Advances in Biological Chemistry
Vol.3 No.2(2013), Article ID:30826,10 pages DOI:10.4236/abc.2013.32030
Microbial analysis and surface characterization of SABIC carbon steel corrosion in soils of different moisture levels
![]()
1Biological Science Department, Microbiology Section, King Abdulaziz University, Jeddah, Saudi Arabia
2Chemistry Department, King Abdulaziz University, Jeddah, Saudi Arabia
Email: aamaljudaibi@kau.edu.sa, ahm13988@hotmail.com
Copyright © 2013 Awatif Al-Judaibi, Aisha Al-Moubaraki. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 15 January 2013; revised 16 March 2013; accepted 20 April 2013
Keywords: Effects of Strain; SEM; Steel; Crevice Corrosion; Microbiological Corrosion; Polymer Coatings
ABSTRACT
We tested the effect of three types of soil in Saudi Arabia on SABIC carbon steel grade X60 (SCSX60) specimens. The results showed that the environment effect of different condition was very clear, indicating that the studied soils were very corrosive SCSX60 specimens. The composition and morphology of corrosion were different in the tested soil based on moisture content and immersion period. In addition, the results showed that bacteria play an important role in the corrosion of SCSX60. The morphologies of corrosion products were analyzed using scanning electron microscopy to further elucidate the complex systems found in the studied soil.
1. INTRODUCTION
In oilfield operations, deteriorating effects such as the corrosion of equipment and installations, plugging of petroleum formation, and souring of the reservoir and fluids are caused by the activity of microorganisms [1-3]. For example in the oil and gas industry, 34% of corrosion damage experienced by one oil company was believed to be related to microorganisms [4-5].
Corrosion is an electrochemical process consisting of two partial reactions: an anodic reaction in which metal becomes corroded and a cathodic reaction where certain species are reduced. When pure metals or their alloys are exposed to soil, corrosion occurs immediately. In some cases, microorganisms from a biofilm on the metal surface, thereby contributing to corrosion reactions [6-8].
All materials can be inhabited by microorganisms, including metals, minerals, organic materials, and plastics. The dissolution of metals both directly and indirectly related to the activities of microorganisms is known as biocorrosion or microbiologically influenced corrosion (MIC). This process is not a distinct mechanism of corrosion; rather, it consists of the activity of microorganisms during normal corrosion processes [9-11]. MIC can result in increased costs in individual companies or sectors of the industry [6], and there have been different estimations for the cost associated with MIC. One of the most useful methods for studying the behavior of steel in soil with different concentrations of the masstour is the activity of microorganisms in soil.
Soil is attractive environment for microorganisms, which can live in soil at different concentrations of moisture [12]. The aim of this study was to elucidate the effect of the environment on SABIC carbon steel grade X60 (SCSX60) specimens, which are a pipeline type of steel that extend beneath the soil in several areas of Saudi Arabia. In addition, this study investigated the effect of the types of soil and exposure conditions (moisture content and immersion period) on the composition and morphology of SCSX60 in different areas. Lastly, the role of bacteria in the corrosion of SCSX60 was assessed in the studied soils.
2. MATERIALS AND METHODS
2.1. Specimens and Surface Pretreatment
Commercial carbon steel API-5L grade (X60) manufactured at Saudi Basic Industries Corporation (SABIC) from Al-Jubail, Saudi Arabia was used in this study. The steel type (X60) is manufactured according to chemical composition for use in pipelines and gas transmission (Table 1).
Specimens were cut into small rods of 1.0 cm in length and 1.0 cm in diameter. Prior to any experiment, the specimens were mechanically abraded using a series of emery papers, ranging from 100 to 1200 grits. The specimens were then washed with de-ionized water, degreased with acetone, and then air-dried. Finally, each specimen was weighed accurately and immersed in the specified test soil.
2.2. Soil Samples
2.2.1. Geographic Locations and Samples Collection
Soil samples were obtained from the various regions of the Kingdom of Saudi Arabia. All soils under investigation were collected from three different cities: Riyadh, Rabigh, and Jeddah. The geographic locations of these cities are shown in Figure 1.
At each of the selected sites, five samples were collected at a depth of approximately 1.5 m, which is approximately the same level as a buried pipeline. The soil samples were taken in polyethylene bags to the laboratory where they were air-dried at room temperature and then sieved. Every soil sample was then homogenized by putting the sample into a large polyethylene bag and rolling it in the bag. The whole sample was then poured into a clean polyethylene container.
2.2.2. Mechanical and Chemical Analysis
The classification of soils is based on different internal properties, such as color, texture, and structure. The texture of the soil refers to the relative amount of clay, silt, and various grades of sand and gravel which compose the soil mass [2]. The texture of the soil samples was determined in accordance with the Saudi Geological Survey laboratories in Jeddah. The results are listed in Table 2.
Table 1. Chemical composition of the studied specimens (wt%).
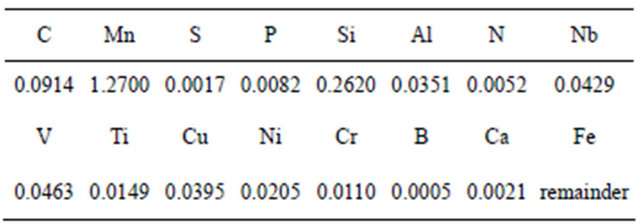
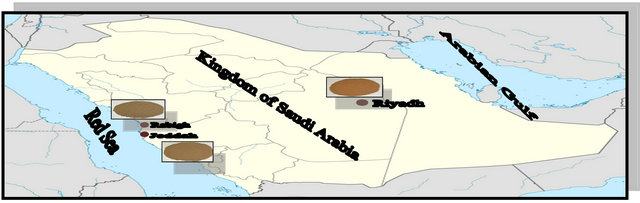
Figure 1. The geographic locations of these cities.
