International Journal of Clinical Medicine
Vol.2 No.5(2011), Article ID:8530,5 pages DOI:10.4236/ijcm.2011.25097
Integrating Complimentary and Alternative Medicine in Form of Active Hexose Correlated Compound (AHCC) in the Management of Head& Neck Cancer Patients
![]()
1Department of Radiation Oncology, North Eastern Indira Gandhi Regional Institute of Health & Medical Sciences, Shillong, India; 2Amino up Chemical Co. Ltd., Sapporo, Japan; 3National Institute of Biomedical Innovation, Osaka, Japan.
Email: *dkparida@hotmail.com
Received September 4th, 2011; revised October 17th, 2011; accepted October 26th, 2011.
Keywords: Head and Neck Cancer, Quality of Life, AHCC
ABSTRACT
Objectives: The Active Hexose Correlated Compound (AHCC), is produced from mushroom mycerium and rich in alpha glucans was administered to the cancer patients along with chemotherapy to see if it is having any beneficial effects on the final outcome in terms of reducing side effects of chemotherapy, maintaining the general condition and having effect on tumor control. Methods: Twenty five patients were administered AHCC along with conventional palliative chemotherapy regimen out of which sixteen patients received paclitaxel, and cisplatinum/carboplatin, nine patients received combination of cisplatim and 5-Flurouracil. All the patients were having advance stage (T3/T4) head and neck cancers. Thirteen patients were cancer of cheek, followed by cancer of tongue (4), oro-pharyngeal cancer (6) and cancer of naso-pharynx (2). Results: All the patients tolerated AHCC well with no added symptoms. Twenty patients reported that they are feeling stronger than before at the time of initiation of chemotherapy cycles. Almost all the patients reported to have better appetite after they started taking AHCC. Twelve patients who required blood transfusion before chemotherapy cycles, decrease in the rate of fall in hemoglobin was observed in these patients and only three patients required blood transfusion before subsequent chemotherapy cycles. In 22 patients definite reduction of chemotherapy side effects like nausea, vomiting, drop in total leucocytes count, loose motion/constipation etc. were observed, which reduced the hospital stay of these patients. Tumor regressed in 11 patients, 8 patients had stable disease and in rest of the patients, the disease progressed. Conclusions: AHCC up to 3 g is safe to administer and definitely helps cancer patients in reducing side effects of chemotherapeutic drugs, getting a sense of wellbeing and improved intake maintains general condition as well as prepare them to continue and tolerate further cycles in a better way.
1. Introduction
India contributes to maximum number of Tobacco Related Cancer Deaths in the world [1]. The use of tobacco also attributes towards tuberculosis, heart diseases and other lung conditions in addition to neoplastic diseases [1,2]. According to the national cancer registry (http:// www.ncrpindia.org/) data, the incidence of cancer of head and neck region is highest in male, where as cancer of esophagus is highest in female in the state of Meghalaya, one of the north-eastern states of India [1]. North eastern region (NER) of India consists of eight small states namely Assam, Arunachal Pradesh, NagalandMeghalaya, Manipur, Mizoram, Tripura and Sikkim. These states are mainly home to various tribal populations, belonging to various ethnic origins. As their domicile changes, their living condition, food pattern, disease spectrum and life span changes. Evidences show that majority of the population inhabiting NER use tobacco in some or other forms in addition to alcohol and supari (arica-nut) and combined use of all these factors are responsible for initiation of Oropharyngeal, Lungs and upper aerodigestive tract cancers. Amongst all these NER states the incidence of tobacco related cancers (TRC) are very high. According to the national cancer registry programme (NCRP) data, the difference of incidence of TRC in NER is too high in comparison to rest of the country [1].
Majority of these patients present with an advance stage disease, where the nutrition status of these patients remain low. Other poor prognostic features like old age, stage of the disease at presentation, neck node status, presence/absence of other metastatic sites are also very important, while considering these patients for any kind of treatment. With above mentioned features, many times it becomes difficult for the patients to tolerate various forms of anticancer treatment. Consequently it becomes very important to maintain the general condition of the patients at an optimum level so that the patients can tolerate further treatment. Therefore this trial was undertaken to see if complimentary and alternative medicine (CAM) in form of AHCC can maintain the nutrition/ immunity at optimum level, so the cancer patients having advance stage disease shall be able to tolerate further treatment like chemotherapy and radiotherapy for better tumor control. AHCC has never been tried in the management of head and neck cancer in the clinical study.
