American Journal of Analytical Chemistry
Vol. 3 No. 1 (2012) , Article ID: 16798 , 7 pages DOI:10.4236/ajac.2012.31011
Remediation of Malathion Contaminated Soil Using Zero Valent Iron Nano-Particles
1Analytical Chemistry Division, Bhabha Atomic Research Centre, Mumbai, India
2Environmental Sciences, S. V. University, Tirupati, India
Email: *rsinghal@barc.gov.in
Received September 19, 2011; revised October 24, 2011; accepted November 8, 2011
Keywords: Malathion; Zero Valent Iron; Nano Particle; Soil; Contamination
ABSTRACT
In this study, iron nano-particles were used to remediate malathion contaminated soil in the concentration range of 1 - 10 µg·g–1. The zero valent iron nano-particles were prepared by reducing ferric chloride solution with sodium borohydride for remediation of the soil. The optimized quantity of iron nano particles was found to be 0.1 g·kg–1 of soil contaminated with 10 mg·g–1 of malathion. Malathion was determined in the soil after leaching to water at pH 8.2 and followed by its oxidation with slight excess of N-bromosuccinimide (NBS). The unconsumed NBS was estimated by measuring the decrease in the color intensity of rhodamine B. Degradation product formed during the oxidation of malathion by zero valent iron was monitored by the Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR). The results clearly showed that quantitative oxidation of malathion was achieved within eight minutes after the addition of zero valent iron nano particles.
1. Introduction
Organophosphates are esters of phosphoric acid, thiophosphoric acid and other phosphoric acids, which are widely used as insecticides and acaricides. [1,2] Malathion {Diethyl 2-[(dimethoxyphosphorothioyl)sulfanyl] butanedioate}, commonly referred to as organophosphorous pesticides (OPPs) is used as insecticides for control of insects on fruits and vegetables. Organophosphorus pesticides (OPPs) are a class of chemicals that generally act as cholinesterase inhibitors and have been widely used in agriculture as the replacement for persistent organochlorinated pesticides [3]. However, organic phosphate pesticides are of great environmental concern primarily because they are toxic to mammals and birds [4]. Malathion is a non systemic wide-spectrum organophosphate insecticide and is one of the earliest organophosphate insecticides developed in 1950. It is used for the control of sucking and chewing insects on fruits and vegetables, and also used to control mosquitoes, flies, household insects, animal parasites (ectoparasites). (Malathion may also be found in formulations with many other pesticides).
Pesticides, like other organic pollutants, are retained by the soil according to their physicochemical properties, nature and composition [2]. Malathion is of low persistence in soil with reported retention half-lives of 1 to 25 days. Its degradation in soil is well known and rapidity of its degradation is related to the degree of soil binding. Breakdown occurs by a combination of biological degradation and nonbiological reaction with water. If released to the air atmosphere, malathion will break down rapidly in presence of sunlight, with a reported half-life of 1.5 days [3-5]. The fate of malathion in soil has been studied extensively. It has been reported that the rate of its degradation increases with increasing moisture/alkaline conditions [6]. It is moderately bound to soil and is soluble in water. Therefore, it may contaminate groundwater or surface water in situations which are less conductive to their breakdown [5].
Due to the environmental concerns associated with the accumulation of the organophosphorus pesticides in food products and water supplies, efforts are focussed to develop safe, convenient and economically feasible methods for pesticides detoxification during the last two decades [7,8]. Malathion, despite of its high toxicity is still extensively used throughout the world [7]. Various environmental friendly materials like soil micro flora and rhizophus oryzae biomass, which are efficient adsorbents for malathion, have been suggested for the decontamination of organophosphorous pesticides (OPPs) from soil [9,10]. Shan et al. [11] studied the removal efficiency of malathion from wastewater of organophosphate pesticide mill, using a bacterium, Acinetobacterjohnsonii MA19 [2,11].
In this study, zero valent iron nanoparticles have been used to degrade malathion in ambient environment. It was reported that the addition of iron compounds (Fe0, Fe2+) enhanced the degradation efficiency of OPPs [12- 14]. The present paper describes the investigation on the remediation of malathion contaminated soil using iron nano particles.