Table 2. Mechanical analysis of soil samples.

Soil physico-chemical parameters such as pH, electrical conductivity, electrical resistivity, redox potential, and oxides and salts content were estimated by the Saudi Geological Survey laboratories. The chemical analyses were duplicated, and the mean values of the resultant data are given in Tables 3 and 4.
2.2.3. Scanning Electron Microscopy (SEM)
The surface morphology of the steel specimens after prolonged immersion (5 weeks) in the studied soils at different moisture contents (2.5%, 10% and 20% wt) was assessed using SEM (Scanning electron microscope Quanta FEG 450).
2.3 Microbial Analysis
2.3.1. Under Aerobic Condition
Sabouraud dextrose agar was used as the medium with an OXOID code of CM41 [13]. This medium has an acid pH for the isolation of molds, other fungi, and yeasts.
Thiobacillus sp. was cultivated in thiosulfate medium with the following composition (per L): 10.0 g of Na2S2O3·5H2O, 4.0 g of KH2PO4, 4.0 g of K2HPO4, 1.0 g of NH4Cl, and 0.4 g of MgC12·6H20, with 0.5 mL of trace element solution added. The pH was 6.7 [14].
2.3.2. Under Anaerobic Condition
The medium used for anaerobic condition was similar to that used in the report by [15] and has the following composition (per L): 4.0 mL of sodium lactate, 1.0 g of yeast extract, 0.1 g of ascorbic acid, 0.2 g of heptahyate magnesium sulfate, 0.01 g of potassium hydrogen phosate (anhyd), 10.0 g of sodium chloride, 0.1 g of ferrous ammonium sulfate hexahydrate, 15.0 g of agar.
The polished SCSX60 specimens were placed in closed polyethylene conical flasks containing 100 g of soil samples with different moisture contents (2.5%, 5.0%, 10.0%, 15.0%, and 20.0%) for different immersion periods (1, 5 and 10 weeks) and incubated at 30˚C ± 1˚C. At the end of each immersion period, the specimens were carefully removed from the soil; the soil samples surrounding them were taken and placed into sterilized
Table 3. Chemical analysis of soil extracted solutions (mg/L).
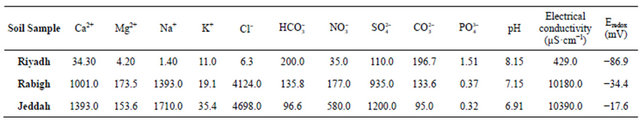
Table 4. Percentage of oxides in soil samples.

test tubes for microbial analysis. SCSX60 specimens were washed with tap water and immersed for 5 minutes in 20% NaOH containing 200 g/L of zinc dust at room temperature [16]. After removing from the pickling solution, the specimens were rinsed thoroughly with de-ionized water and acetone and finally dried with a stream of air.
Isolated bacteria, yeast and fungi were identified at laboratory [17-18], a sub cultures from each isolated microorganisms were made and identified at the Cairo MIRCEN, Ain Shams University, Cairo, Egypt to confirm the identifications. SRB and IRB were counted by a colony counter.
3. RESULTS
To fully elucidate the effect of soil treatment on the corrosion rate of SCSX60, the presence of microorganisms (fungus and bacteria) in the soil environment around the metal surface was analyzed for each soil condition at different treatments and immersion times. The resultant data is given in Table 5. The density of microorganisms differed depending on the type of soil quality and time of metal incubation.
The fungi Aspergillus sp. such as A. niger, A. flavus, A. fumegatus and Fusarium sp. Rhizopus sp. were isolated, and the isolated bacteria were bacilli and cocci aerobic and anaerobic. In addition, some of the soil specimens contained Saccharomyces cerevisiae yeast. Analysis of the isolated microorganisms from the studied soil showed the following species: Aspergillus niger, Aspergillus sydowii, Aspergillus flavus, Penicillium jensenii, Cladosporium herbarum, Emericella rugulosa, Emericella nidulans, Fusarium dimerum, Rhizopus stolonifer, Thiobacillus thiooxidans and T. ferrooxidans from most of the soil studied. The most common bacteria was Bacillus sp. which was identified as Bacillus megaterium
Table 5. Microorganisms isolated in aerated conditions.
Bacillus licheniformis, Bacillus subtilis and Bacillus thuringiensis.
Figures 2-4 show the effect of these bacteria on corrosion by variety of polymers from the metabolism produced of results in a scaly, nodule, and porous corrosion product.