2. Materials and Methods
2.1. Cases
Twenty five patients of advance stage (T3: 13 & T4: 12) Head & Neck Cancer Patients were enrolled in this project. Thirteen patients were cancer of Cheek, followed by cancer of tongue (4), oro-pharyngeal cancer (6) and cancer of naso-pharynx (2) (Table 1). All the patients were either having residual or recurrent tumors subsequent to their primary treatment. Hence, these patients were managed with palliative chemotherapy treatment.
2.2. Active Hexose Correlated Compound (AHCC)
AHCC is an enzyme fermented extract of the mycelia of Basidiomycetes mushroom obtained through the mushroom (Lentinus edodes), containing a mixture of polysaccharides, amino acids, lipids and minerals. The final product is obtained by hot water extraction after culturing media and then treating them with enzymes. The predominant components of AHCC are oligosaccharides of which major portions are alpha-glucans having an effect on the immune system. It has been proven as a biological response modifier in experimental animals as well as human being. AHCC samples were provided by Amino Up Chemical Co., Ltd., Sapporo, Japan for conducting this trial.
Majority of the patient received Taxane based chemotherapy along with Platinum (Cispliatin/Carboplatin) (16). The dose of chemotherapy was customized depending on the general condition of each of the patients. Rest patients received platinum with 5 Fluorouracil combinations. Twelve patients also received targeted Monoclonal Antibody treatment in the form of epidermal growth factor receptor (EGFR) inhibitor. Some of the patients were heavily pre-treated with very low general condition. As such patients with head and neck cancers present with low general conditions because of less oral intake. Previous treatment history of the patients includes 6 patients had undergone surgery, 12 patients had history of radiotherapy and 16 patients had history of chemotherapy, prior to recruitment under this AHCC trial (Table 2). These patients acted as their own control.
All the patients were administered AHCC 3 g of dried extract every day morning 3 days prior to the chemotherapy with water scheduled date and followed up to one week post chemotherapy either with water or milk. The reasons being maximum toxicity of the chemotherapy drugs are observed within first one week following administration.
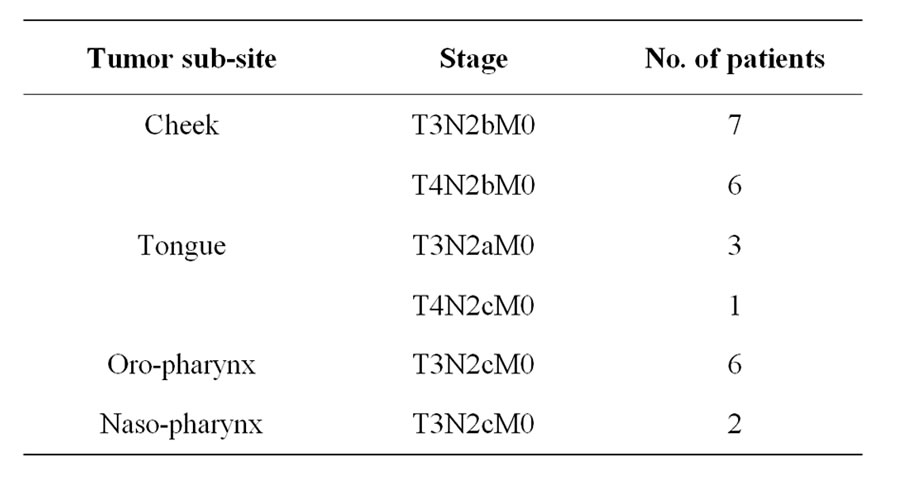
Table 1. Division of patients according to primary tumor site.
