due to genotoxic alteration, the exact genes that are mutated or altered in each type of tumor are unknown.
The mechanism of tumor induction by 1,3-butadiene in rodents and humans may be due to its metabolism to DNA-reactive intermediates, resulting in genetic alterations in protooncogenes and/or tumor suppressor genes. What is known, however, is that there is a quantitative relationship between exposure to 1,3-butadiene, its genotoxicity, and the induction of cancer in occupationally exposed male workers. To date, there have been no studies that examined the effects of 1,3-butadiene and its metabolites Butadiene Diepoxide (BDO2) on male reproductive development or development of the prostate gland. BDO2, the most active metabolite of 1,3-BD, a potential human carcinogen, is released into the environment as a result of petroleum byproducts, smoking or combustion of gasoline products. Once in ambient air, it readily enters the body by several routs such as inhalation or absorption through the skin where it is metabolized to a BDO2 by cytochrome P450s [4,13]. In the body, BDO2 may induce reproductive toxicity in target tissue such as ovaries, testis, and prostate [1,8,14]. However, the biochemical mechanism of BDO2 toxicity in prostate and BDO2’s effects on prostate cell function are undefined. Therefore, the objective of this study was to define some of the cellular changes that are associated with BDO2 toxicity in prostate cells under androgen sensitive (LNCaP(AR+)) and androgen insensitive DU-145 (DU145(AR−)) conditions. We examined the effect of butadiene diepoxide in prostate by assessing its effect on the growth of LNCaP(AR+) cells, production and secretion of prostate secretary protein, androgen receptor status, and induction of androgen dependent genes.
2. Material and Methods
2.1. Cell Culture
LNCaP(AR+) and DU145(AR−) cells were obtained from ATCC (Rockville, MD). Cells were maintained in RPMI 1640 (LNCaP(AR+) or Kaighn’s modification of Ham’s F-12 (F12-K) medium supplemented with 10% FBS, 0.2 mM glutamine, 100 U/ml of penicillin, and 100 mg/ml streptomycin. Cells were kept in 5% CO2 in a waterjacketed incubator and were passaged using a trypsin/ EDTA solution (Sigma-Aldrich, Inc.) when they reached 80% - 90% confluence.
2.2. Cell Viability Analysis
For experiments involving cell growth and gene induction, LNCaP(AR+) or DU145(AR−) cells were grown for five days in RPMI 1640 medium containing 5% FBS that was stripped three times with dextran-coated charcoal. Cells were then grown for 24 hr in Cellgro® serum free-medium. Cells were plated in 96-well plates (8 × 105 cells/well) and allowed to attach overnight. BDO2 in 0.1% DMSO was added in a series of concentrations (0, 10, or 100 nM) to a 96-well plate. As a control and a reference, 10−8 M DHT and 100 ng/ml TNF-α were added to separate wells of each plate. Each treatment and time point had eight replicates. In each treatment, the final concentration of vehicle solvent did not exceed 0.01% v/v in the medium. After 24 h exposure to the test compounds, the effect on cell viability and gene expression was determined. Cytotoxicity was determined by the CellTiter 96Ò AQueous One Solution cell proliferation assay (Promega, Madison, WI) according to the manufacturer’s instructions. After incubation with 3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), absorbance at 490 nm was measured using a ELX800UV universal microplate reader (Bio-Tek, Inc.). Cell viability was calculated as .
.
For Tryphan blue staining, cells were plated in 12-well plates (10,000 cells/well) and induced with 100 nM BDO2 for 24 hr. After induction, cells were harvestedstained with Tryphan blue, and the number of cells was determined using a hemocytometer.
2.3. RNA Extraction and Real Time RT-PCR Analysis
Total RNA was obtained from cells treated with 0, 10, or 100 nM BDO2, 10 nM DHT or 5000 nM Resveratrol in the presence or absence of 50-fold flutamide for 24 hr by lysing with 1.0 ml of TRI reagent (in vitrogen). RNA was isolated and Taqman PCR was performed on the cDNA samples using an ABI PRISM 7500 Sequence Detection system (Applied Biosystems) as previously described [15,16]. Briefly, for each gene tested (see Table 1), PCR was carried out in a multiplex mode with each 25 μl reaction containing 5 μl of cDNA reaction (~100 ng), An increase in fluorescence was obtained at the annealing and extension step at 60˚C. The relative level of expression of each gene in the samples was determined using the relative 2DDCt expression method as described in detail in the ABI PRISM Sequence Detection system User Bulletin 2 [17].
2.4. Fluorescence Microscopy
For microscopy, 5 × 105 cells (LNCaP(AR+) or DU145(AR−) were grown on microscope slides and induced with 0, 10 and 100 nM of BDO2 for 24 h. On each slide, cells were stained for 5 min with 5 µl of a 0.1 μg/μl solution of acridine orange and ethidium bromide. Two fluorescence parameters, green emission from acridine orange (525 nm) and red emission from ethidium bromide (620 nm) were examined under a fluorescence light microscope (Nikon Optiphot, Melville, NY, USA) for the nuclear changes that are typically associated with apoptosis. An index of apoptosis was calculated as the ratio of the number of cells per microscopic field with early and late apoptosis characteristics in treated samples relative to the total number of cells per microscopic field.