Microscopic Observations of SCSX60 Specimens (SEM)

(A)
(B)
(C)
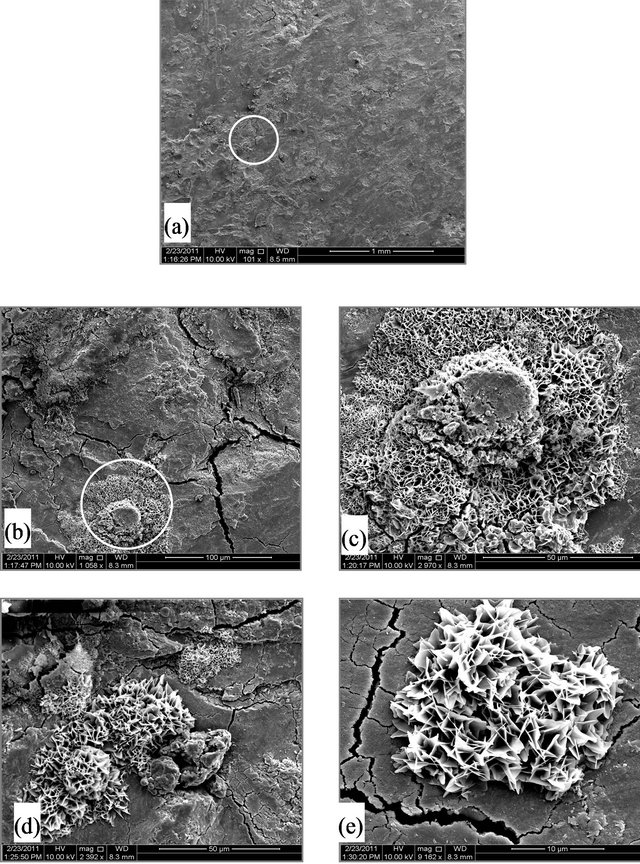
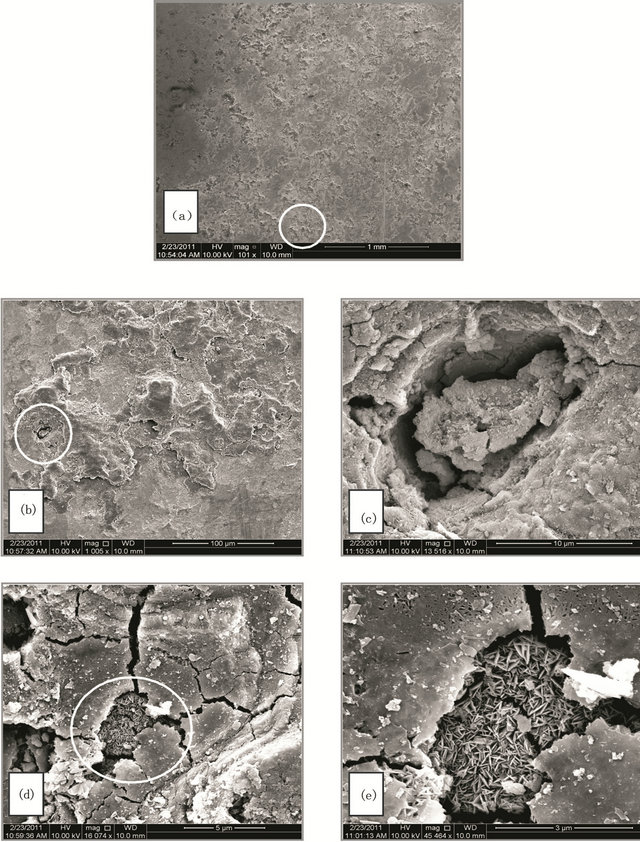
Figure 2. (A) SEM micrographs for SCSX60 surface after exposure in Riyadh soil at 2.5% moisture content for 5 weeks (the circled panels indicate the zoom on area); (B) SEM micrographs for SCSX60 surface after exposure in Riyadh soil at 10% moisture content for 5 weeks (the circled panels indicate the zoom on area); (C) SEM micrographs for SCSX60 surface after exposure in Riyadh soil at 20% moisture content for 5 weeks (the circled panels indicate the zoom on area).
SEM micrographs can macroscopically identify the corrosion products observed on the SCSX60 surface. The corrosion of iron and steel produces a wide variety of chemical compounds, the precise identity of which can provide critical information as to the corrosive conditions responsible for the damage.
Table 6 presents the bacteria species that were isolated in anaerobic conditions at different moisture contents in Riyadh, Rabigh and Jeddah soils after immersion for 1, 5, and 10 weeks. Iron-reducing bacteria (IRB) and sulfate-reducing bacteria (SRB) were found in all the studied soils at most moisture contents.
Figures 2-4 provide the different features of the SCSX60 surface after immersion for 5 weeks in Riyadh, Rabigh and Jeddah soils, respectively. The following sections represent a summary of these features and the possible types of corrosion products that appear on the metal surface for each soil under study.
(A)
(B)

(C)
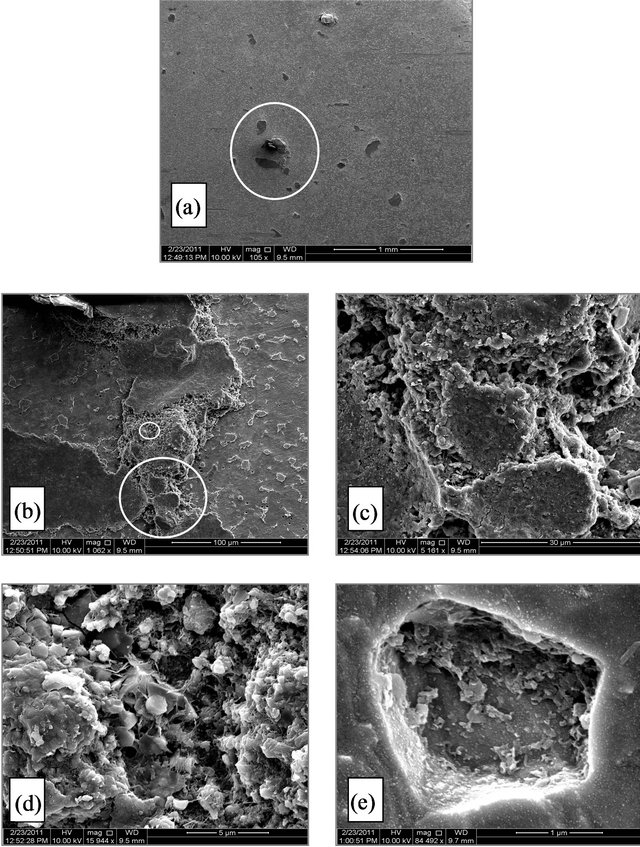
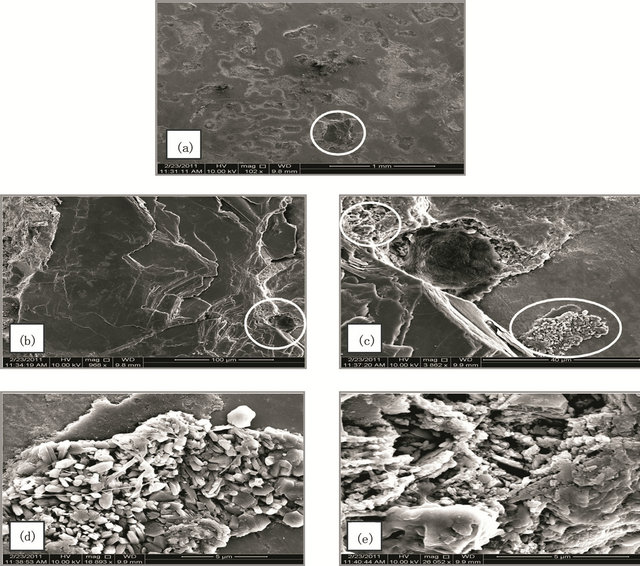
Figure 3. (A) SEM micrographs for SCSX60 surface after exposure in Rabigh soil at 2.5% moisture content for 5 weeks. (the circled panels indicate the zoom on area); (B) SEM micrographs for SCSX60 surface after exposure in Rabigh soil at 10% moisture content for 5 weeks (the circled panels indicate the zoom on area); (C) SEM micrograph for SCSX60 surface after exposure in Rabigh soil at 20% moisture content for 5 weeks (the circled panels indicate the zoom on area).
Table 6. Microorganism count (105) isolated in different conditions.

Riyadh