2. Materials and Methods
2.1. Chemicals and Reagents
Malathion used in this study was obtained from Sigma Aldrich and was used as received. Doubly distilled water (Millipore) was used throughout the experiment for the preparation of the reagents. All other reagents were of analytical grade and were used without further purification. Stock solution of malathion, was prepared by dissolving 100 mg of pesticides (technical forms and formulations) in minimum amount of glacial acetic acid [Merck] and then diluting to 100 mL with distilled water. Working standards were prepared by appropriate dilution. An aqueous solution of 0.01% (w/v) N-bromosuccinimide (Merck.), 0.02% (w/v) rhodamine B and 4 mol·L–1 hydrochloric acid were prepared. Freshly prepared NBS solution was used. Glacial acetic acid (Merck) was used.
2.2. Synthesis of Iron Nano Particles
The nano-particles of Fe were prepared by reducing ferric chloride solution (FeCl3·6H2O) with sodium borohydride (NaBH4). 1.6 M sodium borohydride solution was added drop wise to the 0.2 M ferric chloride solution under stirring. The high concentration difference between the two solutions leads to the formation of iron nanoparticles. Optimum stirring speed of the magnetic stirrer and concentration of FeCl3·6H2O were arrived at by carrying the experiments by varying these two parameters and arriving at desired size nano particles [15,16].
2.3. Measurement of Hydrodynamic Diameter of Fe Nanoparticles
Malvern, Zeta-sizer nano zs with MPT-2 Auto-titrator was used for the measurement of hydrodynamic diameter of the Fe nano particles. 1.5 mL of the aliquot was taken in the transparent cell for recording the particle size distribution using Dynamic Light Scattering [NIBS,{Non Invasive Back Scattered}]. By this technique the hydrodynamic diameter in the range of 0.6 nm - 6 mm could be measured. Details of the same are discussed elsewhere. [17] The measurements of zeta potential at different pH values showed that the iso-electric-point of iron nanoparticles occurred around 8.1 - 8.2.
2.4. Simulation Experiments for Contamination of the Soil with Malathion
Soil was contaminated with malathion under ambient atomspheric conditions, where 1000 g of the soil was mixed with a stock solution of malathion. This process was carried out by forming slurry followed by slow addition of malathion under continuous stirring. The soil was then dried, hand crushed and passed through the 2 mm sieve. Unused malathion was quantified as discussed below.
2.5. Determination of Malathion
Malathion was estimated indirectly by spectrophotometry. The N-bromosuccinimide (NBS) was used to gradually oxidise malathion [6,18]. Excess NBS, when reacted with rhodamine B, decrease its absorbance for which maximum absorbance was at 550 nm [18]. The decrease in the absorbance is directly proportional to the amount of NBS. From these values, reacted NBS and in turn amounts of malathion were calculated. Beer’s law was obeyed in the concentration range of 0.1 - 1.0 µg·mL–1 [6,18]. All the measurement of malathion have Instrumental RSD < 0.5% whereas the method RSD is <2%.
2.6. Mixing of Malathion Contaminated Soil with Zerovalent Iron Nanoparticles
0.1 g of iron nanoparticle powder was mixed manually with 1000 g of dry soil. To have a moisture equivalent of about 20%, 200 mL of deionised water was added dropwise to this soil. Then it was homogenized with a Teflon rod attached to a mechanical stirrer for 30 minutes and kept for equilibration for 12 h. A control soil sample, without adding iron nanoparticles, was prepared in a similar manner.
2.7. Leaching of Malathion from the Soil
Malathion was leached from the contaminated and control soil samples using ground water having pH in the range of 7.3 - 8.5. The protocol for the leaching of the malathion from the soil was standardized by using an inhouse soil standard. Entire set of experiments consisting of preparation of malathion contaminated soil, control sample and leaching of malathion were completed within two days to avoid possible degradation of malathion in soil.
3. Results and Discussion
Various physicochemical characteristics of the soil and groundwater used in the present study are given in the Tables 1 and 2 respectively. Four different types of soils have been used for this study. No major variation of various soil parameters were observed among these soils (Table 1). Ground water samples also did not show major variation in the characteristics like pH, conductance and redox potential as well as levels of different elements present.