2.5. Analysis of Caspase-3/7 Activation
Caspase 3/7 activity was determined using an Apo-OneÒ Homogeneous Caspase-3/7 Assay kit (Promega, Madison, WI) as previously described [16]. Caspase-3/7-like activity was determined based on proteolytic cleavage of rhodamine 110, bis-(N-CBZ-L-aspartyl-L-glutamyl-L-valylL-aspartic acid amide, Z-DEVD-R110) using 100 µg of total protein.
2.6. Western Blot Analysis
Immunoblot of the LNCaP(AR+) or DU145(AR−) protein fraction was performed as previously described [18,19]. The membranes were immunostained with the following antibodies: anti-Bcl2 0.5 µg/ml and anti-Bax 0.5 µg/ml Table 1. Regulation of androgen receptor target genes by butadiene diepoxide (BDO2) in prostate cancer cells.
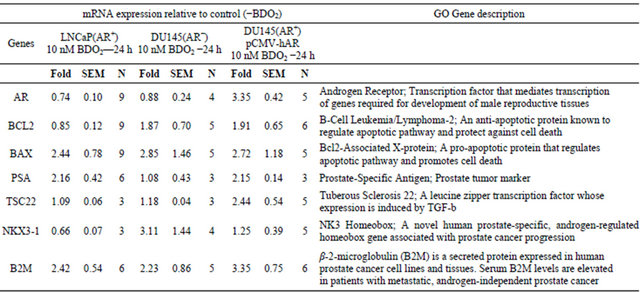
Gene expression was measured using the two-step Taqman Real Time RT-PCR as described in Materials and Methods. The relative expression of each gene was calculated by the 2∆∆Ct method. First, relative quantitation mRNA expression was performed by first normalizing the Ct values of the particular gene amplification against the Ct values of endogenous 18 S rRNA then the resulting Ct values were normalized using the Ct value of the vehicle control sample. The relative expression of the 0.0 mM BDO2 control for each gene was set to zero.
(Santa Cruz Biotechnology Inc., Santa Cruz, CA), antiAR 1.5 µg/ml, anti-PARP 0.5 µg/ml and anti-GDPH 0.5 µg/ml (Cell Signaling, Danvers, MA). The immunoblot signal was captured using an AlphaInnotech Fluorochem HD 9900 (Alpha Innotech, San Leandro, CA) equipped with a CDD camera. The images were analyzed with the AlphaEaseFC software (AlphaInnotech, San Leandro, CA) and curves and graphs were fitted with GraphPad Prism 5.0 software (GraphPad, San Diego, CA) [20].
2.7. Immunofluorescence Staining
DU-145 cells were transiently transfected with AR expression plasmid (0.79 µg/µl pCMVh-AR) and 72-hr post transfection cells were induced with 100 nM BDO2. Twenty-four hours post BDO2 induction, cells were fixed with 100% methanol (−20˚C for 10 min) and then cross linked with 4% paraformaldhye at room temp for 10 min. The slides were blocked with 1% rabbit serum solution at room temperature for one hour. Slides were probed with anti-AR at a 1:100 dilution for 1 hr at room temperature, washed and then incubated with fluorescence-labeled anti-rabbit IgG (1:5000 dilution) for an additional hour at room temperature. For dual antibody staining, slides were washed with TBS-T and blocked in 10% sheep serum for 1 hr and probed with anti-tubulin (1:200) for 1 hr. The tubulin signal was developed by addition of Cy3-labeled anti-mouse IgG for 1 hour. Slides were washed with TBS and stained with prolong gold anti-fade reagent containing DAPI (4,6-diamidino-2-phenylindole). Slides were visualized using a Nikon Optiphot fluorescent microscope with green fluorescent (525 nm) and red fluorescent (620 nm) filters.
2.8. Statistical Analysis
All numerical data were expressed as mean ± SEM. In each assay, three or four measurements were made. Means for the treatment groups were compared using analysis of variance and Duncan’s multiple range test (P < 0.05). To analyze the absorbance density from western blot data, a two-tailed t test (P < 0.05) was used to compare the mean (n = 3) for each treatment group with the mean for the untreated control group. The GraphPad Prism 5.0 software program (GraphPad, San Diego, CA) was used for the statistical analyses [20].