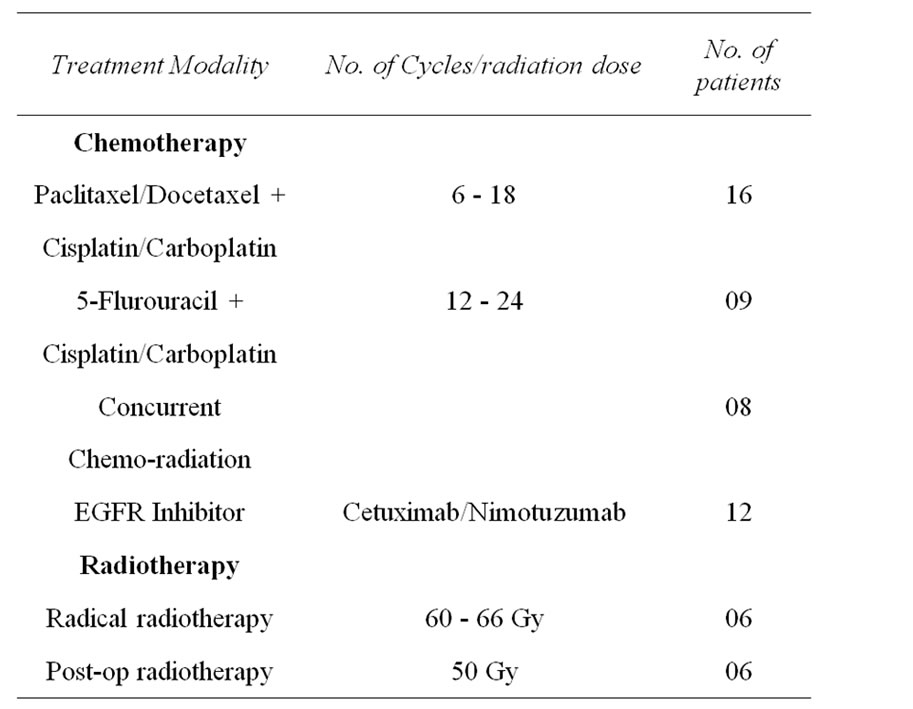
Table 2. Treatment history.
2.3. Assessment
The hematological parameters like hemogram, liver and kidney function tests were performed before each cycle of chemotherapy and followed 3 days after completion of chemotherapy. CT scan was done after completion of three cycles and two weeks after sixth cycle of chemotherapy to evaluate the tumor response. The patients were given a questionnaire on the quality of life issues and asked specific questions regarding their general feeling, sleep pattern, social interaction etc.
3. Results
All the patients tolerated AHCC well with no added symptoms. Twenty patients reported that they are feeling better and stronger than before at the time of initiation of chemotherapy cycles (Figure 1). In most of the patients, the sleep pattern became regular than before and the patients started interacting with visitors normally than before (Table 3). Almost all the patients reported to have better appetite after they started taking AHCC. No patients with AHCC required appetizer.
Sixteen patients who required blood transfusion before chemotherapy cycles, decrease in the rate of fall in hemoglobin was observed in these patients and only three patients required blood transfusion before subsequent chemotherapy cycles. Only 7 patients required growth factor with AHCC compared to 12 patients without it. Also no patients required platelet concentrate transfusion in AHCC group compared to 3 in without AHCC. The comparative charts are given in Figure 1. In 22 patients definite reduction of chemotherapy side effects like nausea, vomiting, loose motion/constipation etc. were observed, which reduced the hospital stay of these patients. While the requirement of antiemetic dropped from 7 - 14 days before AHCC group to 3 - 5 days in AHCC group, only 2 patients required hospitalization because of loose motion in AHCC group compared to 6 patients without AHCC group (Figure 2 and Table 3). Patiaents were evaluated both clinically as well as radiologically to determine tumor response. CT scan/MRI of the primary tumor as well as neck nodes were performed after two weeks of completion of at least six cycles of chemotherapy. Tumor regressed in 11 patients, 8 patients had stable disease and in rest of the patients, disease progressed (Table 4).
4. Discussion
AHCC is an alpha-glucan rich nutritional supplement extracted from the mycelia of shiitake (Lentinula edodes) of the basidiomycetes family of mushrooms. These intercellular chemical messengers trigger white blood cell production and activity [3,4]. The therapeutic effect is
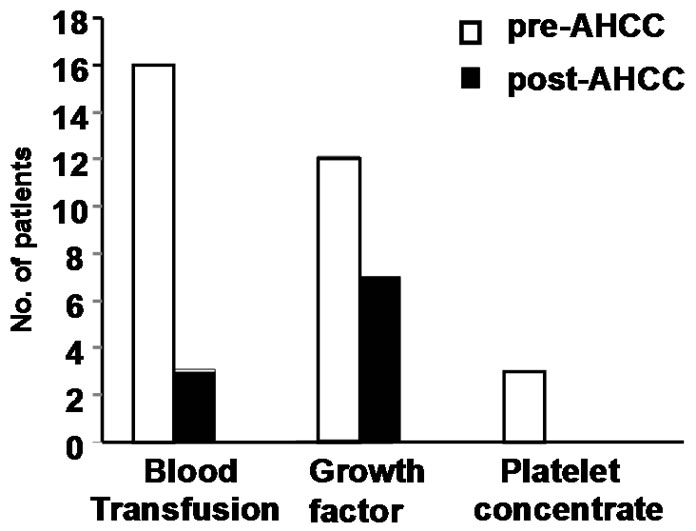
Figure 1. Comparison of hematological parameters in patients with and without AHCC, bringing down the blood transfusion rate (16 vs 3). Total leukocyte and platelet count also showed a slight fall in the patients with AHCC requiring no platelet concentrate transfusion and only 7 patients required growth factor supplement with AHCC.
