Open Journal of Veterinary Medicine
Vol. 3 No. 2 (2013) , Article ID: 32699 , 7 pages DOI:10.4236/ojvm.2013.32025
Is Bat Guano a Reservoir of Geomyces destructans?
1Karst Research Institute, Research Centre of the Slovenian Academy of Sciences and Arts, Postojna, Slovenia
2Institute of Specific Prophylaxis and Tropical Medicine, Medical University of Vienna, Vienna, Austria
Email: *janez.mulec@guest.arnes.si
Copyright © 2013 Janez Mulec et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 28, 2013; revised April 28, 2013; accepted May 28, 2013
Keywords: Geomyces destructans; Bat Guano; Cave; Slovenia
ABSTRACT
Bat guano from six different karst caves in Slovenia was screened by PCR for the presence of Geomyces destructans [ ADDIN EN.CITE
1. Introduction
White-nose syndrome (WNS) is a widespread, epizootic disease in bats associated with the fungus Geomyces destructans (Ascomycota, Helotiales) [2]. This psychrophilic fungus exhibits pronounced proteolytic activities and invades living tissue of cave-roosting bats during hibernation. G. destructans collected from the environment shows slow recovery rates under laboratory conditions (4 - 8 weeks), with the highest growth rate around 12˚C - 15˚C [3,4]. WNS causes unprecedented morbidity and mortality (often >90%) in bats in North America. The fungus is always found on bats with WNS where large numbers of hibernating bats have died [5]. G. destructans appears to have been already present in Europe before the disease outbreak in the USA [6,7]. While the naïve bat populations in North America die easily from infection [6,8], in contrast, in Europe no mass mortality in bats linked to WNS has been reported, although G. destructans is widespread. Many bat species from the genus Myotis are infected on both continents. In different European countries eight Myotis species, particularly M. myotis, have been observed with visible fungal growth usually colonizing wings, tail, ears and muscles [7,9-12]. It is suspected that shorter winter periods in warmer regions in Europe are associated with lower incidence of G. destructans in bats. In Europe, bats with fungal mycelia are first seen in January; in February the number increases and reaches a peak in March, then drops again in April, when bats emerge from hibernation [10].
Because bats play major roles in the ecosystem, such as plant pollination, seed dissemination, forest regeneration and insect control [13], their health and endangerment should be of high concern. Cave walls and hibernacula could serve as passive vectors and/or reservoirs for G. destructans [10,14-16]. Bat guano is known to harbor several bats and human pathogens, such as the fungus Histoplasma capsulatum [17,18]. The guano of insectivorous bats is rich in organic matter, especially keratin [19]. Therefore, bat guano is a potential habitat or reservoir for G. destructans. The idea of detecting G. destructans in guano is appealing for several reasons. First, guano is found in close proximity to bat roosts and is likely to have exposure to bat pathogens. Sampling of guano is less invasive than direct sampling of bats and may be conducted at times when bats are absent from hibernacula. Finally, the chemical and physical characteristics of guano make it appear to be a suitable substrate for G. destructans growth or maintenance. However, although there are several studies from Europe reporting the presence of Geomyces in bat guano, for example G. pannorum var. pannorum from Domica Cave in Slovakia [20], very few investigations of G. destructans in guano have been reported [21]. Perhaps the particular soil and debris material of guano are not optimal for isolation of G. destructans, or guano is unsuitable for sustaining growth of G. destructans.
Detection methods for G. destructans remain in development. Definitive identification may be done by culturing, but requires appropriate culture facilities as well as attention to pathogen containment protocols. In contrast to culturing techniques, molecular methods offer greater sensitivity and the advantage of detecting the fungus from samples in which the fungus is nonviable. Specific real-time PCR assays have recently been developed [22,23], but real-time PCR requires substantial monetary resources for reagents and access to specialized equipment. Conventional PCR, though less specific, allows more cost-effective screening of environmental samples for G. destructans genomic DNA, and may be followed with sequencing for species verification. In a recent study, a G. destructans-specific polymerase chain reaction (PCR) using primers developed by Lorch and co-workers [1] had 100% diagnostic specificity and 96% diagnostic sensitivity in bat tissue. On the other hand these primers lacked the specificity to distinguish between G. destructans and closely related Geomyces spp. in environmental samples [16]. Nonetheless, the advantages of a PCR-based approach remain apparent for allowing rapid screening when culturing facilities are unavailable.
G. destructans has been detected in many European countries, but no reports exist from Slovenia on either its presence in caves or association with geomycosis in bats. The objective of this study was to screen bat guano as a potential habitat for the presence of G. destructans and to unravel its ecology and distribution in Slovenian karst caves. Bat guano samples were screened for G. destructans by conventional PCR in combination with DNA sequencing. Two out of six guano samples from Slovene caves contained sequences closely related to several unidentified Geomyces clones and to pathogenic G. destructans. This is one of the first published investigations of the presence of Geomyces in bat excrement.