3.1. Optimization of Leaching of Malathion from the Soil
Leaching of malathion was carried out using groundwater in the pH range of 4.5 to 8.5 to arrive at optimum pH. The concentration values as a function of pH are plotted in Figure 1. From the figure it is clear that leaching was low at pH 4.5 (30%) and gradually increased with pH. It was observed that at pH 8.2 about 80% of the spiked malathion was leached from the soil and thereafter percentage of leaching of malathion from soil remained nearly constant. Recovery of malathion from the soil was verified from the results of freshly prepared in-house soil standard and the trends were similar. The cause of preferential leaching of malathion in groundwater at pH 8.2 could be due to preferential binding of OH– over the malathion with the organic matter of the soil. Once the pesticide enters the soil it is partitioned between soil particles and soil solution. In soil particles, malathion particularly binds with the soil organic matter. If organic matter is associated with soil particles, then it is favorable for binding pesticides. However when pH is increased from 4.5 to 8.2, the adsorbed pesticide molecules on soil organic matter are desorbed [1,19].
3.2. Characterization of Zerovalent Iron Nanoparticles
Distribution of hydrodynamic diameter of the zerovalent iron nanoparticles in aqueous suspension is shown in Figure 2 and it suggest that the hydrodynamic diameter was 33 nm. The size standardization was done by using latex particles dispersed in 0.92% NaCl medium having a mean hydrodynamic diameter of 60 nm. The suspension has been monitored for 16 h by taking the aliquots at 1 hour intervals. The various measurements show similar size distribution indicating the stability of the suspension.
The XRD diffractogram of freshly synthesized zero valent iron nanoparticle is shown in Figure 3. The XRay Diffraction (XRD) pattern recorded using Cu Ka radiation in the 2q range between 10˚ - 90˚. The figure indicates that iron is mainly in its Fe0 state, characterized by the basic reflection appearing at a 2-theta value of 44.9˚ and additional peaks at 65.22˚, and 82.50˚ These peaks represent bcc (body-centered cubic crystal) Fe(0) lattice planes(110), bcc Fe(0) (200), and bcc Fe(0) (211).
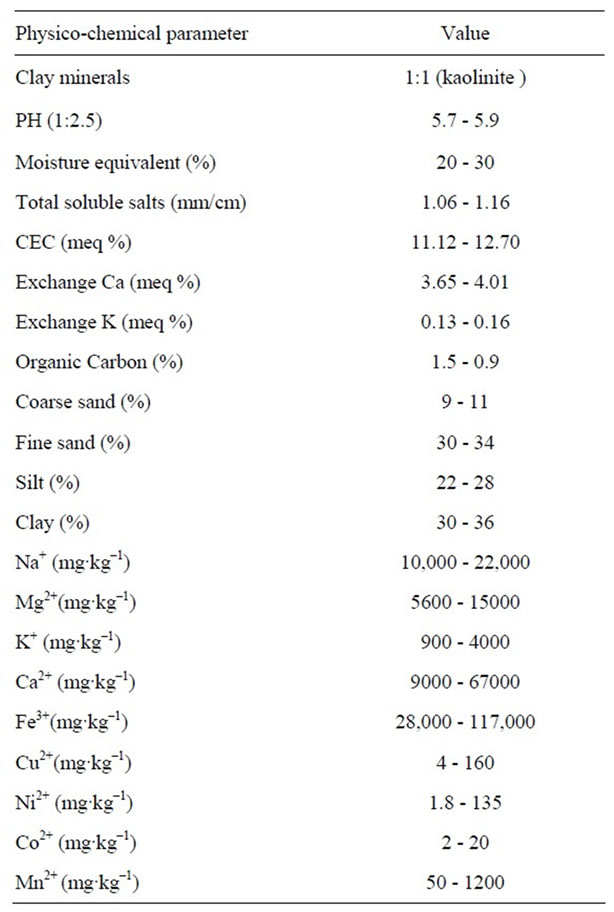
Table 1. Range of physicochemical parameters observed in case of four different soil.

Table 2. Physicochemical characteristics of the ground water.