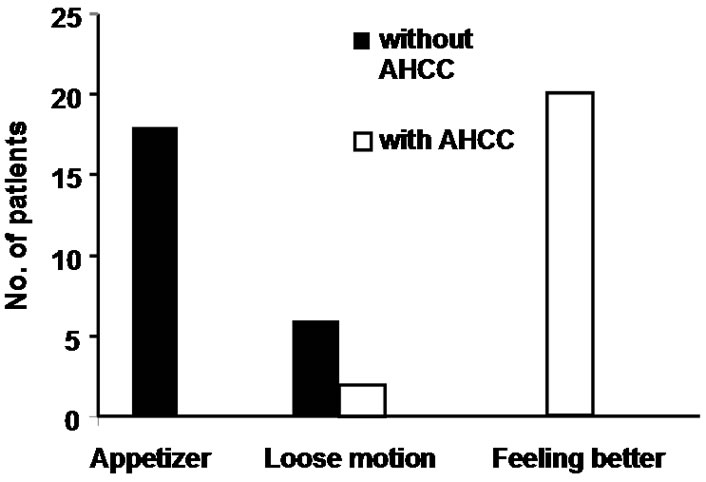
Figure 2. Comparison of quality of life concepts in patients requiring appetizer, having loose motion and better general condition with and without AHCC. While no patients with AHCC required any appetizer and only 2 patients were hospitalized for loose motion.
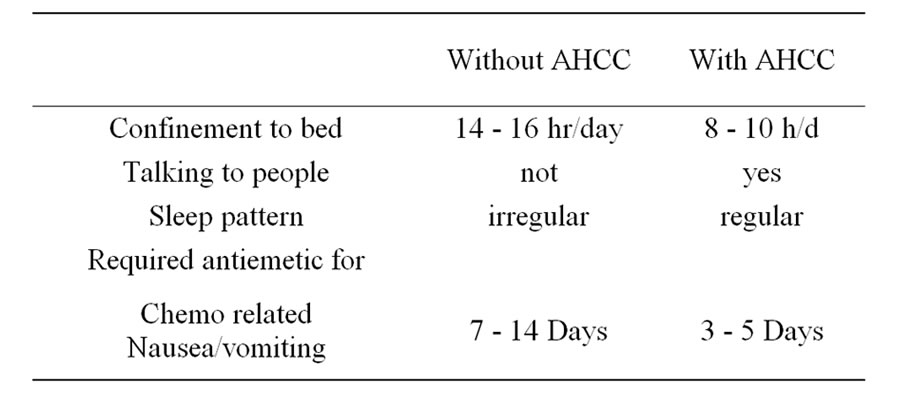
Table 3. Quality of life concept.
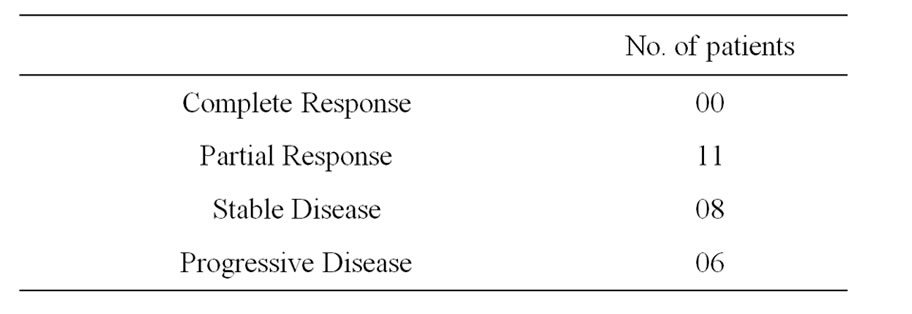
Table 4. Tumor response of the patients.