2. Material and Methods
2.1. Caves and Bats
In Slovenia many caves host bats, but large quantities of bat guano are preserved only in some of them. For this study five caves with bats and significant amounts of guano were selected. Air temperature was measured using a portable Kestrel 4500 Pocket Weather Tracker (USA). Huda luknja Cave (Huda luknja, no. 413 in the cave register of the Karst Research Institute ZRC SAZU and Speleological Association of Slovenia) has a total length of 2339 m, a maximum depth of 119 m, and the cave entrance is at an altitude of 508 m above sea level (date of sampling 19 January 2010, air temperature 7.2˚C). The chamber with bat guano is regularly populated by bats. Predjama Cave (Predjama, cave register no. 734, sampled 20 June 2011, air temperature 13.4˚C) is the third largest Slovenian cave, totalling 13.1 km long and 143 m deep. The entrance to the cave is at an altitude of 490 m a.s.l. The section with guano, named Netopirjev rov (Bat’s passage), is historically known to be densely populated by bats, but unfortunately guano heaps were almost completely removed from the cave several decades ago by local people. Spodnja klevevška Cave (Spodnja klevevška jama, cave register no. 410, sampled 18 July 2011, air temperature 18.1˚C) has a thermal spring (~20˚C), is 318 m long and 14 m deep, and the cave entrance is at an altitude of 177 m a.s.l. Due to water oscillations guano heaps in the cave are usually no older than one season; nevertheless scattered bat droppings can be found throughout the entire cave. Škocjan Caves (Škocjanske jame, cave register no. 735) have a total length of 5.8 km, and the entrance is at an altitude of 425 m. Škocjan Caves are listed in the UNESCO World Heritage list and are the first underground karst wetland under the Ramsar Wetland Classification System. In these caves two guano heaps were sampled on 21 June 2011. The first sample (ŠK1) was taken from guano which was abandoned by a bat colony in the years 1975/76 after the installation of a lighting system at this site; however some individuals are sporadically seen at the site. The second guano sample (ŠK2) was taken from guano in a chamber where bats roost during the winter. Temperature conditions in a guano heap, correlated to the surrounding cave atmosphere, were monitored at site ŠK2 in Škocjan Caves. Temperature data loggers (Anset StowAway TidbiT Temp LoggerTBI32-20 + 50, USA) were placed 5 cm deep in the guano and 20 cm above the guano heap, exposed to the cave atmosphere. Turjeva Cave (Turjeva jama, cave register no. 821, sampled 2 February 2011, air temperature 10.4˚C) is 375 m long and 5 m deep with the entrance at an altitude of 253 m a.s.l. In recent years, no bats have been observed above this guano heap.
Fresh guano samples were characterized by fresh rodshaped excrement indicating the roosting colony of bats at the site. An attribution of “recent” was given to those samples which contained some relatively recent rod-shaped excrement over old guano base, and samples were considered “old” if guano had no characteristics of fresh and/ or recent rod-shaped excrement, and if within a recent time period, usually a few decades, no bat colony was recorded above the guano heap (Table 1).
Twelve different bat species have been identified in Huda luknja Cave, eleven in Predjama Cave, five in Spodnja klevevška Cave, twelve in Škocjan Caves, and five in Turjeva Cave [24,25]. The most numerous bat species