3. Results
3.1. The Androgen Receptor Negative Prostate Cells Are More Sensitive to BDO2 Exposure
In the body, BDO2 may induce reproductive toxicity in target tissue such as ovaries, testis, and prostate (Anderson 1998; Boffetta et al. 2009; Schmiederer et al. 2005). However, the biochemical mechanism of BDO2 toxicity in prostate and BDO2’s effects on prostate cell function are undefined. Therefore, the objective of this study was to define some of the cellular changes that are associated with BDO2 toxicity in prostate cancer cells under androgen sensitive (LNCaP(AR+)) and androgen insensitive DU-145 (DU145(AR−)) conditions. The first objective was to establish an appropriate response of BDO2 in prostate cells by determining whether micro-doses of BDO2 exhibit any cellular effect on prostate derived LNCaP(AR+) and DU145(AR−) cells. To answer this question, we assessed whether micro-doses of BDO2 were toxic to both LNCaP(AR+) and DU145(AR−) prostate cells. Exposure of prostate cancer cells to 10 nM BDO2 for 24 hr, a dose similar to 6 hr of exposure to the parent 1,3-BD compound, significantly decreased cell viability in both cells lines (Table 2). We observed that 10 nM BDO2 induced a 25% - 30% decrease in cell viability in LNCaP(AR+) and DU145(AR−) cells. In control experiments, exposing both cell lines to 100 ng/ml TNF-α (as a positive control for cell death in these cell lines) resulted in a 56% ± 11% and 41% ± 5.3% decrease in the cell viability of LNCaP(AR+) and DU145(AR−) cells, respectively. Surprisingly, exposure of prostate cells to a high concentration (100 nM) of BDO2, produced only a marginal decrease (~7.0%) in cell viability in LNCaP(AR+) cells but a significant decrease (41%) in DU145(AR−) cells after a 24-hr exposure. These data demonstrate a dose-dependent effect of BDO2 on cell viability in DU145(AR−) and suggest that these cells are more sensitive to BDO2 than LNCaP(AR+).
3.2. BDO2 Induces Apoptosis in Prostate Cancer Cells at Low Concentration
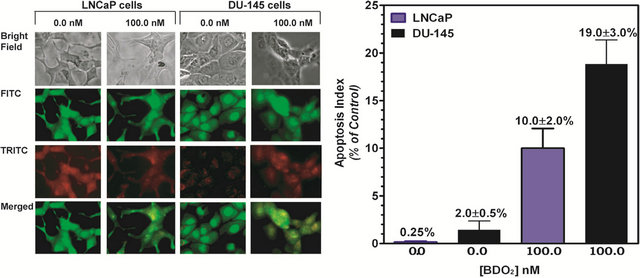
To corroborate the viability assessment of BDO2 in prostate cells and reconcile the apparent difference in sensitivity between the two-cell lines, we investigated whether the cytotoxicity of BDO2 was related to the apoptosis status of these cells. LNCaP(AR+) or DU145(AR−) cells (5 × 105) were grown on microscope slides, induced with 100 nM BDO2, then prepared for microscopic examination as described in Materials and Methods.
Slides were examined under a fluorescent light microscope for the nuclear changes that are typical of necrotic or apoptotic cells (Figure 1). Fluorescent staining with acridine orange and ethidium bromide revealed signs of nuclear condensation, nuclei fragmentation, and membrane budding, which are all hallmark features of apoptosis, and fewer cells showing signs of necrosis. Microscopic examination of apoptotic cells revealed that there was a dose-dependent increase in the number of apoptotic cells following 24 hr exposure to BDO2 (Figure 1). Examination of cells treated with BDO2 under phasecontrast microscopy showed a dramatic decrease in cell number and cellular shrinkage in DU145(AR−) cells as compared to LNCaP(AR+) cells Figure 1(a). The apoptotic index was determined by quantifying the relationship between concentration of BDO2 and the number of apoptotic cells Figure 1(b). We calculated the apoptotic index by averaging the number of apoptotic cells per field (60 - 70 cells) then dividing by the total number of cells per field (120 - 140 cells). A concentration of 100 nM BDO2 resulted in a 19% ± 3% increase in apoptosis in DU145(AR−) cells as compared to an increase of 10% ± 2% in LNCaP(AR+) cells Figure 1(b). Thus, DU145 (AR−) cells were twice as sensitive to BDO2 than LNCaP(AR+), suggesting that the absence of the AR rendered these cells more susceptible to BDO2-induced cytotoxicity.
3.3. Androgen Receptor Protects Prostate Cells from BDO2-Induced Toxicity
To demonstrate the importance of AR in the BDO2-induced cellular effects in prostate cells, we transiently transfect the AR negative DU145(AR−) cells with the full-length human wild-type AR cDNA pCMV expression plasmid. The transfected DU145(AR+) cells were treated with and without BDO2 for 24 hr and then cells were examined for morphological changes. In these experiments, un-transfected LNCaP(AR+) and DU145(AR−) cells were induced for 24 hr with and without BDO2 and serve as positive and negative controls, respectively. (Figure 2, panels (a) and (b)). Immunocytochemistry analysis was performed on transfected and untransfected cells using anti-AR and anti-tubulin. Growth of untransfected DU145(AR−) cells under serum starved condition (3X DCC media) or 3X DCC media plus BDO2 for 24 hrs resulted in increased cell loss and disturbances of DU145(AR−) cellular morphology (Figure 2, panels (b) and (c)). Transient transfection of these cells with a functional AR restored cell-cell contact, cell organization and increased cell numbers in the presence of BDO2 (Figure
Table 2. Cytotoxicity of butadiene diepoxide (BDO2) in prostate cells at micro-dose levels.