Figure 2(A)-(C) show SEM micrographs for SCSX60 specimens after immersion for 5 weeks in Riyadh soil at three different moisture contents: 2.5%, 10% and 20%. As observed, the morphology and composition of the corrosion products formed on SCSX60 specimens vary according to the studied moisture contents. In 2.5% moisture content, small cracks are visible in the layer of corrosion products (Figures 2(Ab) and 2(Ac)). If the film growth continues beyond this critical thickness, cracking occurs. With a high power zoom (Figures 2(Ad) and 2(Ae)), substances such as jelly glue can be observed among “stone like” corrosion products. Figure 2(Ad), shows a “cornflake-like” In addition, the cracks are also observed in the film on SCSX60 specimens formed by the corrosion products after immersion in Riyadh soil with 10% moisture content (Figure 2(B)).
(A)
(B)

(C)
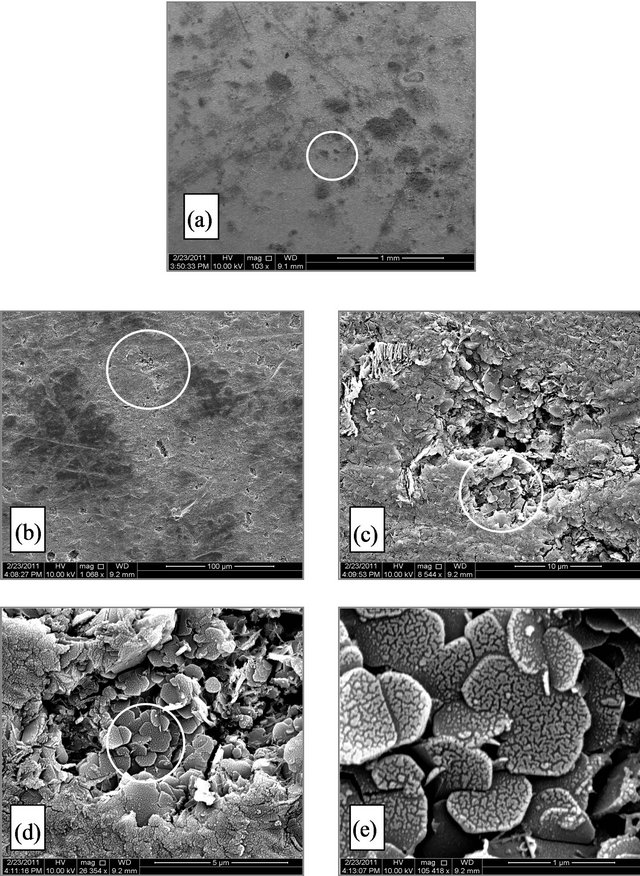
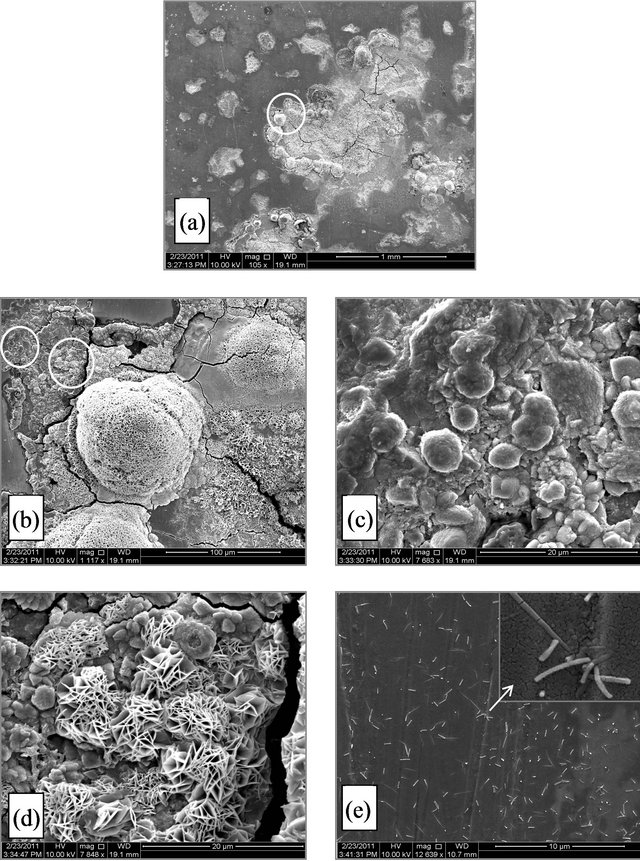
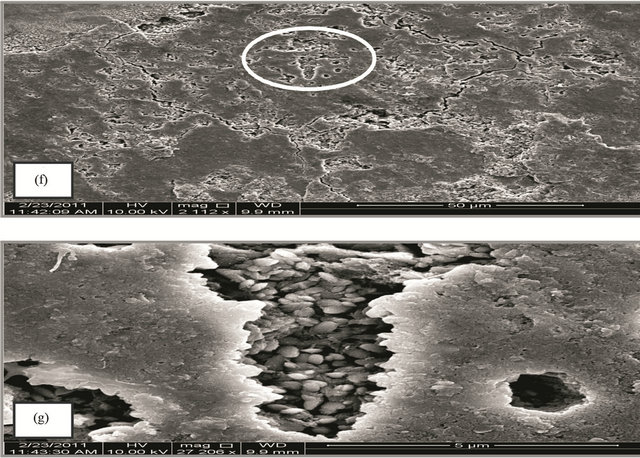
Figure 4. (A) SEM micrograph for SCSX60 surface after exposure in Jeddah soil at 2.5% moisture content for 5 weeks (the circled panels indicate the zoom on area); (B) SEM micrograph for SCSX60 surface after exposure in Jeddah soil at 10% moisture content for 5 weeks (the circled panels indicate the zoom on area); (C) SEM micrograph for SCSX60 surface after exposure in Jeddah soil at 20% moisture content for 5 weeks (the circled panels indicate the zoom on area).
A thin plate of overlapping crystals that looks like a “flowery” structure, can be seen in Figures 2(Bb)-2(Be).
In 20% moisture content, a low density of corrosion products can be seen (Figure 2(Ca)). By comparing between the structural features of SCSX60 after immersion for 5 weeks in Riyadh soil of 2.5%, 10%, and 20% moisture contents (Figures 2(Aa)-2(Ca)), Riyadh soil of 20% moisture showed the lowest density of corrosion products, which is in agreement with the corrosion rate measured at various moisture contents.
Rabigh Figures 3(A)-(C) show a representative SEM micrograph for SCSX60 specimens after immersion for 5 weeks in Rabigh soil at three different moisture contents: 2.5%, 10%, and 20%. In 2.5% moisture small pits with a distinct shape were visible as shown on the electron micrographs (Figure 3(Ab)). These pits were filled with corrosion products. A closer view of one pit (Figures 3(Ac)-3(Ae)) reveals hexagonal plates with several shapes.