predominantly seen in higher basidiomycete family [5]. Studies show that AHCC also enhances production of cytokines, including interferon γ, tumor necrosis factor-α and interleukins (IL-2,4,6,10) [4]. In this present study majority of the patients, who received AHCC along with chemotherapy showed less fall in their hemoglobin level and total leukocyte count. However, researchers have found the influence of AHCC upon the innate immune system in animal studies and published the results in 1992. AHCC significantly increased natural killer (NK) cell activity in cancer patients, and also enhanced the effects of killer T-cells, and cytokines (interferon, IL-12, TNF-alpha) [3]. AHCC stimulates cell-mediated immunity by activating the white blood cells, particularly natural killer cells and macrophages, which directly attack abnormal cells, virus-infected cells or external vital and bacterial pathogens that enter the body. The fundamental mechanism of activating immunity is by means of stimulating the number of dendritic cells as these cells control the activities of B & T lymphocytic cells who are the ultimate mediators of immunity and hence, affected by AHCC. It also exhibits immunomodulating effects partially by regulating thymic apoptosis [6]. Nomura T et al. has published that besides immunogenic, AHCC is also having anti-teratogenic effects in animal models [7]. Effective immunity has got direct impact on tumor control and better tolerability of chemotherapeutic agents. In our series, it was significant that almost all the patients who received AHCC, tolerated chemotherapy better compared to their previous cycles of chemotherapy they had received without AHCC. At least eighteen out of the twenty-five patients acted as control of their own.
One major retrospective study suggests that AHCC intake has a preventive effect in postoperative hepatocellular carcinoma patients [8,9]. The study has compared the outcomes of 113 post-operative liver cancer patients taking AHCC with 156 patients in the control group. The results showed the rate of recurrence of malignant tumors was significantly lower (34.5% versus 66.1%) and patient survival was significantly higher in the AHCC group (79.6% vs. 53.2%). We tried AHCC for the first time in the patients suffering from cancers of head & neck region and obtained good results.
AHCC has been studied extensively for safety in human trials as well as safety with conventional chemotherapy [10,11]. There are also few studies regarding the interaction of AHCC with various kinds of chemotherapeutic agents [12-15]. There is a great deal of scientific evidence that AHCC not only helps to prevent the side effects of chemotherapy, but enhances its primary effectiveness as well. Several animal studies have shown that AHCC was able to relieve the side effects of several standard chemotherapy drugs like 5-FU, cisplatin, cyclophosphamide, mercaptopurine, methotraxate etc. “Severe” (50% to 100%) hair loss or alopecia caused by cytosine arabinoside (Ara-C) was reduced to slight, when AHCC was taken simultaneously. The ability of AHCC to enhance the effectiveness of chemotherapy was demonstrated in a study where rats were implanted with a cell line of spontaneous mammary adenocarcinoma. In the present study, the patients were administered AHCC along with chemotherapeutic agents like paclitaxel, docetaxel, cisplatin, 5-FU and certain monoclonal antibodies like cetuximab and nimotuzumab (Table 2). All the patients tolerated AHCC well. Routine side effects like nausea, vomiting, loose motions were less in these patients.
5. Conclusions
It can be concluded that AHCC is safe to administer and definitely helps cancer patients in reducing side effects of chemotherapeutic drugs, getting a sense of wellbeing and improved intake maintains general condition as well as prepare them to continue and tolerate further cycles in a better way. In advance stage disease achieving partial response/stable disease is also of significance, particularly when all the patients were having either recurrent or residual disease. Whether AHCC is responsible for the regression of the tumors, further trials are required to see the effects of AHCC on tumor control. Also dose enhancement trial has to be undertaken.
6. Acknowledgements
This work was carried out as a collaborative project between the North Eastern Indian Gandhi Regional Institute of Health and Medical Sciences and National Institute of Biomedical Innovation. This project was supported by the grant from the MEXT and MHLW, Japan and Amino up chemical Co. Ltd, Sapporo, Japan. We thank Haruko Ryo, National Institute of Biomedical Innovation for her critical observations and proof reading.