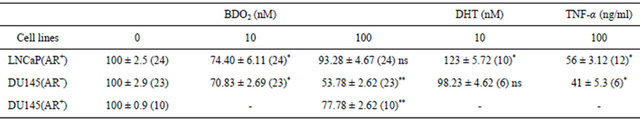
Cytotoxicity of BDO2 in prostate cells was determined using MTT cell viability assay as described in Material and Methods. Note, results are reported as mean value ±SEM. (), number of sample. *p < 0.05, control versus BDO2 treatment in each cell line. Statistically significant differences were obtained using bonferroni’s multiple test analysis. ns, not significant.
 (a) (b)
(a) (b)
Figure 1. BDO2 induces apoptosis in prostate cancer cells at micro-dose levels. Cellular and nuclear morphology indicative of apoptosis induced by BDO2 was examined as described in “Material and Methods”. (a) Nikon Optiphot fluorescent microscope images taken with a 550 nm or a 620 nm filter. (First panel: Bright field, Second panel: Acridine Orange, Third panel: Ethidium Bromide, Fourth panel: a merger of second and third panels); (b) Apoptotic index. We calculated the apoptotic index by averaging the number of apoptotic cells per field (30 - 50 cells) then dividing by the total number of cells per field (120 - 140 cells). The results are shown as % change with respect to untreated cells. The values are the means ± SEM of three separate experiments performed in triplicate where five separate microscopic fields were examined.
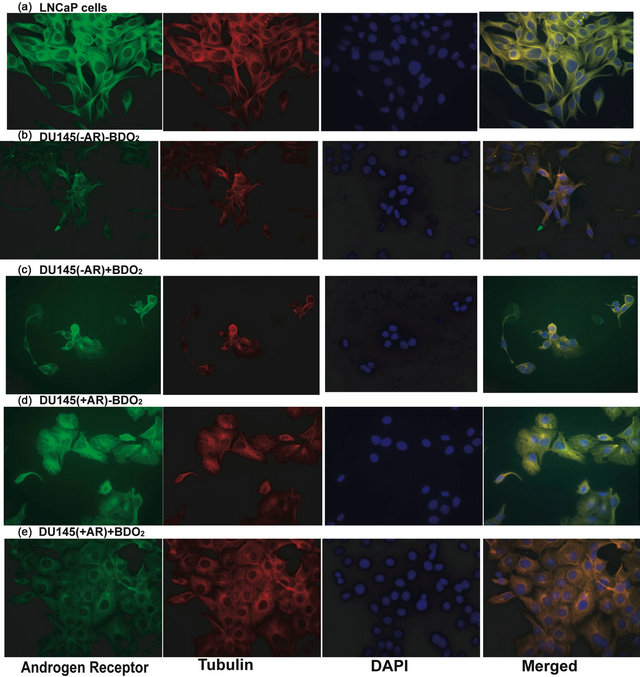
Figure. 2. Activation of androgen receptor function in cells transiently transfected with a CMV-hAR expression following BDO2 exposure. AR transfected cells were induced with and without BDO2, fixed with methanol and paraformaldehyde, stained with either anti-AR or FTCT-labeled anti-Tubulin antibody then the immunoflourescence detected using Cy3-labled secondary antibodies.
2, compare panels (c) and (e)). To corroborate our immunocytochemistry observations, we determined if transfection of a functional AR in DU145(AR−) cells would render them less sensitive to BDO2 exposure as observed in LNCaP cells. Therefore, DU145(AR+) cell sensitivity to BDO2 was assessed using cell viability and cell counting. Treatment of DU145(AR+) cells with BDO2 resulted in decreased toxicity (77.78% viable) as compared to untransfected DU145(AR−) cells (54% viable) after 24 - 48 hr of exposure (Table 2).
3.4. BDO2 Modulated the Expression of Down-Stream Apoptotic Executor Proteins
The process of apoptosis is well conserved in eukaryotic cells and involves both a receptor-mediated and a mitochondrial-mediated pathway. In prostate cancer cells, the presence of AR has been associated with increased cell survival [23]. Therefore, the levels of down-stream apoptotic executor caspase-3/7 and PARP cleavage were analyzed in both cell lines (Figure 3). The level of active caspase-3/7 was measured by proteolytic cleavage of rhodamine 110, from bis-(N-CBZ-L-aspartyl-L-glutamylL-valyl-L-aspartic acid amide) in the Z-DEVD-R110 substrate and PARP cleavage was assayed by Western blot. Significant activation of caspase-3/7 was observed in both cell lines treated with BDO2 (Figure 3). However, the DNA repair enzyme PARP was not activated following BDO2 treatment as evidenced by the absence of the 89 kDa digest band (Figure 4(b)). We found that caspase activation paralleled the observed apoptotic effect of BDO2 determined by morphological assessment (Figure 1). There was a 34% ± 7% and 60% ± 4% increase in caspase levels in LNCaP(AR+) and DU145(AR−) cells, respectively. Introduction of a function AR into DU145
(AR−) cells resulted in a significant decrease in caspase expression and activity (Figure 3). There was a 10% to 12% decrease in caspase activity relative to control, which was similar to that observed with the RES, an know caspases inhibitor in prostate cells. These data strongly suggest that the presence of AR in DU145(AR−) cells protects them from BDO2-induced toxicity and/or improves cell morphology and integrity in the presence of BDO2.