Interestingly, in the case of the 10% moisture content, cracks observed in a layer of corrosion products that have a “cotton ball-like” structure (Figure 3(Ba)). In a high power zoom view (Figure 3(Bb)), the cotton ball structures were found to consist of FeS crystals joined to one another. A high magnification of the circle panels in Figure 3(Bb) shows FeS as amorphous round shape formations (Figure 3(Bc)) and as crystals (Figure 3(Bd)). Rod and spherical-shaped microbes (SRB) on the corrosion products film can be clearly observed (Figure 3(Be)).
A micrograph of the SCSX60 specimen exposed to 20% moisture is presented in Figure 3(C). The corrosion products are randomly distributed on the surface of the specimen (Figure 3(Ca)), and “cotton ball-like” structures of FeS could be observed in a high power zoom (Figures 3(Cb) and 3(Cc)).
Jeddah Figures 4(A)-(c) present the SEM micrograph for the SCSX60 specimens after immersion for 5 weeks in Jeddah soil at three different moisture contents: 2.5%, 10%, and 20%. The film of corrosion products that formed on the SCSX60 specimen in 2.5% moisture is inhomogeneous, and several pits can be observed (Figure 4(Ab)). When a high power zoom was taken (Figure 4(Ac)), the pits are deep, irregular and filled with corrosion products, Such as with crystals of FeS (Figures 4(Ad) and 4(Ae)). Several cracks can be observed in the corrosion film.
The environmental effect of these microorganisms is very clear in the case of 10% moisture content (Figure 4(Ba)). When high power zoom was taken (Figure 4(Bb)), layers of corrosion products observed. Moreover, the high power zoom (Figure 4(Bd)) showed rhombohedral structures associated with hexagonal plates of pyrrhotite and exopolymer substances. Jelly glue-like extracellular polymer substances are also observed among the corrosion products in this study (Figure 4(Be)). In another location on the specimen (Figure 4(Bf)), cracks and irregular deep pits are observed. Figure 4(Bg) shows a high magnification view of one pit filled with corrosion products.
In the case of 20% moisture content, characteristics of the substances observed in 2.5% and 10% moisture content disappeared. Small pits and cracks can be observed in the corrosion film (Figure 4(Cb)). When a high power zoom was taken (Figures 4(Cc) and (Ce)), the pits are filled with corrosion products. Some hexagonal plates with scales structures as corrosion products can also be observed (Figure 4(Cd)).