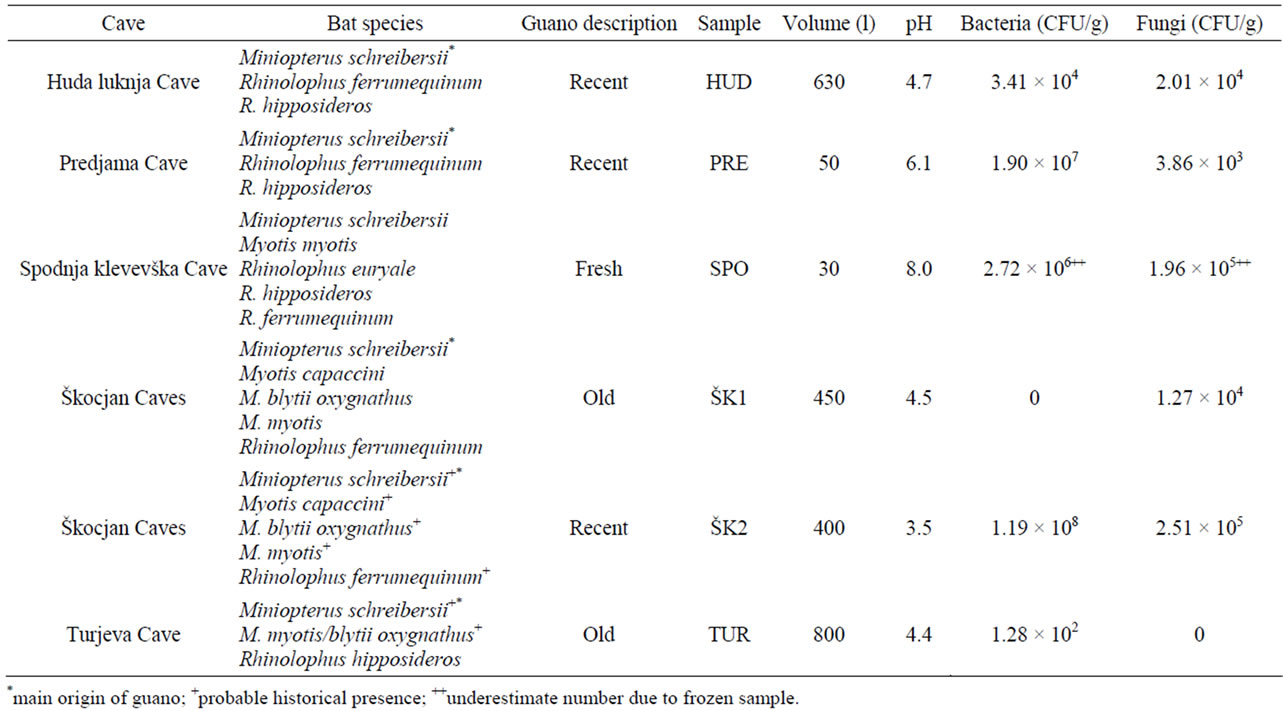
Table 1. Prevalent bat species [24] and characteristics of guano.
and some characteristics of the sampled guano heaps are summarized in Table 1. For analyses, guano heaps were aseptically sampled with a spoon in a range from zero to five cm depth. Each sample was a combination of the top five centimetres. The pH was measured using pH indicator strips (EMD Chemicals, Germany) after homogenization of guano with an equal part of deionised water. In the laboratory guano samples were screened for culturable microbiota. For molecular analyses guano was stored at −20˚C until DNA was isolated.
2.2. Estimation of Culturable Microbes
To estimate the quantity of culturable microbiota, guano samples were screened for bacteria and fungi using RIDA®COUNT test plates. This system has already been used successfully on various cave samples [26]. All guano samples were screened after sampling, except the sample from Spodnja klevevška Cave, which was processed after freezing (Table 1). Fifteen millilitres of sterile physiological saline were added to approximately 2 g of guano and vigorously vortexed. This mixture was serially diluted up to 10−5. One millilitre of these serial dilutions was applied onto RIDA®COUNT test plates. RIDA®COUNT Total Aerobic Count test plates were used for total bacterial counts and RIDA®COUNT Yeast & Mold Rapid for total counts of yeasts and moulds. Counts of bacterial colonies were made after 48 hours of incubation at 35˚C, and of yeasts and moulds after 72 hours at 25˚C [27].
2.3. DNA Isolation, PCR and DNA Sequencing
High molecular weight genomic DNA was isolated from bat guano using the NucleoSpin® Soil kit with SL2 lysis buffer (Macherey-Nagel, Germany). DNA purity and concentration were determined spectrophotometrically using standard absorbance at 260 nm and 280 nm on a NanoDrop ND-1000 UV/Vis spectrophotometer (NanoDrop Technologies, USA). 260/280 absorbance ratio ranged from 1.82 to 1.92.
A Geomyces destructans-specific diagnostic PCR [1] was performed with slight modifications. Amplifications were carried out in 50 µl reaction mixtures containing 1 µl of DNA extract (at 15 - 30 ng/µl), 10 pmol/µl of each primer, nu-SSI(1506)-184-5’-Gd (GGGGACGTCCTAAAGCCT) and nu-5.8S-144-3’-Gd (TTGTAATGACGCTCGGAC), and 0.5 µl of the HOT FIREPol® DNA Polymerase (Solis BioDyne) with a standard PCR programme: initial denaturation at 95˚C for 15 min, followed by 45 cycles of denaturation at 95˚C for 1 minute, annealing at 50˚C for 2 min, and extension at 72˚C for 3 min, followed by a final extension step of 72˚C for 10 min. The amplicons were electrophoresed on 2% agarose in TAE buffer, visualized by Midori Green Advance DNA Stain (Biozyme), and compared to a Step ladder 50 - 3000 bp marker (Sigma-Aldrich). Amplified PCR products of proper size (~620 bp) were purified using the QIAquick Gel Extraction Kit (USA).The purified amplified fragments were sequenced using the same primers to confirm the specificity of the PCR by direct sequencing of the PCR products using the ABI PRISM® BigDye sequencing kit (PE Applied Biosystems, Langen, Germany). Sequencing was carried out in a 310 ABI PRISM® automated sequencer; sequences were obtained from both strands in two independent setups. The nucleotide sequences were assembled in GeneDoc. Multiple sequence alignment was performed by pairwise alignments using the CLUSTAL algorithm.