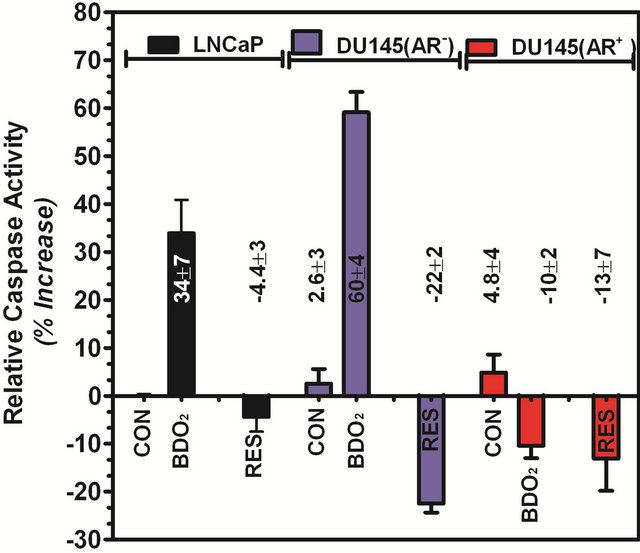
Figure 3. The presence of androgen receptor facilitates BDO2-induced caspase activity in prostate cells. Cells (1E6) were grown in test medium, induced with 100 nM BDO2 or 5 mM resveratrol (RES) for 24 hr and the resulting caspase- 3/7 activity was determined using the Apo-One Homogeneous Caspase-3/7 assay as described in Materials and Methods. Caspase-3/7 activity was expressed as percent increase relative to untreated controls. The values are the means ± SEM of three separate experiments performed in quadruplets.
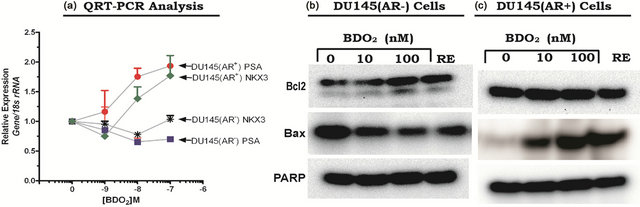
Figure 4. Activation of androgen receptor function in cells transiently transfected with a CMV-hAR expression following BDO2 exposure. (a) Activation of androgen receptor related gene expression in DU145 cells following BDO2 exposure. DU145(AR−) cells or DU145(AR+) cells were induced with 100 nM BDO2 and DNase I-treated RNA, isolated from induced or un-induced cells, was subjected to two-step Taqman Real Time RT-PCR (see Materials and Methods). The relative expression of PSA mRNA expression was calculated by the 2∆∆Ct method. DU145(AR−) (b) and DU145(AR+) (c) cells were grown in serum-free medium, treated with BDO2 or resveratrol (RES) for 24 hr, and the levels of Bcl2, Bax, and PARP proteins were assessed by Western blot analysis. Details of the experiments are presented in Materials and Methods.
3.5. Androgen Receptor Restored Gene Expression but Not PSA Secretion in DU145(AR+) Cells
To support the observation that AR mediates BDO2 action and to validate the function of AR transfected into DU145(AR−) cells, we examined two other AR-dependent processes, namely PSA secretion and AR-dependent gene expression (Figure 4). The parental DU145(AR−) cells do not secret PSA due to the absence of functional AR protein in these cells. Therefore, we hypothesized that a gain of AR would restore PSA expression and secretion in DU145(AR−) cells transfected with the wild type AR. To test this hypothesis, mRNA expression levels of two AR-responsive genes, PSA and NKX3-1, were measured by real-time quantitative RT-PCR in DU145 (AR−) and DU145(AR+) cells treated with BDO2 for 24 hrs (Figure 4, and Table 2). There was reduction in the relative expression of PSA and NKX3-1 in the untransfected DU145(AR−) cells following exposure to BDO2 (Figure 4). Transfection of a functional AR into these cells resulted in a dose-dependent increase in the relative expression of PSA and NKX3-1 after 24 hr exposure to BDO2 (Figure 4(a)). Transfection of AR into DU145(AR−) cells increased the basal levels of both PSA and NKX3-1 by 1.5 to 2-fold above that observed in untransfected cells (Figure 4(a)). Analysis of the expression pattern of the preand pro-apoptotic proteins Bcl2 and Bax in DU145(AR+) showed a doses-dependent increases in Bax protein levels in the presence of BDO2. In DU145(AR−) cells, we observed a high basal level of Bcl2, which increases by 50% - 60% after 100 nM BDO2 treatment. The apparent increase in Bcl2 levels in DU145(AR−) cells was transient and absence from DU145(AR+) cell treated with BDO2. In contrast, in DU145(AR+) cells, we observed an decreases in Bcl2 levels with a corresponding increases in Bax protein expression (Figures 4(b) and (c)).