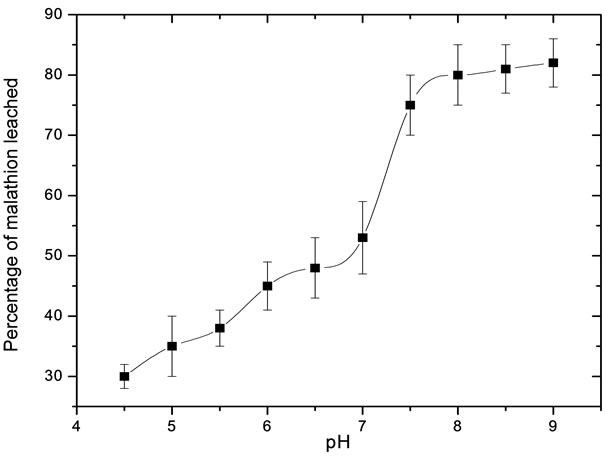
Figure 1. Variation of the percentage leached malathion as a function of pH.
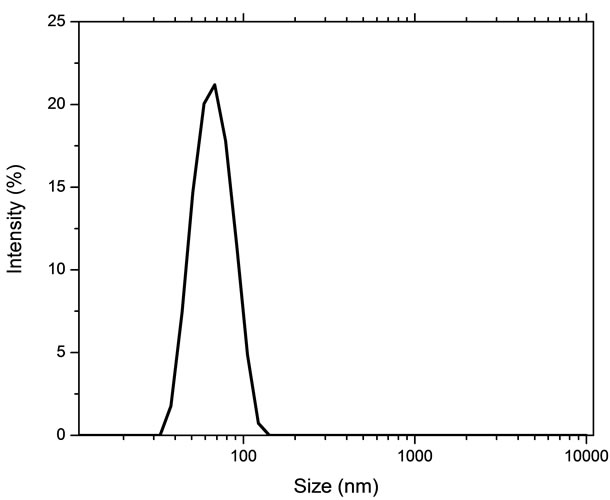
Figure 2. Hydrodynamic particle size distribution of iron nanoparticles.
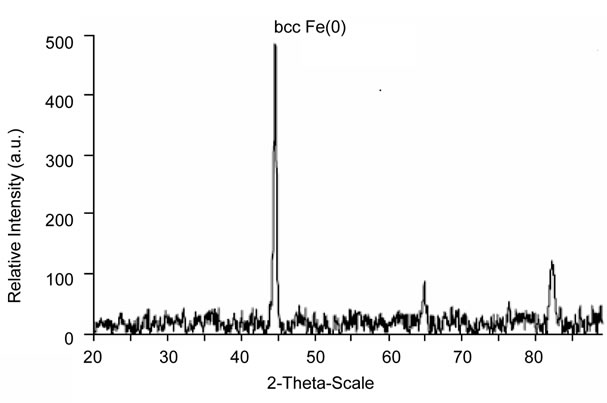
Figure 3. XRD pattern of zero valent iron nanoparticles.
The particles were further characterize by measuring the BET surface area of the particles which comes out to be 23 - 25 m2·g–1.
3.3. Optimization of Concentration of Zerovalent Iron Nanoparticles
Soil contaminated with 10 µg·g–1 of malathion was treated with zero valent Fe nanoparticles in the range of 10 - 130 mg·kg–1 of the soil. It is observed from the Figure 4 that there is a decrease in the concentration of malathion with increase in the concentration of Fe nanoparticles upto 100 mg·kg–1 of the soil and malathion is completely degraded. Therefore, it can be concluded that the optimum value of zero valent Fe nanoparticles is 0.1 g·kg–1 of the soil. This is mainly due to oxidation potential [Fe(0) to Fe(+2) is 0.44 V)] generated by 100 mg·kg–1 of zero valent iron nanoparticles is sufficient to oxidise 10 mg (10 µg·g–1 × 1000 g) malathion in soil.
3.4. Validation of Optimized Concentration of Fe-Nanoparicles
Figure 5 gives the percentage degradation of malathion keeping the concentration of Fe nanoparticles as 0.3 g·kg–1 of the soil. The concentration of malathion was varied from 10 to 50 mg·kg–1 of the soil. It was observed that 100% degradation of malathion was there up to 30 mg·kg–1 and thereafter degradation was only 80% and 60% respectively in the soils having malathion contamination of 40 and 50 mg·kg–1.