4. DISCUSION
The effect of microorganisms in this study on the tested metal depended on the moisture, temperature, and availability of oxygen, condition which allow the microorganisms to produce organic acids, amino acids, and other chemical products. Microbial metabolism uses oxygen, and the availability of organic compound stimulates the emergence of other types of microorganisms that may not be as abundant before incubation. All these metabolic reactions and the production of organic acids react with the iron, causing corrosion of the study metal, leading to an increasing number of bacteria to oxidize or reduce iron. This is clear by the initiation of growth of these microorganisms that started mainly in the 3rd week of incubation. This may be because bacterial spore formation cannot be completed if the level of nutrients is too low for the energy-requiring sporulation process, meaning that the interior of the endospore, the core, is very dry and resistant to moisture [16]. In Figures 2-4 a compared to figures a-d, a variety of polymers from the metabolism produced of Thiobacillus sp. results in a scaly, nodule, and porous corrosion product. This is consistent with previous studies [4,19] showing that the most important and most dangerous biocorrosion is caused by sulfate reducing bacteria (SRB), especially under conditions of temporary oxygen availability. The mechanism of metal corrosion by SRB is likely analogous to the one by Acidithiobacilus ferrooxidans, which causes metal sulfide dissolution [20-21].
In natural environments [22-23] the corrosion products that form on iron and steel surfaces can be extremely complex mixtures of sulfides, oxides, carbonates, and chlorides. According to Anderko and Shuler [24] magnetite and siderite are precursors to iron sulfide species and smythite is a less stable form of iron sulfide. Mackinawite (tetragonal) is a particular form of iron sulfide that occurs frequently in immersion corrosion studies and is produced easily from iron and iron oxides by a consortium of microorganisms, including SRB [25]. Although mackinawite and pyrrhotite (hexagonal) are the most common forms of iron sulfide corrosion products, pyrrhotite is considered to be more thermodynamically favored in anoxic environments [24,26].
Videla [27] proposed that the morphology of the corrosion product is influenced by iron bacterial species. Bacterial activity can usually be confirmed by the measurement of carbon consumption, waste product generation, iron reduction, and/or growth.
IRB is a very important part of the soil microbial community. As most of the IRBs are facultative anaerobes, oxygen availability is preferential for their growth, but they maintain while the ability to grow under anaerobic conditions. IRBs act by reducing the generally insoluble Fe3+ compounds to the soluble Fe2+, thereby exposing the ferric oxide protective layer of metal beneath the soil to the corrosive environment [5,28,29].
IRBs are capable of making the environment suitable for SRB. In a mixed population of microorganisms in a biofilm, the redox potential starts to decrease as oxygen is consumed so that nitrate, ferric ion, and the sulfate are reduced [8,30]. Beech and Gaylarde [6] have shown that metal corrosion is more severe if SRBs are present in the bacterial consortium than if there is a single SRB species. In the presence of SRBs, the main corrosion products that form on the iron surface are iron sulfides. Iron sulfide has the characteristics of a semiconductor, acting as a medium for the transportation of electrons in a galvanic couple with the steel substance, enhancing the anodic and cathodic reaction [31]. Previous studies [26,32] have comprehensively examined the galvanic couple between pipes buried in soil and the sulfide-rich corrosion deposit that sustains the high corrosion rates observed at field sites. The main suggested mechanism of corrosion by SRB in an anaerobic environment is the effect of sulfide ion production by the reduction of sulfate by bacterial metabolic activity and its oxidation and reduction products [33-35]. There are various mechanisms that suggest a role for SRB in the corrosion process of iron.
According to Hausler et al. [36], the corrosion product forms a film that grows until a critical thickness. The products showed in Figure 2 are extracellular polymer substances resulting from the metabolic activity of mi- croorganisms. The formation can be observed clearly through the extracellular polymer substances. In the microscopically studied [10], the interaction between corrosion-causing bacterium and the corroding mild steel coupon. The authors found that surface of mild steel coupon changed from loss or deposition of corrosion products and from the attachment of the bacteria and its exopolysaccharide fibers to the coupon surface.
In Figure 2 Similar structures were previously observed on X65 carbon steel samples during its corrosion in H2S/CO2 environments for various periods of time [37]. The crystals that appear in Figure 2(B) are a form of iron sulfide FeS. The formation of FeS may be generated by a microbial reduction of 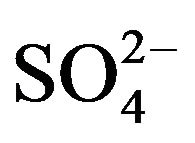 to
to 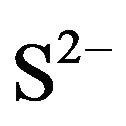 [3,38, 39]. Zhao et al. [40] reported that in an environment containing SRB, the original corrosion products are mainly iron (oxyhydr)oxide. In the presence of
[3,38, 39]. Zhao et al. [40] reported that in an environment containing SRB, the original corrosion products are mainly iron (oxyhydr)oxide. In the presence of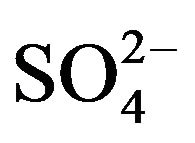 , (oxyhydr)oxide transforms to sulfide with the reaction of biogenic hydrogen sulfide, extracellular polymer substances, and organic acid. The authors of a previous study studied the transformation by SRB of iron to iron sulfide through iron oxyhydro (oxide) in a marine environment [19,22,37,41].
, (oxyhydr)oxide transforms to sulfide with the reaction of biogenic hydrogen sulfide, extracellular polymer substances, and organic acid. The authors of a previous study studied the transformation by SRB of iron to iron sulfide through iron oxyhydro (oxide) in a marine environment [19,22,37,41].
A recent study of C-steel corrosion in natural sea water over 1 and 2 years immersion periods evaluated the appearance of hexagonal plates with attached rod-shaped bacteria, which was identified as SRB [7], while other study observed hexagonal plates of FeS on an API X70 steel surface after 24 h of immersion in a H2S -saturated solution at room temperature [42].