4. Discussion
Prostate cancer (PCa) development is influenced by factors such as increased age, ethnicity and exposure to environmental factors. Exposure of prostate cells to environmental compounds such as vinclozoline, PCB and cadmium has been characterized [30-32]. These compounds contribute to PCa through modulation of ARdependent and estrogen-dependent pathways. We report for the first time a direct cellular effect of BDO2 on prostate cancer cell function at micro-dose levels. We demonstrate that BDO2 induces cellular toxicity by increasing apoptotic processes, while modulating the expression of genes involved in prostate cancer progression. We found that BDO2 is able to modulate PCa cell activity via an apparent AR-pathway, although there are no known studies that indicate a direct binding of BDO2 to AR. The AR-dependent activity of BDO2 observed is best explained by BDO2 covalently binding to AR through bifunctional cross linking, leading to ligand independent receptor activation. Such activation of AR would exemplify itself through increased gene expression of the AR cascade. We found that BDO2 increased the expression of PCa tumor makers PSA, B2M and NK3. These data suggest that micro-doses of BDO2 modulate prostate cell function at several levels and imply that BDO2 is involved either in tumor induction in prostate cells or in tumor progression of (AR+) prostate cancer cells.
One hypothesis for the induction of tumors by environmental compounds such as BDO2 is that they function as tumor promoters by inhibiting the normal apoptotic processes in cells. The process of apoptosis is well conserved in eukaryotic cells and involves both a recaptor-mediated and a mitochondrial-mediated pathway. In prostate cancer cells, the presence of AR has been associated with increased cell survival because of enhanced Bcl2 protein expression [23]. To gain insight into the mechanism of BDO2-induced cytotoxicity in LNCaP(AR+) and DU145(AR−) cells the dose-dependent expression levels of key apoptosis-related proteins were examined. Micro-dose concentrations of BDO2 down-regulated antiapoptotic Bcl2 expression and enhanced both the expression of the pro-apoptotic Bax protein and the activity of caspase-3/7. Interestingly, the activation of caspase- 3/7 did not lead to PARP activation in either of the two prostate cell lines, despite an increase in apoptotic bodies in the presence of BDO2. Failure to activate PARP under conditions where there is an increase in caspase-3/7 activity, a decrease in cell viability and an increase in apoptotic bodies suggests that some type of compensatory mechanism, which protects prostate cancer cells from the BDO2-induced, mitochondrial-mediated apoptosis pathway, may be operating in these cells. We are currently testing the hypothesis that in BDO2-treated cells, some type of autophagocytosis accompanies the activation of the mitochondrial apoptosis pathway or that BDO2 is directly inducing autophagocytosis.
Tobacco smoke is an environmental source of BDO2 which contributes directly to an increase in PCa [33]. Tobacco smoking produces constant low amounts of BDO2 resulting in micro-dose exposure. Recent studies support the idea that smokers are more likely to develop aggressive PCa as compared to non-smokers [33]. Couple to this our current study, which provides evidence that exposure to micro-doses of BDO2 may contribute to or modulate the development or progression of PCa. BDO2 exposure increases cellular toxicity and alters apoptosis in prostate cells by increasing caspase activation without change in down stream targets such as PARP. Class I carcinogens such as BDO2 have been shown to induce apoptosis via death receptor pathways leading to inhibition of apoptosis. Thus, our work introduces the possibility that micro-doses of carcinogens such as BDO2 may promote prostate tumors by modulation specific cellular activity.
5. Acknowledgements
This work was supported in part by the National Institutes of Health National Center for Research Resources Grant No. P20RR16456-02-R138954.
REFERENCES
- D. Anderson, “Butadiene: Species Comparison for Metabolism and Genetic Toxicology,” Mutation Research, Vol. 405, No. 2, 1998, pp. 247-258. doi:10.1016/S0027-5107(98)00142-0
- D. Anderson, “Genetic and Reproductive Toxicity of Butadiene and Isoprene,” Chemico-Biological Interactions, Vol. 135-136, 2001, pp. 65-80. doi:10.1016/S0009-2797(01)00171-5
- M. Christian, “Review of Reproductive and Developmental Toxicity of 1,3-Butadiene,” Toxicology, Vol. 113, No. 1-3, 1996, pp. 137-143. doi:10.1016/0300-483X(96)03438-5
- E. Delzell, N. Sathiakumar, J. Graff, M. Macaluso, G. Maldonado and R. Matthews, “An Updated Study of Mortality among North American Synthetic Rubber Industry Workers,” Research Report (Health Effects Institute), Vol. 132, 2006, pp. 1-63, 65-74.