Sequence data were deposited in the GenBank database under the following accession numbers: JX467177 (sample ŠK2 designated as SK2011) and JX467178 (sample HUD designated as HUD2010).
3. Results and Discussion
3.1. Analyses of Geomyces Sequences
PCR with primers reported to be specific for G. destructans [1] was performed on total DNA isolated from guano samples collected from the upper five centimetres of guano heaps. Several independent PCR reactions were carried out and all gave the same results. Altogether, two samples were positive for the expected PCR product size; these were a recent guano sample from Škocjan Caves, sample ŠK2, and a recent guano sample from Huda luknja Cave, sample HUD (Figure 1).
The PCR primers used in the study amplify a 624-bp segment of the ribosomal RNA gene region that consists of the 1506 intron for which no unambiguous sequence could be obtained, a small portion of SSU (small 18S ribosomal subunit), ITS 1, 5.8 S and part of ITS 2 [1]. Two bands of appropriate size (~620 bp) were bidirectionally sequenced. Sequences were obtained from both strands; from both positive PCR products only a 364 bp fragment could be sequenced. The sequences from the two Geomyces-positive samples, SK2011 and HUD2010, are both 364 bp long and 100% identical to one another. These two sequences showed highest sequence identity, 100% (364/364 bp), to several unidentified Geomyces
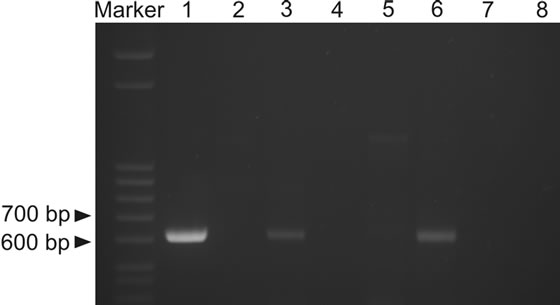
Figure 1. PCR-based detection of Geomyces destructans in guano (Marker-Step ladder, Sigma-Aldrich, 1-positive control using genomic DNA of G. destructans as a template, 2-ŠK1, 3-ŠK2, 4-PRE, 5-TUR, 6-HUD, 7-SPO, 8-negative control). A 624-bp band matches the expected size for G. destructans for samples 3 and 6.
clones detected in soil from bat hibernacula in the USA (GenBank accession numbers: HM848968, HM848971, HM848981, HM848983, HM848985, HM848988; [16]). The next highest sequence identity (363/364 bp) was to two strains of G. pannorum, one isolated from arctic cryopegs and marine deposits (DQ189224; [28] and one from wheat rhizosphere in the UK (AJ938165), and, among others, to several clones/strains of G. destructans isolated from bats in Germany (e.g. JF502400-JF502402 [10], HM222616 [7]) and in the Czech Republic (e.g. HM584949-HM584975; [12]). Because PCR products were directly sequenced rather than being first cloned, if G. destructans were present in low abundance it could easily be masked by other related strains.
3.2. Bats, Guano and Geomyces
To date there has been no report of fungal growth characteristic of G. destructans on bats in these two caves. This is somewhat surprising given the prevalence of the fungus in other European countries, but could be attributed to a rather low abundance of Myotis (Primož Presetnik, personal communication) and/or to poor surveillance of bats in Slovenian caves. Our results neither rule out nor confirm the presence of G. destructans in Slovenia, but do indicate that very closely related strains are detectable in bat guano of Slovene caves.
It is reasonable that guano could be suited for the growth or propagation of G. destructans because in guano a variety of keratin substrata are available, such as bat hairs and partly digested insect fragments including wings, wing parts and leg scales [19,29]. Other Geomyces species are known to be keratinophilic, and thus G. destructans is presumed to be as well. Once G. destructans has been introduced to a guano heap, this could represent a permanent source of infection for hibernacula, particularly if these heaps are close to a bat colony. Movement of air masses caused by bats is not negligible, and air draught and water splashing are two important factors for spreading aerosolized microbial spores in caves [30].
Interestingly, two guano heaps only 450 m apart in Škocjan Caves were analysed, but we obtained positive PCR only for the heap containing recent bat droppings. The guano that had been abandoned by the bat colony in 1975/76, presumably derived from the same bat species roosting at the other site today (Primož Presetnik, personal communication), was negative by PCR. This finding could suggest a recent introduction of these Geomyces species to Slovenia (less than ~40 years) by migratory bats, but, more likely, indicates that the detected Geomyces have a limited life in guano and will only be detected in relatively fresh samples. If conditions in aging guano do not support its growth, guano could not be a long-term reservoir of this fungus. Recent analysis of sediments from bat hibernacula in the United States confirms that G. destructans can persist in these substrates for at least several months [14]. The average sedimentation rate of bat excrement in guano heaps from Domica Cave in Slovakia is 0.99 mm per year [19]. By analysing guano in a range from zero to five cm depth, and if we assume a similar deposition rate in Slovenian caves due to similar size of guano and number of bats, the age of screened guano in the 5-cm deep sample ranged from 0 up to 50 years, much longer than the time period tested in US sediments.