REFERENCES
- National Cancer Registry P Publication, 2007. http://www.ncrpindia.org
- P. Jha, B. Jacob, V. Gajalakshmi, P. C. Gupta, N. Dhingra, R. Kumar, D. N. Sinha, R. P. Dikshit, D. K. Parida, R. Kamadod, J. Boreham, R. Peto and RGI-CGHR Investigators, “A Nationally Representative Case-Control Study of Smoking and Death in India,” New England Journal of Medicine, Vol. 358, No. 11, 2008, pp. 1137-1147. doi:10.1056/NEJMsa0707719
- Y. Gao, D. Zhang, B. X. Sun, H. Fujii, K. Kosuna and Z. Yin, “Active Hexose Correlated Compound Enhances Tumor Surveillance through Regulating both Innate and Adaptive Immune Responses,” Cancer Immunology Immunotherapy, Vol. 55, No. 10, 2006, pp. 1258-1266. doi:10.1007/s00262-005-0111-9
- N. Terakawa, Y. Matsui, S. Satoi, et al., “Immunological Effect of Active Hexose Correlated Compound (AHCC) in Healthy Volunteers: A Double-Blind, Placebo-Controlled Study,” Nutrition and Cancer, Vol. 60, No. 5, 2008, pp. 643-651. doi:10.1080/01635580801993280
- S. Wasser and A. Weis, “Therapeutic Effects of Substances Occurring in Higher Basidiomycetes Mushrooms: A Modern Perspective,” Critical Reviews in Immunology, Vol. 19, No. 1, 1999, pp. 65-96.
- R. Burikhanov, K. Wakame, Y. Igarashi, S. Wang, S. Matsuzaki, “Suppressive Effect of Active Hexose Correlated Compound on Thymic Apoptosis Induced by Dexamethasone in the Rat,” Endocrine Research, Vol. 34, 2000, pp. 181-188.
- T. Nomura, et al., “Antiteratogenic Effects of Tumor Inhibitors, Caffeine, Antipain, and Retinoic Acid in Mice,” Cancer Research, Vol. 43, 1983, pp. 5156-5162.
- Y. Matsui, J. Uhara, S. Satoi, M. Kaibori, H. Yamada, H. Kitade, A. Imamura, S. Takai, Y. Kawaguchi, A. Kwon and Y. Kamiyama, “Improved Prognosis of Postoperative Hepatocellular Carcinoma Patients When Treated with Functional Foods: A Prospective Cohort Study,” Journal of Hepatology, Vol. 37, No. 1, 2002, pp. 78-86. doi:10.1016/S0168-8278(02)00091-0
- C. Suwanna, et al., “Prognostic Improvement of Patients with Advanced Liver Cancer after Active Hexose Correlated Compound (AHCC) Treatment,” Asian Pacific Journal of Allergy & Immunology, Vol. 24, No. 1, 2006, pp. 33-45.
- E. L. Spierings, H. Fujii, B. Sun and T. Walshe, “A Phase I Study of the Safety of the Nutritional Supplement, Active Hexose Correlated Compound (AHCC) in Healthy Volunteers,” Journal of Nutritional Science & Vitaminology, Vol. 53, No. 6, 2007, pp. 536-539. doi:10.3177/jnsv.53.536
- I. Hyodo, N. Amano and K. Eguchi, “Nationwide Survey on Complementary and Alternative Medicine in Cancer Patients in Japan,” Journal of Clinical Oncology, Vol. 23, No. 12, 2005, pp. 2645-2654. doi:10.1200/JCO.2005.04.126
- K. Matsushita, et al., “Combination Therapy of Active Hexose Correlated Compound (AHCC) Plus UFT Significantly Reduces the Metastasis of Rat Mammary Carcinoma,” Anti-Cancer Drugs, Vol. 9, 1998, pp. 343-350. doi:10.1097/00001813-199804000-00008
- A. Hirose, E. Sato, H. Fujii, S. Buxiang, H. Nishioka and O. I. Aruoma, “The Influence of Active Hexose Correlated Compound (AHCC) on Cisplatin-Evoked Chemotherapeutic and Side Effects in Tumor-Bearing Mice,” Toxicology and Applied Pharmacology, Vol. 222, No. 2, 2007, pp. 152-158. doi:10.1016/j.taap.2007.03.031
- C. Mach, H. Fujii, K. Wakame and J. Smith, “Evaluation of Active Hexose Correlated Compound Hepatic Metabolism and Potential for Drug Interactions with Chemotherapy Agents,” Journal of the Society for Integrative Oncology, Vol. 6, No. 3, 2008, pp. 105-109.
- K. Shigama, A. Nakaya, K. Wakame, H. Nishioka and H. Fujii, “Alleviating Effect of Active Hexose Correlated Compound (AHCC) for Anticancer Drug-Induced Side Effects in Non-Tumor-Bearing Mice,” Journal of Experimental Therapeutics and Oncology, Vol. 8, No. 1, 2009, pp. 43-51.