- E. Delzell, N. Sathiakumar, M. Hovinga, M. Macaluso, J. Julian, R. Larson, P. Cole and D. Muir, “A Follow-Up Study of Synthetic Rubber Workers,” Toxicology, Vol. 113, No. 1-3, 1996, pp. 182-189. doi:10.1016/0300-483X(96)03443-9
- M. Goggin, J. A. Swenberg, V. E. Walker and N. Tretyakova, “Molecular Dosimetry of 1,2,3,4-Diepoxybutane-induced DNA-DNA Cross-Links in B6C3F1 Mice and F344 Rats Exposed to 1,3-Butadiene by Inhalation,” Cancer Research, Vol. 69, 2009, pp. 2479-2486. doi:10.1158/0008-5472.CAN-08-4152
- D. Anderson, “Male-Mediated Developmental Toxicity,” Toxicology and Applied Pharmacology, Vol. 207, No. 2, 2005, pp. 506-513. doi:10.1016/j.taap.2005.01.022
- P. Boffetta, H. O. Adami, P. Cole, D. Trichopoulos and J. S. Mandel, “Epidemiologic Studies of Styrene and Cancer: A Review of the Literature,” Journal of Occupational & Environmental Medicine, Vol. 51, No. 11, 2009, pp. 1275-1287. doi:10.1097/JOM.0b013e3181ad49b2
- H. Ma, T. G. Wood, M. M. Ammenheuser, J. I. Rosenblatt and J. B. Ward Jr., “Molecular Analysis of hprt Mutant Lymphocytes from 1,3-Butadiene-exposed Workers,” Environmental and Molecular Mutagenesis, Vol. 36, 2000, pp. 59-71.
- F. Pacchierotti, C. Tiveron, R. Ranaldi, B. Bassani, E. Cordelli, G. Leter and M. Spanò, “Reproductive Toxicity of 1,3-Butadiene in the Mouse: Cytogenetic Analysis of Chromosome Aberrations in First-Cleavage Embryos and Flow Cytometric Evaluation of Spermatogonial Cell Killing,” Mutational Research, Vol. 397, No. 1, 1998, pp. 55-66.
- J. Huff, R. Melnick, H. Solleveld, J. Haseman, M. Powers, and R. Miller, “Multiple Organ Carcinogenicity of 1,3-Butadiene in B6C3F Mice after 60 Weeks of Inhalation Exposure,” Science, Vol. 227, No. 4686, 1985, pp. 548-549. doi:10.1126/science.3966163
- M. Macaluso, R. Larson, E. Delzell, N. Sathiakumar, M. Hovinga, J. Julian, D. Muir and P. Cole, “Leukemia and Cumulative Exposure to Butadiene, Styrene and Benzene among Workers in the Synthetic Rubber Industry,” Toxicology, Vol. 113, No. 1-3, 1996, pp. 190-202. doi:10.1016/0300-483X(96)03444-0
- E. Mylchreest, L. A. Malley, A. J. O’Neill, T. A. Kegelman, G. P. Sykes and R. Valentine, “Reproductive and Developmental Toxicity of Inhaled 2,3-Dichloro-1,3-butadiene in Rats,” Reproductive Toxicology, Vol. 22, No. 4, 2006, pp. 613-622. doi:10.1016/j.reprotox.2006.04.002
- M. Schmiederer, E. Knutson, P. Muganda and T. Albrecht, “Acute Exposure of Human Lung Cells to 1,3-Butadiene Diepoxide Results in G1 and G2 Cell Cycle Arrest,” Environmental and Molecular Mutagenesis, Vol. 45, No. 4, 2005, pp. 354-364. doi:10.1002/em.20099
- S. Naragoni, S. Sankella, K. Harris and W. G. Gray, “Phytoestrogens Regulate mRNA and Protein Levels of Guanine Nucleotide-Binding Protein, Beta-1 Subunit (GNB1) in MCF-7 Cells,” Journal of Cellular Physiology, Vol. 219, No. 3, 2009, pp. 584-594. doi:10.1002/jcp.21699
- R. Solipuram, S. Koppula, A. Hurst, K. Harris, S. Naragoni, K. Fontenot and W. Gray, “Molecular and Biochemical Effects of Kola Nut Extract on Androgen Receptor-Mediated Pathways,” Journal of Toxicology, Vol. 2009, 2009, Article ID: 530279. doi:10.1155/2009/530279
- A. Biosystem, “User Bulletin #2: ABI PRISM 7700 Sequence Detection System,” 2001.
- S. Stahl, T. Y. Chun and W. G. Gray, “Phytoestrogens Act as Estrogen Agonists in an Estrogen-Responsive Pituitary Cell Line,” Toxicology and Applied Pharmacology, Vol. 152, No. 1, 1998, pp. 41-48. doi:10.1006/taap.1998.8500
- W. Washington, L. Hubert, D. Jones and W. G. Gray, “Bisphenol a Binds to the Low-Affinity Estrogen Binding Site,” In Vitro & Molecular Toxicology, Vol. 14, No. 1, 2001, pp. 43-51. doi:10.1089/109793301316882531
- P. I. GraphPad, “GraphPad,” 5th Edition, GraphPad Software, Inc., San Diego, 1997.