3.5. Impact of Particle Size on the Degradation of Malathion
Kinetics of degradation of malathion has been investigated by varying the size of the zero valent iron nanoparticles. Results obtained are given in Figure 6 which shows the time for the complete degradation of malathion as a function of particle size of the zero valent iron nanoparticle. From this figure it is also clear that in the size range of 33 to 78 nm, with the lower size particles, time taken for the total degradation of malathion has been low indicating that surface area favours degradation reaction. After 78 nm, degradation of malathion was not 100% and the kinetic have reached a constant with increase in particle size. The results clearly indicated that that required potential for oxidation of Fe0 to Fe2+ is there in all the cases for degradation of the malathion. Degradation kinetics was slow in case of larger size Fe nanoparticles indicating surface area dependency of the reaction. This clearly pointed out that higher specific surface area of iron particles is desired for faster degradation [14, 20].
3.6. Mechanism of Degradation of Malathion with Zerovalent Fe Nanoparticles
Iron is one of the major elements present in the soil mostly in the forms of hydroxi-des/oxides/chlorides. Ge-
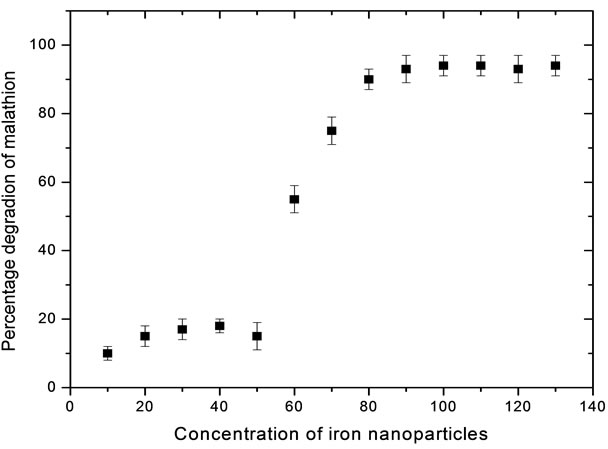
Figure 4. Optimization of concentration of Fe nanoparticles for 1 kg of the soil having 10 µg·g–1 malathion.
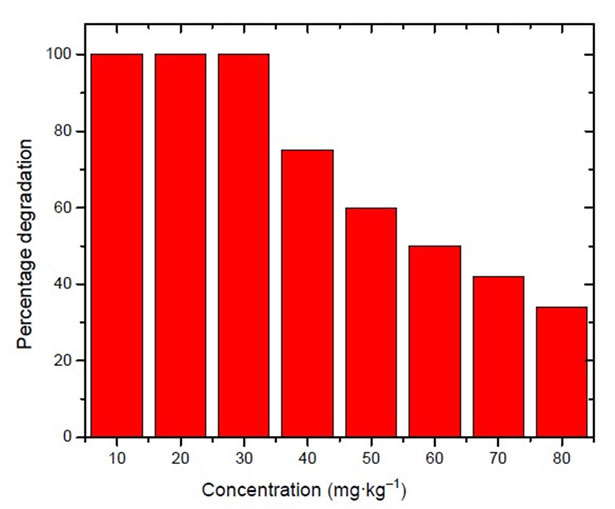
Figure 5. Variation in degradation of malathion in soil having malathion in different concentration and constant concentration of zero valent nanoparticles.
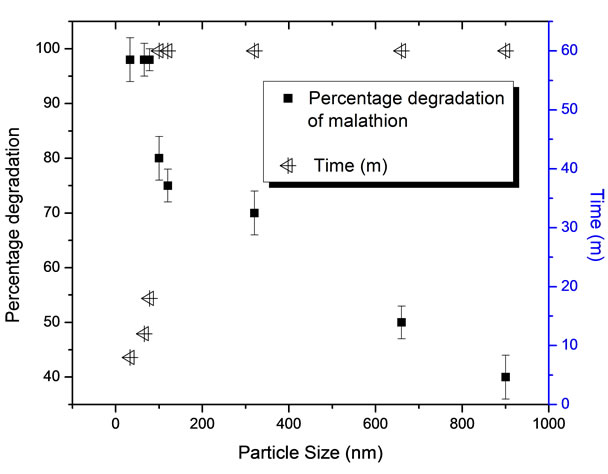
Figure 6. Impact of size of Iron nanoparticles on the degradation of malathion.