The rhombohedral showed in Figure 4 structures may be smythite, a naturally occurring rhombohedral sulfide. Smythite is formed by the sulfidation of siderite (ferrous carbonate FeCO3). McNeil et al. [41] used mineralogical data determined by X-ray crystallography, thermodynamic stability diagrams (Pourbaix diagrams), and the simplexity principle for precipitation reactions to evaluate corrosion product mineralogy. The authors concluded that many sulfides under near-surface natural environmental conditions could only be produced by microbiological action on metals.
5. CONCLUSION
The aim of this study was to elucidate the effect of the environment on SABIC carbon steel grade X60 (SCSX60) specimens, which are a pipeline type of steel that extend beneath the soil in several areas of Saudi Arabia. The study investigated the effect of the types of soil and exposure conditions (moisture content and immersion period) on the composition and morphology of SCSX60 in different areas, and the role of bacteria in corrosion of SCSX60 was assessed in the studied soils. The results showed that the type of corrosion product that formed on the SCSX60 specimens depends on the soil type and exposure conditions. In addition, the bacteria play an important role in the corrosion of SCSX60 in the studied soils. We recommend that these results can be used in industry to better the properties of steel plates.
6. ACKNOWLEDGEMENTS
This work has been supervised by Dr. Ehteram Noor, associate professsor of physical chemistry, chemistry dep., King Abdulaziz University. Would like to thank her for her technical help during field and laboratory experiments.
We are grateful to King Abdul Aziz City for Science and Technology (KACST) for funding this study by grand number AT-17-198. Also, we would like to thank Saudi Basic Industries Corporation (SABIC) for supplying carbon steel specimens of grade X60 that have been used in the completion of this study.
REFERENCES
- Rassenfoss, S. (2011) From bacteria to barrels: Microbiology having an impact on oil fields. Journal of Petroleum Technology, 32, 32-39.
- Rassenfoss, S. (2011) From bacteria to barrels: Microbiology having an impact on oil fields. Journal of Petroleum Technology, 32, 32-39.
- Puls, R.W., Paul, C.J. and Powell, R.M. (1999) The application of in situ permeable reactive (zero-valent iron) barrier technology for the remediation of chromate-contaminated groundwater: A field test. Applied Geochemistry, 14, 989-1000.
- Gittel, A., Sørensen, K.B., Skovhus, T.L., Ingvorsen, K., and Schramm, A. (2009) Prokaryotic community structure and sulfate reducer activity in water from high-temperature oil reservoirs with and without nitrate treatment. Applied and Environmental Microbiology, 75, 7086-7096. doi:10.1128/AEM.01123-09
- Obuekwea, C.O., Westlake, D.W.S., Plambeck, J.A. and Cook, F.D. (1981) Corrosion of mild steel in cultures of ferric iron reducing bacterium isolated from crude oil I. polarization characteristics. Corrosion, 37, 461-467. doi:10.5006/1.3585992
- Obuekwea, C.O., Westlake, D.W.S., Plambeck, J.A. and Cook, F.D. (1981) Corrosion of mild steel in cultures of ferric iron reducing bacterium isolated from crude oil I. polarization characteristics. Corrosion, 37, 461-467. doi:10.5006/1.3585992
- Duan, J., Wu, S. Zhang, X., Huang, G., Du, M. and Hou, B. (2008) Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater. Electrochimica Acta, 54, 22-28. doi:j.electacta.2008.04.085
- Schwermer, C.U., Lavik, G., Abed, R.M.M., Dunsmore, B., Ferdelman, T.G., Stoodley, P., Gieseke, A. and De Beer, D. (2008) Impact of nitrate on the structure and function of bacterial biofilm communities in pipelines used for injection of seawater into oil fields. Applied and Environmental Microbiology, 74, 2841-2851. doi:10.1128/AEM.02027-07
- Jack, R.F., Ringelberg, D.B. and White, D.C. (1992) Differential corrosion rates of carbon steel by combinations of Bacillus sp., Hafnia alvei and Desulfovibrio gigas established by phospholipid analysis of electrode biofilm. Corrosion Science, 33, 1843-1853. doi:0010-938X(92)90188-9
- Obuekweb, C.O., Westlake, D.W.S., Cook, F.D. and Costerton, J.W. (1981) Surface changes in mild steel coupons from the action of corrosion-causing bacteria. Applied and Environmental Microbiology, 41, 766-774.
- Thierry, D. and Sand, W. (2002) Microbially influenced corrosion: Corrosion mechanisms in theory and practice. In: Marcus, P. Ed., CRC Press, 16, 563-603.
- Maier, R.M., Pepper, I.L. and Gerba, C.P. (2009) Environmental microbiology. 2nd Edition, ELSEVIER. 387- 439. doi:B978-0-12-370519-8.00020-1
- Maier, R.M., Pepper, I.L. and Gerba, C.P. (2009) Environmental microbiology. 2nd Edition, ELSEVIER. 387- 439. doi:B978-0-12-370519-8.00020-1
- Maier, R.M., Pepper, I.L. and Gerba, C.P. (2009) Environmental microbiology. 2nd Edition, ELSEVIER. 387- 439. doi:B978-0-12-370519-8.00020-1
- Allred, R.C. (1958) Methods used for the counting of sulfate-reducing bacteria and for the screening of bactericides. Producers Monthly, 22, 32-34.
- Oguzie, E.E., Agochukwu, I.B. and Onuchukwu, A.I. (2004) Monitoring the corrosion susceptibility of mild steel in varied soil textures by corrosion product count technique. Matererial Chemistyr Physics, 84, 1-6. doi:10.1016/j.matchemphys.2003.09.002
- Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T. and Williams, S.T. (1994) Bergey’s manual of determinative bacteriology. 9th Edition, Williams and Wilkins.