- C. Lee, D. M. Sutkowski, J. A. Sensibar, D. Zelner, I. Kim, I. Amsel, N. Shaw, G. S. Prins and J. M. Kozlowski, “Regulation of Proliferation and Production of Prostate-Specific Antigen in Androgen-Sensitive Prostatic Cancer Cells, LNCaP, by Dihydrotestosterone,” Endocrinology, Vol. 136, No. 2, 1995, pp. 796-803. doi:10.1210/en.136.2.796
- T. V. Nguyen, M. Yao and C. J. Pike, Flutamide and Cyproterone Acetate Exert Agonist Effects: Induction of Androgen Receptor-Dependent Neuroprotection,” Endocrinology, Vol. 148, No. 6, 2007, pp. 2936-2943. doi:10.1210/en.2006-1469
- X. B. Liao, S. Q. Tang, T. J. Brantley, L. G. Tomas and B. Y. Li, “Small-Interfering RNA-Induced Androgen Receptor Silencing Leads to Apoptotic Cell Death in Prostate Cancer,” Molecular Cancer Therapeutics, Vol. 4, 2005, pp. 505-515. doi:10.1158/1535-7163.MCT-04-0313
- P. Thelen, P. Burfeind, S. Schweyer, J. G. Scharf, W. Wuttke and R. H. Ringert, “Molecular Principles of Alternative Treatment Approaches for Hormone-Refractory Prostate Cancer,” Der Urologe, Vol. 46, No. 9, 2007, pp. 1271-1274. doi:10.1007/s00120-007-1452-0
- P. Thelen, T. Peter, A. Hunermund, S. Kaulfuss, D. Seidlova-Wuttke, W. Wuttke, R. H. Ringert and F. Seseke, “Phytoestrogens from Belamcanda Chinensis Regulate the Expression of Steroid Receptors and Related Cofactors in LNCaP Prostate Cancer Cells,” BJU International, Vol. 100, No. 1, 2007, pp. 199-203. doi:10.1111/j.1464-410X.2007.06924.x
- W.-M. B. Thomas, Z. Liao, S. Kim, S. Lemeshow, J. W. Erdman and S. K. Clinton, “Prostate Carcinogenesis in N-Methyl-N-nitrosourea (NMU)-Testosterone-Treated Rats Fed Tomato Powder, Lycopene, or Energy-Restricted Diets,” Journal of the National Cancer Institute, Vol. 95, No. 21, 2003, pp. 1578-1586. doi:10.1093/jnci/djg081
- P. Wang, Q. Ma, J. Luo, B. Liu, F. Tan, Z. Zhang and Z. Chen, “Nkx3.1 and p27(KIP1) Cooperate in Proliferation Inhibition and Apoptosis Induction in Human Androgen-Independent Prostate Cancer Cells,” Cancer Investigation, Vol. 27, No. 4, 2009, pp. 369-375. doi:10.1080/07357900802232749
- H. G. Yoon and J. Wong, “The Corepressors Silencing Mediator of Retinoid and Thyroid Hormone Receptor and Nuclear Receptor Corepressor Are Involved in Agonistand Antagonist-Regulated Transcription by Androgen Receptor,” Molecular Endocrinology, Vol. 20, No. 5, 2006, pp. 1048-1060. doi:10.1210/me.2005-0324
- L. Yu, G. L. Blackburn and J. R. Zhou, “Genistein and Daidzein Downregulate Prostate Androgen-Regulated Transcript-1 (PART-1) Gene Expression Induced by Dihydrotestosterone in Human Prostate LNCaP Cancer Cells,” The Journal of nutrition, Vol. 133, No. 2, 2003, pp. 389-392.
- R. Kavlok and A. Cummings, “Androgen Receptor Function—Vinclozolin-Induced Malformations in Reproductive Development,” Critical Reviews in Toxicology, Vol. 35, 2005, pp. 721-726. doi:10.1080/10408440591007377
- P. J. Mink, H.-O. Adami, D. Trichopoulos, N. L. Britton and J. S. Mandel, “Pesticides and Prostate Cancer: A Review of Epidemiologic Studies with Specific Agricultural Exposure Information,” European Journal of Cancer Prevention, Vol. 17, No. 2, 2008, pp. 97-110. doi:10.1097/CEJ.0b013e3280145b4c
- G. S. Prins, “Endocrine Disruptors and Prostate Cancer Risk,” Endocrine-Related Cancer, Vol. 15, 2008, pp. 649-656. doi:10.1677/ERC-08-0043
- L. A. Plaskon, D. F. Penson, T. L. Vaughan and J. L. Stanford, “Cigarette Smoking and Risk of Prostate Cancer in Middle-Aged Men,” Cancer Epidemiology, Biomarkers & Prevention, Vol. 12, 2003, pp. 604-609.