nerally the most dominant oxidation state is Fe3+ and when reducing conditions (like subsurface environment) are prevailing, iron exists as Fe2+. The concentration of iron in four different soils considered under this study varied between 40,000 µg·g–1 to 50,000 µg·g–1. It was experimentally observed that the presence of iron as hydroxide/oxide/chloride was not effective for the degradation of malathion in the soil [20,21]. This fact was verified by contaminating the ground water with 10 µg·mL–1 of malathion and mixing with 1 µg·mL–1 of Fe as FeCl3 solution. Concentration of malathion was monitored upto 60 h and the results indicate that except for a very small decrease (5% to 10%) not much change was observed in the concentration of malathion. However, when 0.1 g of iron nanoparticles was added to 1000 mL solution having concentration of malathion as 10 µg·mL–1, no trace of malathion was detected even after eight minutes. These experimental facts clearly indicated that only zero valent nanoparticles oxidized malathion.
3.7. Characterization of the Degradation Product by FTIR
Malathion has several chemical bonds that are potentially labile under anticipated environmental reaction conditions. Wolfe et al. [22] in their work showed various possible pathways resulting in sulfur-carbon bond cleavage proceeding through an elimination reaction that gives O,O-dimethyl phosphorodithioic acid and diethyl fumarate [22]. Phosphorus-sulfur bond cleavage by water or hydroxide would give diethyl thiomalate and O,O-dimethyl phosphorothionic acid which would be in equilibrium with its tautomer, O,O-dimethyl phosphorothiolic acid. Carboxyl ester hydrolysis would give two possible products, malathion α- and β-monoacids [23]. Another potential reaction under environmental conditions would be oxidation of the sulfur-phosphorus double bond to give malaoxon. The detection of the degradation product was done by recording the FTIR spectrum using ATRFTIR (Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy). In order to get the confirmation of degraded product, malathion was leached in water and mixed with zerovalent iron nanoparticles. Aliquots from this reaction mixture were withdrawn at different time interval sand instantaneously their ATR-FTIR were recorded by transferring a drop of the solution over the diamond crystal. The spectrum was recorded by putting a drop of each solution on the diamond crystal. All ATR FTIR spectra were recorded between 500 - 4000 cm−1 region with spectral resolutions of 4 cm−1. All the spectra were processed by OPUS software (ver.6.5 Build 5.5.97) (copyright Bruker Optics GmbH 2009.). Figure 7 shows the recorded FTIR spectrum for the malathion. The bands located at 1730 cm−1, 1005 cm−1 and 853 cm−1 are the most intense ones. The signal at 1730 cm−1 is due to the
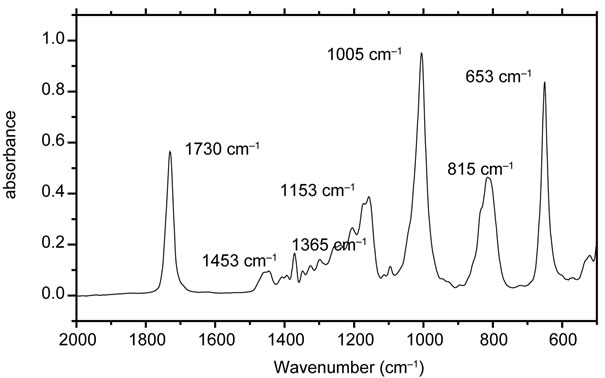
Figure 7. ATR FTIR spectrum of malathion in wavelength region of 2000 - 500 cm−1.