More studies are needed to better understand the ecology and distribution of Geomyces in caves and other underground cavities. Besides G. destructans, additional lineages of psychrophilic and saprophytic fungi exist, and some of these are distributed rather widely in temperate climates [31,32]. Screening of potential habitats of G. destructans with PCR could give us valuable information on its distribution in Slovenia and more widely in Europe. Because geomycosis has been shown to exist in Europe [9] such information may help prevent and/or control WNS outbreak and transmission in bats.
3.3. Microbial Estimators and Conditions in Guano
Guano contains very diverse microbiota [33]. We tested whether the guano sampled for this study contained viable fungi and bacteria. Among the sampled guanos, culturable bacterial and fungal abundances varied by up to four orders of magnitude. The overall culturable fungal densities in the two guano piles with positive PCR results were in the same range as the other tested guano samples from the sampled caves (Table 1). In Huda luknja Cave (HUD) 2.01 × 104 CFU/g and in Škocjan Caves (ŠK2) 2.51 × 105 CFU/g of fungi were found. Changes in composition and abundance of microbiota over time is known to occur in guano, which could also explain several unsuccessful attempts to retrieve culturable bacteria from old guano in Škocjan Caves (ŠK1), although 1.27 × 104 fungal CFU/g were detected on RIDA®COUNT test plates. Due to cultivation conditions for fungal plates (25˚C, 72 hours) and nature of the fungal medium we did not retrieve psychrophilic strains, but we did retrieve an estimate of the abundance of nutrient non-demanding fast growing culturable fungi. Although this guano is clearly able to support fungal growth, we infer from our sequencing results and from the fact that PCR results were positive only with prolonged amplification that G. destructans is probably not very abundant in bat guano. Indeed, even in soil samples Lindner and co-workers reported that G. destructans does not dominate the pool of fungal DNA [16].
Laboratory studies on the temperature optimum for psychrophilic G. destructans showed good growth at 4˚C and a growth optimum around 12˚C - 15˚C, with no growth observed above approximately 20˚C [3,4]. Temperatures in hibernacula affected by WNS usually range between 2˚C and 14˚C [5]. In the current study, temperature conditions in the monitored guano, 10˚C - 14˚C, were favourable for G. destructans. The temperature in guano generally followed the temperature changes in the cave atmosphere. In the colder part of the year the temperature in guano was slightly higher than in the cave atmosphere, while in early summer guano temperature was slightly below cave atmosphere temperature (Figure 2). Besides being a source of nutrients for G. destructans, guano might also serve as an insulator that dampens large temperature changes, which could be especially important where guano is exposed to more extreme atmospheric conditions, for example at cave entrances and at sites with pronounced airflow.
The pH of fresh guano is neutral to alkaline, while older guano of insectivorous bats tends to be acidic [34] (Table 1). Because G. destructans could be isolated on either Sabouraud agar (pH~5.6) or Rose Bengal agar (pH~7.2) [3] it can be expected that the acidic pH of older guano is not a limiting factor for this organism; in fact, both Geomyces-positive samples were considerably more acidic than pH 5.6. These two samples were further characterized by a relatively high quantity of guano material deposited, and by the presence of some relatively fresh bat droppings at the sampling sites. In both chambers in Huda luknja Cave and Škocjan Caves (site ŠK2) where we took samples bats have been observed during bat surveying and sampling (Table 1).
In insectivorous mammals toxic metals bioaccumulate [35]; for this reason guano also contains a high quantity of heavy metals. In guano from Škocjan Caves the quantity of Cu exceeded values for agricultural soil in both samples, and in recent guano (ŠK2) Cd and Zn also exceeded these values [36]. Further studies are needed to
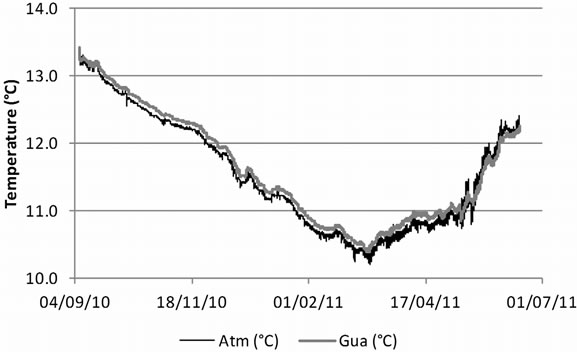
Figure 2. Temperature changes in the bat guano heap (Gua) where G. destructans-specific PCR was positive and in cave atmosphere (Atm) in Škocjan Caves between 7 September 2010 and 16 June 2011.
reveal if heavy metals affect the ecology of G. destructans in guano.