- Watanabe, T. (2002) Pictorial atlas of soil and seed fungi: Morphologies of cultured fungi and key to species. 2nd Ed., CRC Press. doi:10.1201/9781420040821
- Lane, D.J., Harrison, A.P., Stahl, D., Pace, B., Giovannoni, S.J., Olsen, G.J. and Pace, N.R. (1992) Evolutionary relationships among sulfurand iron-oxidizing eubacteria. Journal of Bacteriology, 174, 269-278.
- Hartomo, W.A., Rizki, I.N., Widyanto, B. and Chaerun, S.K. (2010) Microbiologically influenced corrosion (MIC) of AISI 1006 carbon steel by Acidithiobacillus ferrooxidans and Desulvofibrio piger. Proceedings of the Third International Conference on Mathematics and Natural Sciences, 895-905.
- Javaherdashti, R. (2008) Microbiologically influenced corrosion “An engineering insight”. Springer, 1-35.
- Jeffery, R. and Melchers, R.E. (2003) Bacteriological influence in the development of iron sulfide species in marine immersion environments. Corrosion Science, 45, 693-714. doi:S0010-938X(02)00147-6
- Phillips, D.H., Watson, D.B., Roh, Y. and Gu, B. (2003) Mineralogical characteristics and transformations during long-term operation of a zerovalent iron reactive barrier. Journal of Environmental Quality, 32, 2033-2045. doi:10.2134/jeq2003.2033
- Anderko, A. and Shuler, P.A. (1997) Computational approach to predicting the formation of iron sulfide species using stability diagrams. Computers & Geosciences, 23, 647-658. doi:S0098-3004(97)00038-1
- Hamilton, W.A. (1994) Biocorrosion: The action of Sulfate-reducing bacteria. In: Ratledge, C., Ed., Biochemistry of Microbial Degradation, Kluwer Academic, Dordrecht, 555-570. doi:10.1007/978-94-011-1687-9_17
- McNeil, M.B. and Little, B.J. (1990) Mackinawite formation during microbial corrosion. Corrosion, 46, 599-600. doi:10.5006/1.3585154
- Videla, H.A. (1996) Manual of biocorrosion. Lewis Publishers, Boca Raton, 37-67.
- Dubiel, M., Hsu, C.H., Chien, C.C., Mansfeld, F. and Newman, D.K. (2002) Microbial iron respiration can protect steel from corrosion. Applied and Environmental Microbiology, 63, 1440-1445. doi:10.1128/AEM.68.3.1440-1445.2002
- Graff, W.J. (1981) Introduction to offshore structures. Gulf Publishing Co., Houston, Chapter 12.
- Panter, C. (2007) Ecology and characteristics of iron reducing bacteria-suspected agents in corrosion of steels, Mic—An international perspective symposium, extrin corrosion consultants. Curtin University, Perth, 14-15.
- King, R.A. and Mille J.D. (1971) Corrosion by the sulfate reducing bacteria. Nature, 233, 491-492. doi:10.1038/233491a0
- Jack, T.R., Wilmott, A., Stockdale, J., Van Boven, G., Worthingham, R.G. and Sutherby, R.L. (1998) Corrosion consequences of secondary oxidation of microbial corrosion. Corrosion, 54, 246-252. doi:10.5006/1.3284850
- Newman, R.C., Wong, W.P. and Garner, A. (1986) A mechanism of microbial pitting in stainless steel. Corrosion, 42, 489-491. doi:10.5006/1.3583056
- Pourbaix, A., Aguiar, L.E. and Clarinval, A.M. (1993) Local corrosion processes in the presence of sulphatereducing bacteria: Measurements under biofilms. Corrosion Science, 35, 693-698. doi:10.1016/0010-938X(93)90205-U
- Schaschl, E. (1980) Elemental sulfur as a corrodent in deaerated neutral aqueous solutions. Materials Performance, 19, 9-12.
- Hausler, R.H., Goeller, L.A., Zimmerman, R.P. and Rosenwald, R.H. (1972) Contribition to the “filming amine” theory: An interpretation of experimental results. Corrosion, 28, 7-16. doi:10.5006/0010-9312-28.1.7
- Svenningsen, G., Palencsár, A. and Kvarekvål, J. (2009) Investigation of iron sulfide surface layer growth in aqueous H2S/CO2 environments. Corrosion, 09359.
- Gu, B., Watson, D. B., Wu, L., Philips, D.H., White, D.C., and Zhou J.Z. (2002) Microbiological characteristics in a zero-valent iron reactive barier. Environmental Monitoring and Assessment, 77, 293-309. doi:10.1023/A:1016092808563
- Scherer, M.M., Richter, S., Valentine, R.L. and Alvarez, P.J. (2000) Chemistry and microbiology of permeable reactive barriers for in situ groundwater cleanup. Critical Reviews in Environmental Science and Technology, 30, 363-411. doi:10.1080/10643380091184219
- Zhao, X., Duan, J., Houand, B. and Wu, S. (2007) Effect of sulfate-reducing bacteria on corrosion behavior of mild steel in sea mud. Journal of Materials Science and Technology, 23, 323-328.
- McNeil, M.B., Jones, J.M. and Little, B.J. (1991) Mineralogical Fingerprints for Corrosion Processes Induced by Sulfate-Reducing Bacteria. Corrision, 580.
- Arzola, S., Mendoza-Flores, J., Duran-Romero, R. and Genesca, J. (2006) Electrochemical behavior of API X70 steel in hydrogen sulfide-containing solutions. Corrosion, 62, 433-443. doi:10.5006/1.3278280