Figure 8. Decrease in the intensity of absorption band of P-OCH3 at 1005 cm−1 with time after addition of Fe nanoparticles
stretching of carbonyl of the ester group. The absorptions around 1453 cm−1 and 1365 cm−1 are due to the various vibrational modes of -CH2 and -CH3 groups. The intense band at 1005 cm−1 is due to stretching of P-OCH3 group. The degradation of malathion was measured by measuring the decrease in the intensity of this band [23-25]. Figure 8 shows the decrease in the intensity of absorption band with time. Similarly, the kinetics of the degradation of the malathion was studied by taking out the aliquots at different time intervals and subsequently recording their ATR FTIR. No significant change was observed in ATR FTIR spectra up to 5 minutes. But the samples taken after 5 minutes showed a slight variation in their ATR FTIR spectra. ATR FTIR spectra of samples taken after ten minutes showed spectra very close to O,O-dimethyl phosphorodithioic.
4. Conclusion
Experimental work carried out under this programme showed that 80% of the malathion was adhered to the soil particles whereas only 20% was dissolved in the soil solution in case of direct contamination of soil with malathion. Degradation of malathion was only effective with zero valent nanoparticles having a hydrodynamic diameter in the range of 33 - 100 nm. Optimized concentration of zero valent iron nanoparticle for degradation of 1 kg of the soil having 10 µg·g–1 of malathion was 0.1 g. Degradation of malathion became slower as the particle size increased from 33 nm to 100 nm. Malathion adhered to the soil particles were quantitatively leached in groundwater at pH 8.2. Malathion was degraded to O-dimethyl phosphorodithioic, which is not toxic either to human or any other living beings.
5. Acknowledgements
The authors sincerely acknowledge the encouragement provided by Prof. T. Mukherjee, Director chemistry group.
REFERENCES
- W. P. Gibson and R. G Burns, “The Breakdown of Malathion in Soil and Soil Components,” Microbial Ecology, Vol. 3, No. 3, 1977, pp. 219-230. doi:10.1007/BF02010619
- C. A. Edwards, “Environmental Pollution by Pesticides,” Plenum Press, London, 1973, pp. 78-79.
- M. A. Gallo and N. J. Lawry, “Handbook of Pesticides Toxicology,” Academic: E.R. Laws Press, New York, 1991, pp. 3-10.
- R. E. Meye, “Chemistry of Hazardous Materials,” Prentice Hall, Inc., Englewood Cliffs, 1977, pp. 208-210.
- F. Derek, I. Laine and F. Cheng, “The Destruction of Organic Pollutants under Mild Reaction Conditions: A Review,” Microchemical Journal, Vol. 85, No. 2, 2007, pp. 183-193. doi:10.1016/j.microc.2006.07.002
- F. A. Patty, “Industrial Hygiene and Toxicology,” Vol. 2, Interscience Publishers, New York, 1963, pp. 1950-1952
- B. Sunitha, A. Mathew, K. Pillai and V. K. Gupta, “A Rapid Spectrophotometric Assay of Some Organophosphorus Pesticide Residues in Vegetable Samples,” Spectrochimica Acta Part A, Vol. 67, No. 5, 2007, pp. 1430- 1432.
- A. W. Bourquin, “Degradation of Malathion by SaltMarsh Microorganisms,” Applied and Environmental Microbiology, Vol. 33, No. 2, 1977, pp. 356-362.