4. Conclusions
Bat guano in two caves from Slovenia hosted Geomyces strains which are related to the virulent G. destructans that causes WNS. If conditions in guano support viability of G. destructans, guano heaps could represent a permanent reservoir for in situ infection of bats. Screening of guano could help to control WNS outbreaks, and in the USA screening of guano profiles might provide additional information on the approximate period when the European version of G. destructans was introduced.
In sum, our results suggest that G. destructans could be present in Slovenia and that screening of fresh guano samples is a potential technique for identifying it in other caves or places where bats dwell. Molecular analysis of excrement provides an alternative method when direct sampling of bats is impossible.
5. Acknowledgements
This study was partly supported by the program Karst Research P6-0119 and bilateral research project between Slovenia and Austria (2011-2012). Authors gratefully acknowledge Sébastien Puechmaille for providing positive control genomic DNA from Geomyces destructans, Primož Presetnik for valuable data on bats, Andreea Oarga and David C. Culver for field work support, Iveta Haefeli for laboratory assistance, and Robert Krieger (MachereyNagel GmbH & Co.) for optimization of DNA isolation from bat guano.
REFERENCES
- J. M. Lorch, A. Gargas, C. U. Meteyer, B. M. BerlowskiZier, D. E. Green, V. Shearn-Bochsler, N. J. Thomas and D. S. Blehert, “Rapid Polymerase Chain Reaction Diagnosis of White-Nose Syndrome in Bats,” Journal of Veterinary Diagnostic Investigation, Vol. 22, No. 2, 2010, pp. 224-230. doi:10.1177/104063871002200208
- A. Gargas, M. T. Trest, M. Christensen, T. J. Volk and D. S. Blehert, “Geomyces destructans sp. nov. Associated with Bat White-Nose Syndrome,” Mycotaxon, Vol. 108, No. 8, 2009, pp. 147-154. doi:10.5248/108.147
- V. Chaturvedi, D. J. Springer, M. J. Behr, R. Ramani, X. Li, M. R. Peck, P. Ren, D. J. Bopp, B. Wood, W. A. Samsonoff, C. M. Butchkoski, A. C. Hicks, W. B. Stone, R. J. Rudd and S. Chaturvedi, “Morphological and Molecular Characterizations of Psychrophilic Fungus Geomyces destructans from New York Bats with White Nose Syndrome (WNS),” Plos One, Vol. 5, No. 5, 2010, p. 5. doi:10.1371/journal.pone.0010783
- M. L. Verant, J. G. Boyles, W. Jr. Waldrep, G. Wibbelt and D. S. Blehert, “Temperature-Dependent Growth of Geomyces destructans, the Fungus That Causes Bat White-Nose Syndrome,” Plos One, Vol, 7, No. 9, 2012, p. 9. doi:10.1371/journal.pone.0046280
- D. S. Blehert, A. C. Hicks, M. Behr, C. U. Meteyer, B. M. Berlowski-Zier, E. L. Buckles, J. T. Coleman, S. R. Darling, A. Gargas, R. Niver, J. C. Okoniewski, R. J. Rudd and W. B. Stone, “Bat White-Nose Syndrome: An Emerging Fungal Pathogen?” Science, Vol. 323, No. 5911, 2009, p. 227. doi:10.1126/science.1163874
- L. Warnecke, J. M. Turner, T. K. Bollinger, J. M. Lorch, V. Misra, P. M. Cryan, G. Wibbelt, D. S. Blehert and C. K. Willis, “Inoculation of Bats with European Geomyces destructans Supports the Novel Pathogen Hypothesis for the Origin of White-Nose Syndrome. Proceedings of the National Academy of Sciences of the United States of America, Vol. 109, No. 18, 2012, pp. 6999-7003. doi:10.1073/pnas.1200374109
- G. Wibbelt, A. Kurth, D. Hellmann, M. Weishaar, A. Barlow, M. Veith, J. Prüger, T. Görföl, L. Grosche, F. Bontadina, U. Zöphel, H. P. Seidl, H. P. Seidl and D. S. Blehert, “White-Nose Syndrome Fungus (Geomyces destructans) in Bats, Europe,” Emerging Infectious Diseases, Vol. 16, No. 8, 2010, pp. 1237-1242. doi:10.3201/eid1608.100002
- J. M. Lorch, C. U. Meteyer, M. J. Behr, J. G. Boyles, P. M. Cryan, A. C. Hicks, A. E. Ballmann, J. T. Coleman, D. N. Redell, D. M. Reeder and D. S. Blehert, “Experimental Infection of Bats with Geomyces destructans Causes WhiteNose Syndrome,” Nature, Vol. 480, No. 7377, 2011, pp. 376-378. doi:10.1038/nature10590
- J. Pikula, H. Bandouchova, L. Novotny, C. U. Meteyer, J. Zukal, N. R. Irwin, J. Zima and N. Martínková, “Histopathology Confirms White-Nose Syndrome in Bats in Europe,” Journal of Wildlife Diseases, Vol. 48, No. 1, 2012, pp. 207-211.
- S. J. Puechmaille, G. Wibbelt, V. Korn, H. Fuller, F. Forget, K. Muhldorfer, A. Kurth, W. Bogdanowicz, C. Borel, T. Bosch, T. Cherezy, M. Drebet, T. Görföl, A. J. Haarsma, F. Herhaus, G. Hallart, M. Hammer, C. Jungmann, Y. Le Bris, L. Lutsar, M. Masing, B. Mulkens, K. Passior, M. Starrach, A. Wojtaszewski, U. Zöphel and E. C. Teeling, “Pan-European Distribution of White-Nose Syndrome Fungus (Geomyces destructans) not Associated with Mass Mortality,” Plos One, Vol. 6, No. 4, 2011, p. 4. doi:10.1371/journal.pone.0019167
- S. J. Puechmaille, P. Verdeyroux, H. Fuller, M. A. Gouilh, M. Bekaert and E. C. Teeling, “White-Nose Syndrome Fungus (Geomyces destructans) in Bat, France,” Emerging Infectious Diseases, Vol. 16, No. 2, 2010, pp. 290- 293. doi:10.3201/eid1602.091391
- N. Martínková, P. Bačkor, T. Bartonička, P. Blažková, J. Červený, L. Falteisek, J. Gaisler, V. Hanzal, D. Horáček, Z. Hubálek, H. Jahelková, M. Kolařík, L. Korytár, A. Kubátová, B. Lehotská, R. Lehotský, R. K. Lučan, O. Májek, J. Matějů, Z. Rehák, J. Šafář, P. Tájek, E. Tkadlec, M. Uhrin, J. Wagner, D. Weinfurtová, J. Zima, J. Zukal and I. Horáček, “Increasing Incidence of Geomyces destructans Fungus in Bats from the Czech Republic and Slovakia,” Plos One, Vol. 5, No. 11, 2010, p. 11. doi:10.1371/journal.pone.0013853
- T. H. Kunz and M. B. Fenton, “Bat Ecology,” University of Chicago Press, Chicago, London, 2003.
- J. M. Lorch, L. K. Muller, R. E. Russell, M. O’Connor, D. L. Lindner and D. S. Blehert, “Distribution and Environmental Persistence of the Causative Agent of White-Nose Syndrome, Geomyces destructans, in Bat Hibernacula of the Eastern United States,” Applied and Environmental Microbiology, 2012, in press.
- J. M. Lorch, D. L. Lindner, A. Gargas, L. K. Muller, A. M. Minnis and D. S. Blehert, “A Culture-Based Survey of Fungi in Soil from Bat Hibernacula in the Eastern United States and its Implications for Detection of Geomyces destructans, yhe Causal Agent of Bat White-Nose Syndrome,” Mycologia, 2012, in press.
- D. L. Lindner, A. Gargas, J. M. Lorch, M. T. Banik, J. Glaeser, T. H. Kunz and D. S. Blehert, “DNA-Based Detection of the Fungal Pathogen Geomyces destructans in Soils From Bat Hibernacula,” Mycologia, Vol. 103, No. 2, 2011, pp. 241-246. doi:10.3852/10-262
- I. Alteras, “First Romanian Isolation of Histoplasma capsulatum from the Soil,” International Journal of Dermatology, Vol. 5, No. 2, 1966, pp. 69-71. doi:10.1111/j.1365-4362.1966.tb05188.x
- B. Julg, J. Elias, A. Zahn, S. Koppen, C. Becker-Gaab and J. R. Bogner, “Bat-Associated Histoplasmosis Can Be Transmitted at Entrances of Bat Caves and Not Only inside the Caves,” Journal of Travel Medicine, Vol. 15, No. 2, pp. 133-136. doi:10.1111/j.1708-8305.2008.00193.x
- V. Krištůfek, D. Elhottová, L. Kováč, A. Chroňáková, K. Žák and I. Světlík, “Heap of Old Bat Guano in the Cave Domica (NP Slovak Karst) and Electron Microscopy of Bats Excrements/Stáří kopy netopýřího guana v jeskyni Domica (NP Slovenský Kras) a elektronová mikroskopie exkrementoů netopýrů,” Acta Carsologica Slovaca, Vol. 46, No. 1, 2008, pp. 165-172.
- A. Novaková, “Microscopic Fungi Isolated from the Domica Cave System (Slovak Karst National Park, Slovakia),” International Journal of Speleology, Vol. 36, No. 1, 2007, pp. 71-82.
- C. A. Dobony, K. E. Langwig, R. L. von Linden, J. C. Okoniewski, M. L. Verant, R. E. Rainbolt and A. C. Hicks, “White-Nose Syndrome: Lessons Learned at Fort Drum Military Installation, NY,” 5th Annual White-Nose Syndrome Symposium, Madison, 4-7 June 2012, p. 8.