- L. W. Getzin and I. Rosefield, “Organophosphorus Insecticide Degradation by Heat Labile Substances in Soil,” Journal of Agricultural and Food Chemistry, Vol. 16, No. 4, 1968, pp. 598-601. doi:10.1021/jf60158a031
- S. Kumar, K. G. Mukerji and R Lal, “Molecular Aspects of Pesticide Degradation by Microorganisms,” Critical Reviews in Microbiology, Vol. 22, No. 1, 1996, pp. 1-26. doi:10.3109/10408419609106454
- S. Xie, J. X. Liu, L. Li and C. L. Qiao, “Biodegradation of Malathion by Acinetobacter Johnsonii MA19 and Optimization of Cometabolism Substrates,” Journal of Environmental Sciences, Vol. 21, No. 1, 2009, pp. 76-82. doi:10.1016/S1001-0742(09)60014-0
- S. H. Joo, A. J. Feitz and T. D. Waite, “Oxidative Degradation of the Carbothioate Herbicide, Molinate, Using Nanoscale Zero-Valent Iron,” Environmental Science & Technology, Vol. 38, No. 7, 2004, pp. 2242-2247. doi:10.1021/es035157g
- H. M. Hung and M. R. Hoffmann, “Kinetics and Mechanism of the Enhanced Reductive Degradation of CCl4 by Elemental Iron in the Presence of Ultrasound,” Environmental Science & Technology, Vol. 32, No. 19, 1998, pp. 3011-3016. doi:10.1021/es980273i
- W. X. Zhang, “Nanoscale Iron Particles for Environmental Remediation: An Overview,” Journal of Nanoparticle Research, Vol. 5, No. 3-4, 2003, pp. 323-328. doi:10.1023/A:1025520116015
- F. Gilbert, P. Refait, F. Leveque, C. Remazeilles and E. Conforto, “Synthesis of Goethite from Fe(OH)2 Precipitates: Influence of Fe(II)Concentration and Stirring Speed,” Journal of Physics and Chemistry of Solids, Vol. 69, No. 8, 2008, pp. 2124-2130. doi:10.1016/j.jpcs.2008.03.010
- J. Cao, P. Clase and W. Zhang, “Nanoporous Zero-Valent Iron,” Journal of Materials Research, Vol. 20, No. 12, 2005, pp. 32-39. doi:10.1557/jmr.2005.0401
- R. K. Singhal, R. Karpe, K. P. Muthe and A. V. R. Reddy, Pu-239+240 Selectivity for Pseudo-Colloids of Iron in Subsurface Aquatic Environment Having Elevated Level of Dissolved Organic Carbon,” Journal of Radioanalytical and Nuclear Chemistry, Vol. 280, No. 1, 2009, pp. 141-148. doi:10.1007/s10967-008-7390-5
- R. M. Kurakalva, R. Attinti and S. Kalluru, “Extractive Fluorimetric Determination of Malathion Residues in Water,” Analyst, Vol. 125, No. 2, 2000, pp. 323-326. doi:10.1039/a904207e
- W. W. Walker and B. J. Stojanovic, “Microbial versus Chemical Degradation of Malathion in Soil,” Journal of Environmental Quality, Vol. 2, No. 2, 1973, pp. 229-232. doi:10.2134/jeq1973.00472425000200020012x
- C. Wang and W. X. Zhang, “Nanoscale Iron Particles for Reductive Dechlorination of PCE and PCBs,” Environmental Science & Technology, Vol. 31, No. 7, 1997, pp. 2154-2156. doi:10.1021/es970039c
- K. J. Cantrell, D. I. Kaplan and T. W. Wietsma, “Zerovalent Iron for the in Situ Remediation of Selected Metals in Groundwater,” Journal of Hazardous Materials, Vol. 42, No. 2, 1995, pp. 201-212. doi:10.1016/0304-3894(95)00016-N
- N. L. Wolfe, R. G. Zepp, J. A. Gordon, G. L. Baughman, D. M. Cline, “Kinetics of Chemical Degradation of Malathion in Water,” Environmental Science & Technology, Vol. 11, No. 1, 1977, pp. 88-93. doi:10.1021/es60124a001
- M. Khanmohammadi, M. A. Karimi, K. Ghasemi, M. Jabbari and A. G. Bagheri, “Quantitative Determination of Malathion in Pesticide by Modified Attenuated Total Reflectance-Fourier Transform Infrared Spectrometry Applying Genetic Algorithm Wavelength Selection Method,” Talanta, Vol. 72, No. 2, 2007, pp. 620-625. doi:10.1016/j.talanta.2006.11.029
- G. Quintas, S. Garrigue and M. de la Guardia, “FT-Raman Spectrometry Determination of Malathion in Pesticide Formulations,” Talanta, Vol. 63, No. 2, 2004, pp. 345-350. doi:10.1016/j.talanta.2003.11.004
- G. M. Quintás, A. Asunción, S. Sergio and M. D. L. G. Garrigues, “Fourier Transform Infrared Spectrometric Determination of Malathion in Pesticide Formulations,” Analytica Chimica Acta, Vol. 502, No. 2, 2004, pp. 213-220. doi:10.1016/j.aca.2003.10.044
NOTES
*Corresponding author.