- L. K. Muller, J. M. Lorch, D. L. Lindner, M. O’Connor, A. Gargas and D. S. Blehert, “Bat White-Nose Syndrome: A Real-Time TaqMan Polymerase Chain Reaction Test Targeting the Intergenic Spacer Region of Geomyces destructans,” Mycologia, 2012, in press.
- S. Chaturvedi, R. J. Rudd, A. Davis, T. R. Victor, X. Li, K. A. Appler, S. S. Rajkumar and V. Chaturvedi, “Rapid Real-Time PCR Assay for Culture and Tissue Identification of Geomyces destructans: The Etiologic Agent of Bat Geomycosis (White Nose Syndrome),” Mycopathologia, Vol. 172, No. 4, 2011, pp. 247-256. doi:10.1007/s11046-011-9435-5
- P. Presetnik, K. Koselj, M. Zagmajster, N. Aupič, K. Jazbec, U. Žibrat, A. Petrinjak and A. Hudoklin, “Atlas Netopirjev (Chiroptera) Slovenije,” Center za kartografijo favne in flore, Miklavž na Dravskem polju, 2009.
- A. Hudoklin and P. Presetnik, “The Cave Spodnja klevevška jama—Important Bat Roost and Newly Recorded Site of Schreiber’s Bat (Miniopterus schreibersii) in Dolenjska (South-Eastern Slovenia),” Natura Sloveniae, Vol. 7, No. 1, 2005, pp. 31-35.
- J. Mulec, V. Krištůfek and A. Chroňáková, “Comparative Microbial Sampling From Eutrophic Caves in Slovenia and Slovakia Using RIDA®COUNT Test Kits,” International Journal of Speleology, Vol. 41, No. 1, 2012, pp. 1-8. doi:10.5038/1827-806X.41.1.1
- J. Mulec, V. Krištůfek and A. Chroňáková, “Monitoring of Microbial Indicator Groups in Caves through the Use of RIDA®COUNT Kits,” Acta Carsologica, Vol. 42, No. 2-3, 2012, pp. 287-296.
- G. A. Kochkina, N. E. Ivanushkina, V. N. Akimov, D. A. Gilichinski and S. M. Ozerskaia, “Haloand Psychrotolerant Geomyces Fungi from Arctic Cryopegs and Marine Deposits,” Mikrobiology, Vol. 76, No. 1, 2007, pp. 39-47.
- L. J. Maher, “Environmental Information from Guano Palynology of Insectivorous Bats of the Central Part of the United States of America,” Palaeogeography Palaeoclimatology Palaeoecology, Vol. 237, No. 1, 2006, pp. 19- 31. doi:10.1016/j.palaeo.2005.11.026
- J. Mulec, J. Vaupotič and J. Walochnik, “Prokaryotic and Eukaryotic Airborne Microorganisms as Tracers of Microclimatic Changes in the Underground (Postojna Cave, Slovenia),” Microbial Ecology, Vol. 64, No. 3, 2012, pp. 654-667. doi:10.1007/s00248-012-0059-1
- C. H. Robinson, “Cold Adaptation in Arctic and Antarctic Fungi,” New Phytologist, Vol. 151, No. 2, 2001, pp. 341- 353. doi:10.1007/s00248-008-9387-6
- S. K. Schmidt, K. L. Wilson, A. F. Meyer, M. M. Gebauer and A. J. King, “Phylogeny and Ecophysiology of Opportunistic ‘Snow Molds’ from a Subalpine Forest Ecosystem,” Microbial Ecology, Vol. 56, No. 4, 2008, pp. 681-687. doi: 10.1007/s00248-008-9387-6
- A. Chroňáková, A. Horák, D. Elhottová and V. Krištůfek, “Diverse Archaeal Community of a Bat Guano Pile in Domica Cave (Slovak Karst, Slovakia),” Folia Microbiologica, Vol. 54, No. 5, 2009, pp. 436-446. doi:10.1007/s12223-009-0061-2
- R. Shahack-Gross, F. Berna, P. Karkanas and S. Weiner, “Bat Guano and Preservation of Archaeological Remains in Cave Sites,” Journal of Archaeological Science, Vol. 31, No. 9, 2004, pp. 1259-1272. doi:10.1016/j.jas.2004.02.004
- L. A. Walker, V. R. Simpson, L. Rockett, C. L. Wienburg and R. F. Shore, “Heavy Metal Contamination in Bats in Britain,” Environmental Pollution, Vol. 148, No. 2, 2007, pp. 483-490.
- V. Krištůfek, A. Chroňáková and J. Mulec, “The Heavy Metal Content in Bat Guano Heaps in Karst Caves,” Abstract Book: 20th International Conference on Subterranean Biology, Postojna, 29 August-3 September 2010, pp. 101-102.
NOTES
*Corresponding author.

